Abstract
Background
To circumvent the challenges associated with delivering large compounds directly to the brain for the treatment of Parkinson’s disease (PD), non-invasive procedures utilizing smaller molecules with protective and/or restorative actions on dopaminergic neurons are needed.
New Method
We developed a methodology for evaluating the effects of a synthetic neuroactive peptide, DNSP-11, on the nigrostriatal system using repeated intranasal delivery in both normal and a unilateral 6-hydroxydopamine (6-OHDA) lesion rat model of PD.
Results
Normal rats repeatedly administered varying doses of DNSP-11 intranasally for 3 weeks exhibited a significant increase in dopamine (DA) turnover in both the striatum and substantia nigra (SN) at 300 μg, suggestive of a stimulative effect of the dopaminergic system. Additionally, a protective effect was observed following repeated intranasal administration in 6-OHDA lesioned rats, as suggested by: a significant decrease in d-amphetamine-induced rotation at 2 weeks; a decrease in DA turnover in the lesioned striatum; and an increased sparing of tyrosine hydroxylase (TH) positive neurons in a specific sub-region of the lesioned substantia nigra pars compacta. Finally, tracer studies showed 125I-DNSP-11 distributed diffusely throughout the brain, including the striatum and SN, as quickly as 30 minutes after a single intranasal dose.
Comparison with Existing Methods
The results of bilateral intranasal administration of DNSP-11 are compared to our unilateral single infusion studies to the brain in rats.
Conclusions
These studies support that DNSP-11 can be delivered intranasally and maintain its neuroactive properties in both normal rats and in a unilateral 6-OHDA rat model of PD.
Keywords: 6-hydroxydopamine, nigrostriatal pathway, neurochemistry, neuroprotection, peptide, intranasal administration
1. Introduction
Neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) have shown great promise in treating an array of neurodegenerative diseases such as Parkinson’s disease (PD) [1–4]. However, as with many protein biotherapeutics, neurotrophic factors are unable to cross the blood-brain barrier due to their large size and poor bioavailabilty following both oral and systemic administration [5]. To directly target the central nervous system (CNS), neurotrophic factors such as GDNF have been delivered by direct intraparenchymal infusion [3, 4, 6, 7]. However, its poor biodistribution after surgical implantation of a catheter into the brain, likely due to GDNF’s high affinity heparin-binding domains, has resulted in failed phase II clinical trials [4, 8–10]. To overcome the challenges associated with the invasive delivery of neurotrophic factors and other large molecules, numerous studies have shown that intranasal administration is effective in delivering compounds including but not limited to peptides to the CNS [11–17]. Therefore, the discovery and development of smaller molecules with neuroprotective or restorative properties that can be used in a less invasive delivery regimen, such as intranasal administration, is an attractive therapeutic strategy for treating neurodegenerative diseases [18–20].
Dopamine neuron stimulating peptide-11 (DNSP-11) is a synthetic, amidated 11-amino acid neuroactive peptide derived from the human GDNF pro-domain [18–21]. In both E14 primary neurons derived from the ventral mescencephalon of Sprague Dawley rats and dopaminergic neuron cell lines, our team demonstrated that DNSP-11 is able to promote neuronal growth, differentiation, and activate the phosphorylation of ERK1/2 as well as providing protection against apoptosis and cell death through mechanisms not directly involving its binding to the GFRα-1 receptor [18–20]. Furthermore, following a single intranigral infusion of DNSP-11 our team has shown that DNSP-11 increases levels of dopamine (DA) and its active metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic (HVA), and increases potassium evoked release in the striatum of normal Fischer 344 (F344) rats [18, 19], while decreasing apomorphine-induced rotation in a unilateral 6-hydroxydopamine (6-OHDA) rodent model of PD [18]. Based on DNSP-11’s in vitro and in vivo bioactivity [18, 19], lack of heparin binding sites lending to increased biodistribution [18, 20], and stable structure allowing for long term storage [18, 20], we hypothesized that DNSP-11 would be an attractive candidate to test the efficacy of repeated intranasal administration in both normal and a unilateral 6-OHDA striatal lesion rat model that mimics the later stages of PD [22].
In this series of studies, we report the methodology for intranasal delivery of DNSP-11 and demonstrate the efficacy of repeated intranasal administration of DNSP-11 on the nigrostriatal system in both normal and in unilaterally 6-OHDA lesioned F344 rats, using light isoflurane anesthesia. Utilizing the optimal dosage (300 μg) determined from our dose response study in normal rats, we investigated changes in d-amphetamine-induced rotation, DA turnover [(DOPAC + HVA/DA)], and tyrosine hydroxylase (TH) positive (+) neuronal sparing in a unilateral 6 - OHDA lesion model of the striatum [22]. Additionally, we investigated DNSP-11’s distribution in the brain and its uptake into CSF and blood following a single intranasal dose of 125I-labeled DNSP-11 as a function of time. Collectively, our results demonstrate for the first time that DNSP-11 is able to be delivered to the CNS intranasally and maintains its neuroactivity on dopamine neurons in both normal rats and unilaterally 6-OHDA lesioned rats, after repeated intranasal administration.
2. Methods
2.1. Ethics statement
All animal procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee in agreement with AAALAC guidelines.
2.2. Materials
All chemicals were either purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Fisher Chemical Fairlawn, NJ). DNSP-11 was synthesized and purified to >98% purity by Genscript (Piscataway, NJ) for all studies. The modified DNSP-11 sequence (R9K) was synthesized by AAPPTec (Louisville, KY) and iodinated (125I) via the Bolton-Hunter method by PerkinElmer (North Billerica, MA) and purified to ≥ 93 % by reverse phase HPLC. DNSP-11 was dissolved in 0.9% sterile saline for all in vivo studies in which it has previously been shown to be stable [20].
2.3. Intranasal administration of DNSP-11
For all in vivo studies, male F344 rats between 3–8 months old were utilized. Rats were obtained from Harlan Laboratories Inc. (Indianapolis, IN) and were housed on a 12 hour light/dark cycle with water and food provided to rats ad libitum. For all intranasal studies, rats were initially lightly anesthetized using an induction chamber and then transferred to a nose cone where they continued to receive light isoflurane anesthesia (~ 1.0 – 3.0% with 1.0% oxygen) for the duration of the dosing period, typically lasting a total of 20 minutes. While in a supine position, each rat received a total volume of 50 μl of either vehicle (0.9 % sterile saline) or a mixture of DNSP-11 in an equivalent volume of vehicle. Prior to each intranasal dose, the rats were removed from a nose cone and the tip of an Oxford Benchmate pipette (range: 10 – 50 μl) was placed at the tip of the rat’s nare (Fig. 1.A). A total of 12.5 μl was administered to each nare in 3 – 4 μl droplets allowing the rat to inhale the solution over the course of approximately 30 – 45 seconds (Fig. 1.B). After administering 12.5 μl to the first nare (time = 0), the solution was allowed to absorb for 5 minutes prior to dosing the opposite nare. This regimen was repeated a total of 4 times alternating between nares (2 times per nare) [11, 14]. For all intranasal experiments, excluding tracer studies, researchers were blinded to intranasal treatments for all in vivo experiments, excluding tracer studies.
Figure 1. Illustration of intranasal administration of DNSP-11 in F344 rats.
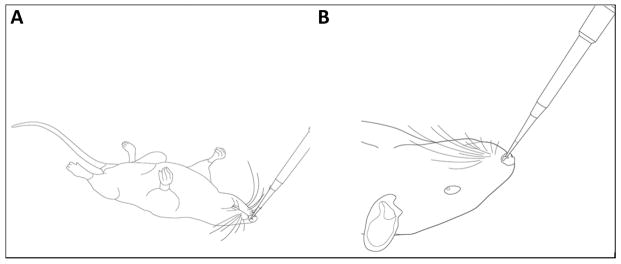
For all in vivo intranasal studies rats were anesthetized with isoflurane (A) and administered either vehicle or DNSP-11 while in a supine position. (B) For intranasal dosing a micropipetter containing 12.5 μl of either vehicle or DNSP-11 in an equivalent volume of vehicle was administered.
2.4. Dose response in normal rats (HPLC-EC neurochemical analysis)
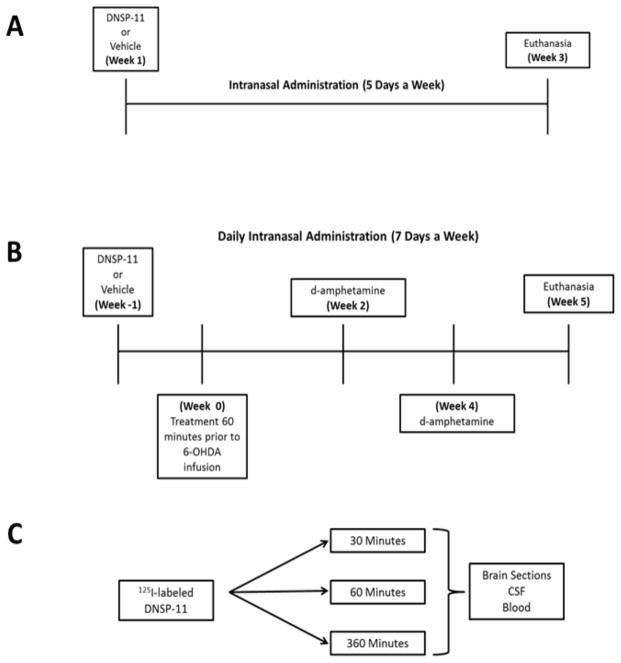
Previous studies in rodents have indicated that generally < 1% of compounds administered intranasally enter the CNS (13, 14, 27–29). To take into account lower levels of delivery to the CNS, we examined half-log steps above and below a 10-fold increase from the most effective DNSP-11 dosage (30 μg) determined from previous infusion studies in rodents [18, 19, 23]. In this study, rats received either 100 μg (n = 6), 300 μg (n = 6) or 1000 μg (n = 6) DNSP-11 in vehicle or vehicle alone (n = 9), 5 days a week for 3 weeks under light isoflurane (~ 1.0 – 3.0% with 1% oxygen) anesthesia (Fig. 2.A).
Figure 2. Experimental timeline for all intranasal studies.
Three major studies designs were used to determine the efficacy of repeated intranasally administered DNSP-11’s effects on the nigrostriatal system in both normal and unilateral 6-OHDA- lesioned rats using isoflurane anesthesia. (A) To determine the efficacy of our intranasal delivery method and adequate dosing, normal rats were subjected to repeated intranasal administration of various doses of DNSP-11, 5 days a week for 3 weeks (21 days). (B) Utilizing the determined dosage from experiment A, the efficacy of intranasally administered DNSP-11’s effects on the nigrostriatal system were examined in a unilateral 6-OHDA (right hemisphere) lesion model of PD where rats were treated with either vehicle or DNSP-11 daily, 1 week (7 days) prior to unilateral 6-OHDA infusion and 5 weeks (35 ± 3 days) post-surgery. Prior to the start of the study baseline d-amphetamine rotation data were determined and at both 2 and 4 weeks post unilateral 6-OHDA infusion to assess lesion severity. (A–B) For experiments A and B, researchers were blinded to the treatments throughout the entirety of the studies. (C) Tracer studies examined 125I-labeled DNSP-11’s distribution at 30, 60, and 360 minutes after the start of the first intranasal dose in blood, CSF and the CNS.
At 3 weeks, rats were euthanized under heavy isoflurane anesthesia (5.0% with 1% oxygen), and the brain was removed, submerged in ice-cold saline and then placed in a brain mold (on dry ice) where 2 mm sections of the entire striatum at the optic chiasm and SN were harvested and frozen at −80 °C for neurochemical analysis [41]. For HPLC-EC analysis, pre-weighed (wet weight) tissue samples from the SN and striatum were thawed and immediately processed, as described previously [24]. A total of 50 μl of filtrate (in mobile phase) was injected onto an HPLC column. Retention times of DA standards and dihydroxybenzylamine (DHBA), which was used as an internal standard, were used to identify peaks and used to determine tissue levels (DA, DOPAC, HVA) found in each brain sample represented as ng/g wet weight of the tissue sample [24].
2.5. Unilateral, 3-site, 6-OHDA striatal lesion
Rats were intranasally pre-treated daily with either 300 μg (selected from the dose response study) of DNSP-11 in vehicle, or vehicle alone, for 7 days prior to 6-OHDA lesion. On the day of surgery, rats received intranasal treatment (DNSP-11 or vehicle) approximately 60 minutes prior to 6-OHDA infusion under isoflurane anesthesia (see section 2.3; Fig. 1A–B). Rats were then placed in a stereotaxic frame and received isoflurane (~ 1.0 – 2.0% with 1% oxygen) throughout the duration of surgery. Using previously published procedures and stereotaxic coordinates [22, 23] to target the dorsal striatum, injection sites for 6 - OHDA infusion included; ((1) AP: + 1.0, ML: − 3.0, DV: − 5.0); (2) AP: − 0.1, ML: − 3.7, DV: − 5.0; (3) AP: − 1.2, ML: − 4.5, DV: − 5.0)). Each injection site received 10 μg/ 2.5 μl of the 6-OHDA in 0.2% ascorbic acid sterile saline solution for a total dose of 30 μg/ 7.5 μl using a 26 gauge Hamilton syringe. Designated blinded intranasal treatments continued post-6-OHDA infusion, 7 days a week for a total of 5 weeks (35 ± 3 days) (Fig. 2B).
2.6. d-amphetamine-induced rotation in 6-OHDA-lesioned rats
Prior to rats entering the study (n = 29), an exclusion criterion was used to test for baseline hemisphere asymmetry with d-amphetamine (2.5 mg/kg, i.p.). Rats were excluded (n = 8) from entering the study if baseline rotation exceeded ≥ 160 turns per hour in either direction (clockwise and/or counterclockwise). After 6-OHDA infusion, rats (n = 21) received d-amphetamine (2.5 mg/kg, i.p.) at both 2 and 4 weeks to assess lesion severity (Fig. 2.B). Rats were placed in automated rotometer bowls and clockwise rotations were recorded over the course of 24 intervals that lasted 300 seconds (5 minutes) each using SDI Rotation (San Diego, CA USA). For analysis, only the peak 60 minute rotation time was used after the first 15 minutes had elapsed [25, 26]. On the day of testing, rats were not anesthetized and/or given their designated blinded intranasal treatment.
2.7. DA and DA metabolite levels in the striatum of 6-OHDA lesioned rats
At 5 weeks post-6-OHDA lesion, rats were heavily anesthetized with isoflurane (5.0% with 1% oxygen) and transcardially perfused using approximately 200 ml of ice-cold saline. The brain removed, placed in an ice-cold saline bath and then placed in an ice-cold brain mold (on dry-ice) where 2 mm thick sections were taken and the entire striatum was removed. Tissue was then flash frozen utilizing dry ice and stored at −80 °C for later HPLC-EC analysis. For HPLC-EC analysis, tissue samples were processed in the same manner as previously discussed (see section 2.4) except a total of either 50 or 25 μl of filtrate in mobile phase was injected onto the HPLC column. If analytes were undetectable at these injection volumes, samples were run on a C18 column (4.6 mm x 75 mm, 3 μm particle size, Shiseido CapCell Pak UG120, Shiseido Co., LTD., Tokyo, Japan) with a more sensitive limit of detection was used [27].
2.8. Tissue processing for TH + neurons in the SN of 6-OHDA lesioned rats
After the striatum was removed for later HPLC-EC analysis the midbrain was blocked off and immersioned fixed in a 4% paraformaldehyde solution for 24 hours. Tissue was then submerged in increasing sucrose concentrations (15 & 30 %) and frozen at −80 °C. Frozen serial coronal sections (30 μm) were cut on a sliding microtome and stored in cryoprotectant solution (30% sucrose, 30% ethyelene glycol in 100 mM sodium phosphate buffered saline (NaPBS), pH 7.2)) at −20 °C [28].
For staining purposes, every sixth series free floating serial coronal section was stained for tyrosine hydroxylase (TH) as previously published with minor adjustments [29]. Sections were treated with the primary antibody against TH overnight (1:10,000; Millipore, Billerica, MA) and then incubated in biotinylated horse anti-mouse secondary antibody (1:5,000; Vector, Burlingame, CA) for 1 hour at controlled room temperature (21 – 22 °C). Tissue was then incubated with avidin-biotin-peroxidase complex using an Elite ABC Vectastain kit (Vector; Burlingame, CA) and TH immunoreactivity was visualized using 3,3′-diaminobenzidine (DAB) with nickel enhancement.
2.9. TH + neurons and fiber density in 6-OHDA lesioned rats
TH + neurons were counted at three different levels: − 5.24 mm, − 5.60 mm and −5.96 mm with respect to bregma using BioQuant Image Analysis System (BioQuant, Nashville, TN USA) [30]. The oculomotor nerve root was used to determine the borders of the substantia nigra pars compacta (SNpc) from the ventral tegmental area [28]. The degree of dopaminergic sparing on the ipsilateral side to the lesion was determined by comparing the average total number of TH+ neurons at each level examined in rats treated with either DNSP-11 or vehicle.
The substantia nigra pars reticulata (SNpr) was also examined to determine TH+ fiber density (total area of the SNpr with TH+ immunoreactive staining) and measured using BioQuant Image Analysis Software at all three levels for rats (n = 5 vehicle; n = 6 DNSP-11) that were perfused, free of background staining due to remnants of blood vessels. Data were average for each section for statistical analysis. One rat was removed from all TH+ neuronal counts and fiber density analysis, as tissue depth was inconsistent because of sectioning problems with the microtome.
2.10. Distribution studies (gamma counting & autoradiography) in normal rats
Rats were administered a one-time dose of 125I - labeled DNSP-11 (50 μl/50 μCi-300 μg DNSP-11) while under light isoflurane anesthesia (Fig. 1A – B). At 30 (n = 4), 60 (n = 3) and 360 (n = 3) minutes after the start of the first intranasal dose (time = 0), CSF was immediately collected (Fig. 2.C). For CSF collection, a modified version presented by Nirogi et al. was used [31]. Rats were placed upright and the back of the head was shaved while a piece of gauze was placed under the neck angling the rat’s head downward 45 degrees. The rat was then deeply anesthetized with isoflurane (~ 5.0 % with 1 % oxygen) and the foramen magnum was located using the wings of the atlas and occipital protuberance as landmarks. A 23 gauge needle (affixed to a short length of tubing connected to a 1.0 ml syringe) was inserted at the midline center point and ~100 – 120 μl of CSF was removed. The flexible tubing attached to the syringe allowed for the syringe to be clamped off using hemostat forceps as a fail-safe prior to blood contaminating the CSF sample.
Prior to transcardial perfusion, 500 μl of blood was removed via aortic puncture and rats were then perfused with 200 ml of ice-cold saline. The brain was removed, the olfactory bulbs were removed at the frontal cortex, and the brain was then placed into a brain mold (on wet ice). Eleven 2 mm thick coronal slabs were then harvested and all brain, CSF, and blood samples were immediately analyzed by gamma counting (Cobra II Auto-Gamma, Packard). Both brain and CSF samples were only quantified by gamma counting if no blood was visible. To determine normalized concentrations of tracer found in each sample, the counts per minute (CPM) values for all samples (brain, CSF, blood) were divided by the CPM value determined for the total 125I-labeled dose (50 μl/ 50 μCi – 300 μg DNSP-11) which was then normalized by the weight (mg) or volume (μl) of the wet sample.
For autoradiography analysis, one rat was administered a one-time 125I-labeled DNSP-11 (50 μl/50 μCi – 300 μg DNSP-11) and euthanized 60 minutes after the start of the first intranasal dose. The rat was transcardially perfused with 200 ml of ice-cold saline and 200 ml of 4 % paraformaldehyde. Frozen sagittal brain sections were taken using a cryostat (0.5 mm thick). Sections were then exposed for 21 days on a GE phosphor screen [11], and analyzed for radioactive signal (Typhoon 9400, GE Health Care).
2.11. Statistics
Prior to statistical analysis one animal was removed from the 6-OHDA study as d-amphetamine-induced counterclockwise rotations exceeded that of clockwise rotations and did not observe a ≤ 90% depletion of striatal DA. Neurochemical (analyte and/or DA turnover) analysis for both normal and 6-OHDA lesioned rats were analyzed by a one-way ANOVA with Bonferroni’s post hoc test. A two-way ANOVA with repeated measures with Bonferroni’s post hoc test was used to analyze d-amphetamine-induced rotation in 6-OHDA lesioned rats. TH+ neurons of the SNpc and fiber density of the SNpr for three different sections (rostral, middle, and caudal) were analyzed by a two-tailed unpaired t-test comparing the ipsilateral sides to the lesion (right hemisphere) of DNSP-11 treated rats versus vehicle. A one-way ANOVA with Bonferroni’s post hoc test was used to analyze gamma counting data examining the average tracer (125I) levels of each brain section (OB-combined left and right, B2 – B11), CSF and blood samples. Statistical significance was defined as p < 0.05 for all analyses.
3. Results
3.1. Dose response of nasal delivery of DNSP-11 in normal rats
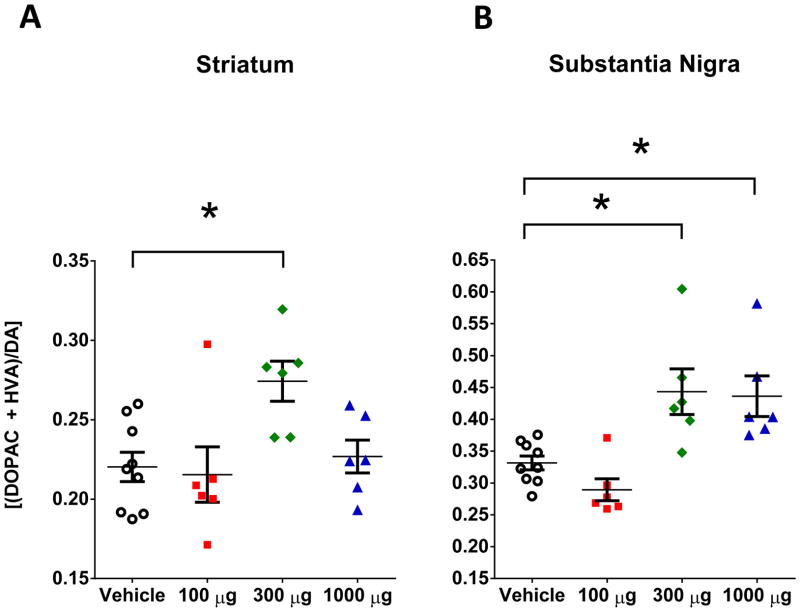
A dose-response study was carried out to determine the minimum dose of DNSP-11that would produce consistent effects on DA neurons, following intranasal administration in normal F344 rats. The levels of DA, DOPAC and HVA in the striatum and SN of normal rats were measured by HPLC-EC following intranasal administration of vehicle or DNSP-11 (100, 300, or 1000 μg) for 5 days a week, for 3 weeks. The 300 μg dosage of DNSP-11 produced significant changes in DOPAC (p < 0.05) and HVA (p < 0.05) in the SN (Table 1). As changes in individual DA and/or metabolite (DOPAC & HVA) levels were observed, DA turnover levels were examined as a more reliable index of changes in DA function following intranasal DNSP-11. Rats treated with 300 μg of DNSP-11 represented the lowest dose that produced significant increases in DA turnover in both the striatum (25%, p < 0.05) and the (34%, p < 0.05) SN, compared to vehicle (Table 1, Fig. 3.A & B). The 1000 μg intranasal dose produced a significant increase in DA turnover in the SN, but not the striatum (Table 1, Fig. 3.A & B), suggesting that higher repeated intranasal dosing is either ineffective and/or region specific in normal rats. Based on these effects, we selected the 300 μg intranasal dose of DNSP-11 for all other subsequent in vivo experiments presented in this manuscript (Fig. 2.B–C).
Table 1. Whole tissues levels of DA and DA metabolites in normal rats after repeated intranasal administration of DNSP-11.
Individual analyte (DA, DOPAC and HVA) levels (ng/g - wet tissue weight) and DA turnover ratios of the whole (combined hemispheres) striatum and SN of normal rats treated with vehicle or DNSP-11 (100, 300, 1000 μg) in an equivalent volume of vehicle 5 days a week for 3 weeks. In normal rats, a stimulation of the DA system is evidenced by increased DA turnover in the striatum and SN for the 300 μg dosage (as well as an increase in DA turnover in the SN for the 1000 μg dosage).
| Brain Region | Neurochemical Analyte | Intranasal Treatment | one-way ANOVA | |||
|---|---|---|---|---|---|---|
| Vehicle (ng/g) | 100 μg DNSP-11 (ng/g) | 300 μg DNSP-11 (ng/g) | 1000 μg DNSP-11 (ng/g) | |||
| Striatum | DA | 12427 ± 564 | 9043 ± 1427 | 11923 ± 904 | 9910 ± 991 | P=0.0527, F(3,23)=2.98 |
| DOPAC | 1919 ± 133 | 1257 ± 174 | 2422 ± 259 | 1574 ± 186 | P=0.0022, F(3,23)=6.63 | |
| HVA | 832 ± 85 | 570 ± 78 | 843 ± 63 | 658 ± 60 | P=0.0555, F(3,23)=2.92 | |
| (DOPAC+HVA)/DA | 0.220 ± 0.009 | 0.216 ± 0.017 | * 0.274 ± 0.013 | 0.227 ± 0.010 | P=0.0124, F(3,23)=4.52 | |
| SN | DA | 620 ± 83 | 481 ± 83 | 815 ± 83 | 416 ± 71 | P=0.0186, F(3,23)=4.07 |
| DOPAC | 151 ± 23 | 103 ± 20 | * 269 ± 29 | 124 ± 27 | P=0.0009, F(3,23)=7.74 | |
| HVA | 55 ± 6 | 37 ± 7 | * 81 ± 7 | 51 ± 4 | P=0.0012, F(3,23)=7.43 | |
| (DOPAC+HVA)/DA | 0.332 ± 0.011 | 0.289 ± 0.017 | * 0.443 ± 0.036 | * 0.436 ± 0.032 | P=0.0002, F(3,23)=9.82 | |
All data were analyzed using a one-way ANOVA with Bonferroni’s post-hoc test
p < 0.05. All data are presented as mean ± SEM, n = 9 vehicle and n = 6 per DNSP-11 treated group.
Figure 3. HPLC-EC studies of DA turnover [(DOPAC+HVA)/DA] following intranasal administration of DNSP-11 or vehicle. of the nigrostriatal system in normal rats.
Normal rats were repeatedly administered either vehicle (open circles), 100 (red squares), 300 (green diamonds) or 1000 (blue triangles) μg of DNSP-11 intranasally 5 days a week for 3 weeks. (A) At 300 μg a significant increase (p = 0.0230) in basal DA turnover was observed compared to vehicle in the striatum (A). (F(3,23)=4.520, p=0.0124) (B) In addition, a significant increase in DA turnover was observed in the SN at both 300 (p = 0.0129) and 1000 μg (p = 0.0217) compared to vehicle (F(3,23)=9.818, p=0.0002). All data were analyzed using a one-way ANOVA with Bonferroni’s post-hoc test *p<0.05. All data are shown as mean ± SEM, n=9 vehicle and n=6 DNSP-11 treated rats.
3.2. d-amphetamine-induced rotations in 6-OHDA lesioned rats
D-amphetamine induced rotation was assessed to determine the severity of the unilateral 6-OHDA lesion [23, 25]. On days of d-amphetamine testing, rats were not intranasally dosed to mitigate effects of isoflurane anesthesia. Analysis of d-amphetamine induced rotations following 6-OHDA lesion showed a significant 55 % decrease at 2 weeks (p < 0.05) and a 35% decrease at 4 weeks (p > 0.05) in d-amphetamine induced rotation, compared to vehicle (Fig. 4). At week 4 of testing, attrition of the rats was observed in both the vehicle (n = 2) and DNSP-11 (n = 4) treated groups a few hours after d-amphetamine injection.
Figure 4. d-amphetamine-induced rotation at 2 & 4 weeks post unilateral 6-OHDA lesion in rats after repeated intranasal administration of DNSP-11 or vehicle.
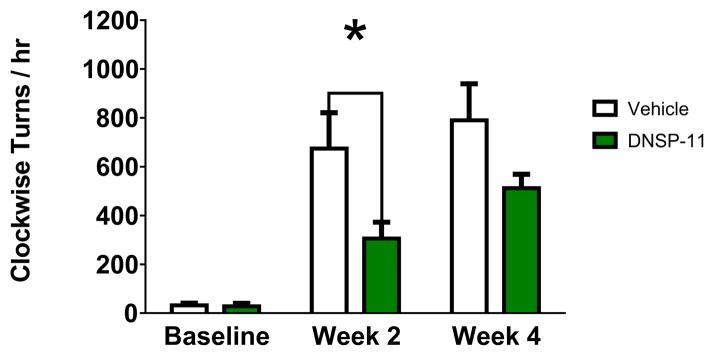
d-amphetamine (2.5 mg/kg i.p.) rotation was assessed prior to 6-OHDA lesion and again at 2 and 4 weeks post-surgery and data are presented as the number of peek clockwise turns per hour. A significant decrease in rotation was observed at 2 weeks (*p = 0.0210) but not at 4 weeks (p = 0.1176) compared to vehicle treated rats. Data were analyzed using a two-way ANOVA with repeated measures (F(1,18)=0.0210, p=0.0283) with Bonferroni’s post hoc test. Animals taken out to 6 weeks (data not shown) observed a 21% reduction in d-amphetamine-induced rotations in DNSP-11 (1069 ± 47; n = 3) compared to vehicle (840 ± 127; n = 2) treated animals. When animals taken out to 6 weeks were added back into the analysis, d-amphetamine-induced rotation became significant at both 2 (p = 0.0180) and 4 (p = 0.0315) weeks post lesion (data not shown) in DNSP-11 (n = 13) compared to vehicle (n = 12) treated rats. Data presented as mean ± SEM, n = 10 vehicle, n = 10 DNSP-11.
3.3 Tissues levels of DA and DA metabolites in the striatum of unilateral 6-OHDA-lesioned rats
To determine lesion severity in terms of neurochemical content, the entire striatum was removed 5 weeks post 6-OHDA lesion (Fig. 2.B). It is important to note that initial HPLC-EC analysis revealed undetectable DA, DOPAC and/or HVA levels in some rats (n = 8). As samples were limited, those which observed undetectable levels were run on a HPLC-EC system with a lower limit of detection. Therefore, comparisons in analyte levels for statistical purposes were reserved to ratios instead of direct comparisons of individual analyte levels (Table 2).
Table 2. Whole tissues levels of DA and DA metabolites in unilaterally 6-OHDA lesioned rats after repeated intranasal administration of DNSP-11.
Individual analyte levels (ng/g – wet tissue weight) of the contralateral (unlesioned) and ipsilateral (lesioned) striatum. DA turnover [(DOPAC + HVA)/DA] ratios were analyzed by a one-way ANOVA with Bonferroni’s post-hoc test
| Brain Region | Neurochemical Analyte | Intranasal Treatment | one-way ANOVA | |
|---|---|---|---|---|
| Vehicle (ng/g) | 300 μg DNSP-11 (ng/g) | |||
| Unlesioned Striatum | DA | 10304±1104 | 12299±555 | |
| DOPAC | 2807±457 | 2422±241 | ||
| HVA | 759±44 | 861±49 | ||
| (DOPAC+HVA)/DA | 0.341±0.047 | 0.257±0.024 | P<0.0001 F(3,24)=24.56 |
|
| Lesioned Striatum | DA | 123±46 | 201±171 | |
| DOPAC | 60±19 | 58±46 | ||
| HVA | 18±7 | 21±20 | ||
| (DOPAC+HVA)/DA | 0.884±0.074 | **0.538±0.062 | P<0.0001 F(3,24)=24.56 |
|
p < 0.05. Intranasal administration of DNSP-11 resulted in a decrease in DA turnover in the lesioned striatum, suggestive of a protective effect on the DA system. All data are presented as mean ± SEM, n = 8 vehicle and n = 6 DNSP-11 treated.
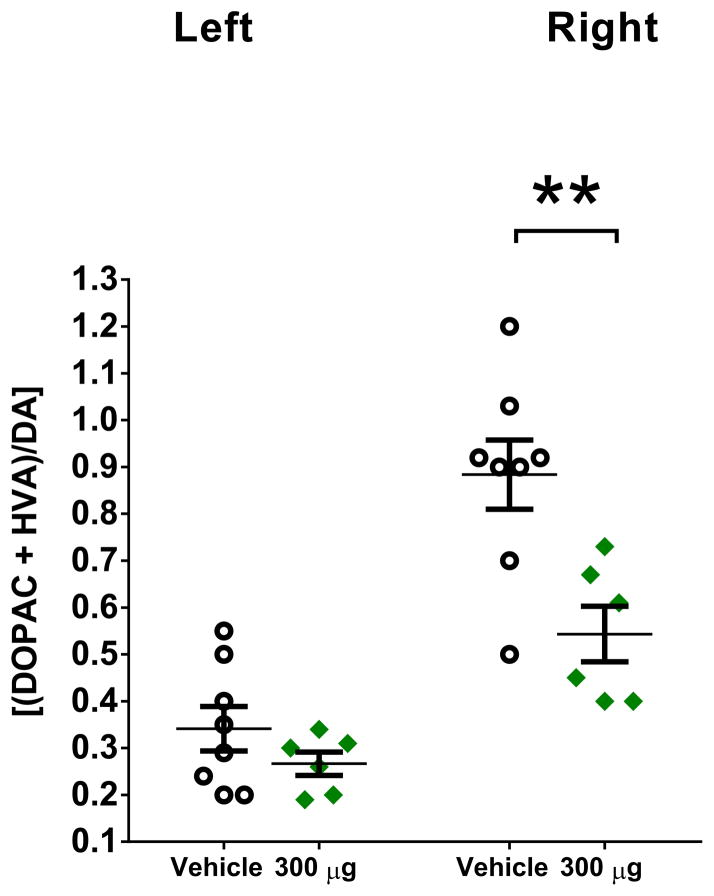
HPLC-EC analysis of striatal tissue revealed at least a ≥ 90% depletion of DA in the ipsilateral side to the lesion (right hemisphere) compared to the contralateral side to the lesion (left hemisphere) in all rats (Table 2). DNSP-11 treated rats exhibited a 39% (p < 0.01) reduction in DA turnover in the ipsilateral side to the lesion compared to vehicle (Table 2, Fig. 5). Similarly, when comparing the ipsilateral to contralateral sides to the lesion, vehicle treated rats exhibited a 159% (p < 0.0001) increase in DA turnover, whereas the DNSP-11 treated rats showed a smaller 59% (p < 0.05) increase (Table 2, Fig. 5), indicating that DA turnover on the ipsilateral side to the lesion was approaching basal levels in comparison to the contralateral sides to the lesion of vehicle or DNSP-11 treated rats (Fig. 5).
Figure 5. HPLC-EC studies of DA turnover (DOPAC + HVA)/DA in unilateral (right) 6-OHDA lesioned rats after repeated intranasal administration of DNSP-11 or vehicle.
DNSP-11 treatment significantly decreased DA turnover in the ipsilateral (right/lesioned) striatum from 0.884 ± 0.074 to 0.538 ± 0.062 ng/g at 5 weeks in 6-OHDA lesioned rats (green diamonds) compared to vehicle (open circles). Data were analyzed using a one-way ANOVA (F(3,24) = 24.62, p < 0.0001) with Bonferroni’s post hoc test **p < 0.01. Data presented as mean ± SEM, n = 8 vehicle, n = 6 DNSP-11.
3.4. TH+ neurons and fiber density
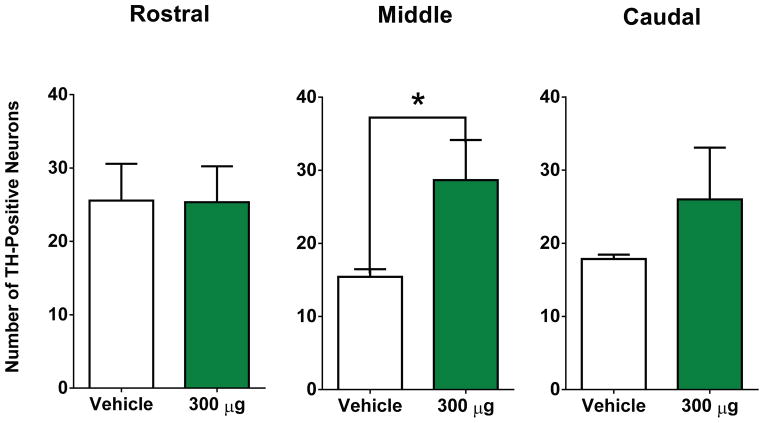
To further evaluate DNSP-11’s effects in a unilateral 6-OHDA lesion model. The SN was processed for TH+ neurons and the average total number of TH+ neurons was determined in sections representing the rostral, middle, and caudal regions (Fig. 6). Analysis indicated statistically significant sparing of TH+ neurons in the middle section/region of the SNpc (29 ± 5) of DNSP-11 treated rats compared to vehicle (13 ± 2; p < 0.05; Fig. 6.A–D & Fig. 7). While sparing was not observed in the more rostral region/section (p > 0.05; Fig. 7), there was a 44% increase in the average total number of TH+ neurons in the caudal region/section (p > 0.05; Fig. 7) of the SNpc in DNSP-11 treated rats compared to vehicle. As topographical organization of the nigrostrial system would suggest, a 6-OHDA lesion of the dorsolateral striatum would likely exhibit greater losses of dopaminergic cell bodies at the more middle and caudal regions of the SNpc, [32] thereby supporting the greater observed loss of TH+ neurons in both the middle and caudal sections of the SNpc (Fig. 7). Conversely, examination of the SNpr fiber density from the same tissue sections used for cell counts exhibited no change (p > 0.05) in any of the three sections (Table 3).
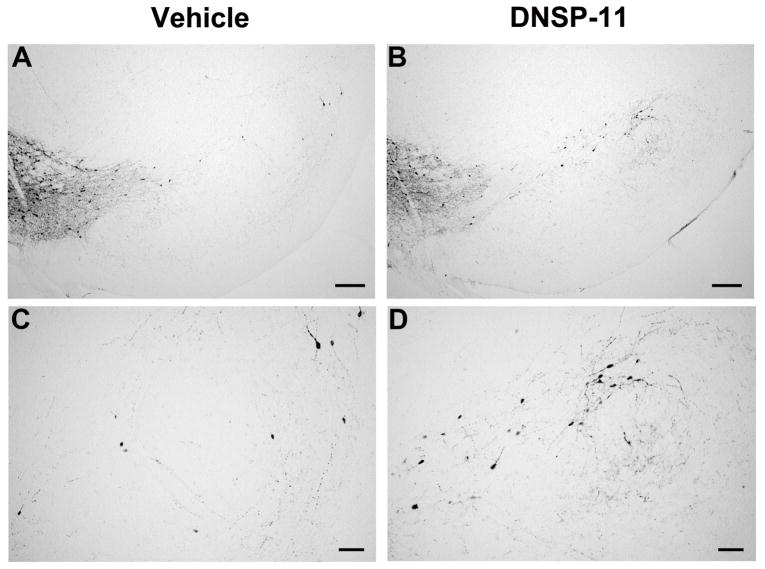
Figure 6. Representative sections of the SNpc used for TH+ neurons and fiber area of the SNpr at three levels.
rostral: − 5.24 mm; (A – D) middle: − 5.60 mm; caudal: − 5.96 mm of the SNpc and SNpr used for cell counting and fiber area. Scale bars = 200 μm in A – B and 100 μm in C–D.
Figure 7. The effects of repeated intranasal administration of DNSP-11 on TH+ dopaminergic neurons in the SNpc and fiber area of the SNpr in 6-OHDA unilaterally lesioned rats.
(A–C) The total number of TH+ neurons were counted from three sections of the lesioned SNpc after repeated intranasal administration of either vehicle or DNSP-11: Rostral (t(11) = 0.2714, p = 0.7911), (A–D) Middle (t(11) = 2.703, p = 0.0205), and Caudal (t(11) = 1.223, p = 0.2468). There was no significant difference found between DNSP-11 treated rats or vehicle in the rostral or caudal regions of the SNpc (mean ± SEM, vehicle: 23 ± 6; DNSP-11: 25 ± 5) and (mean ± SEM, vehicle: 18 ± 1; DNSP-11: 26 ± 7). (B) There was a significant increase observed in the average number of TH+ positive neurons in DNSP-11 treated rats compared to vehicle (*p < 0.05) in the middle region of the lesioned SNpc (mean ± SEM, vehicle: 13 ± 2; DNSP-11: 29 ± 5). The data were analyzed by a two-tailed unpaired t test. Data are shown as mean ± SEM (n = 7 vehicle, n = 6 DNSP-11).
Table 3. The effects of repeated intranasal administration of DNSP-11 on TH + fiber density in the SNpr in unilateral 6-OHDA lesioned rats.
At all three levels examined for TH positive neurons in the SNpc, the SNpr was examined to determine changes in fiber density.
| Fiber Density for the Lesioned SNpr | |||
|---|---|---|---|
| SNpr Region | Vehicle | DNSP-11 | unpaired T-test |
| Rostral | 0.20 ± 0.03 | 0.26 ± 0.09 | t(8) = 0.4987, p = 0.6314 |
| Middle | 0.22 ± 0.07 | 0.41 ± 0.20 | t(9) = 0.8222, p = 0.4322 |
| Caudal | 1.10 ± 0.67 | 0.91 ± 0.41 | t(9) = 0.1947, p = 0.8500 |
All data were analyzed using an unpaired T-test,
p < 0.05. All data are presented as mean ± SEM with n = 5 vehicle treated, and n = 6 DNSP-11 treated.
3.5. Distribution studies following intranasal administration
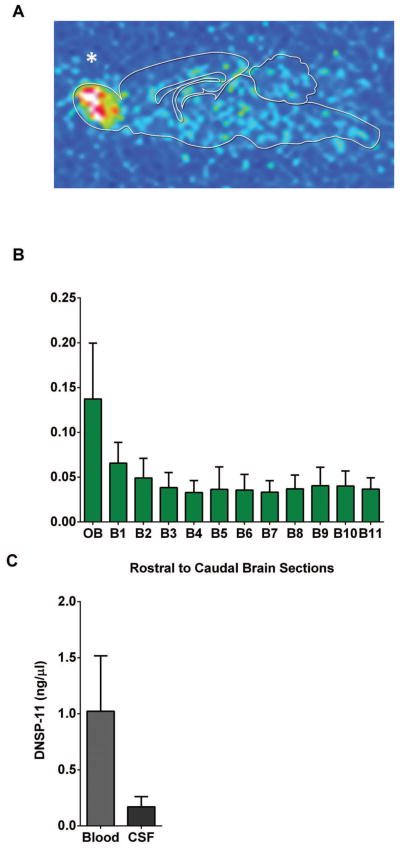
To understand the distribution of DNSP-11 following intranasal administration, F344 rats were administered a single intranasal dose of the 125I-labeled modified DNSP-11(R9K) in vehicle (50 μl/ 50 μCi − 300 μg DNSP-11) (Fig. S.1). Distribution in the CNS, CSF, and blood were analyzed by gamma counting at 30, 60 and 360 minutes after the start of the first intranasal dose (time = 0) (Fig. 2.C). Gamma counting analysis revealed that radioactive signal was detected diffusely throughout the brain at every time point (Table 4), with visibly higher signal found in the olfactory bulbs at 60 minutes (p > 0.05; Table 4). Furthermore, qualitative autoradiography analysis supports this observation, showing radioactive signal diffusely throughout the CNS and at visibly higher levels in the olfactory bulbs at 60 minutes (Fig. 8.A & B). Cumulatively, tracer studies indicated increased radioactive signal in the olfactory bulbs at 60 minutes, and both the CSF and blood 30 minutes after the first intranasal dose (p > 0.05; Fig. 8.A – C; Table 4). Furthermore, radioactive signal was observed in targeted regions of the nigrostriatal system in brain (B) sections (B3–7) in as little as 30 minutes and continuing up to 360 minutes thereafter (Table 4; Fig. 8.A & B). This distribution is similar to other rodent intranasal tracer studies showing CNS distribution in as early as 15 – 30 minutes following intranasal administration of compounds of similar and larger molecular weight [11, 12, 14–16, 33].
Table 4. Tissue 125I levels in normal rats after a one-time dose.
Olfactory bulbs (combined left and right), rostral to caudal 2 mm thick brain sections (B1–B11), CSF and blood were processed for 125I levels by gamma counting at 30 (n = 4), 60 (n = 3) and 360 (n = 3) post the start of the first intranasal dose (t = 0).
| Time point | ||||
|---|---|---|---|---|
| Brain Region | 30 Minutes (ng/mg) | *60 Minutes (ng/mg) | 360 Minutes (ng/mg) | one-way ANOVA |
| OB | 0.083 ±0.026 | 0.137 ±0.062 | 0.079 ±0.019 | P = 0.5346 F(2,7) = 0.6857 |
| B1 | 0.067 ±0.011 | 0.066 ±0.023 | 0.058 ±0.007 | P = 0.9067 F(2,7) = 0.09934 |
| B2 | 0.058 ±0.014 | 0.049 ±0.022 | 0.056 ±0.006 | P = 0.9121 F(2,7) = 0.09319 |
| B3 | 0.048 ±0.020 | 0.038 ±0.017 | 0.059 ±0.007 | P = 0.7320 F(2,7) = 0.3263 |
| B4 | 0.061 ±0.015 | 0.033 ±0.013 | 0.059 ±0.007 | P = 0.3341 F(2,7) = 1.287 |
| B5 | 0.071 ±0.024 | 0.036 ±0.025 | 0.062 ±0.006 | P = 0.5445 F(2,7) = 0.6639 |
| B6 | 0.065 ±0.020 | 0.036 ±0.018 | 0.061 ±0.009 | P = 0.4796 F(2,7) = 0.8177 |
| B7 | 0.063 ±0.019 | 0.033 ±0.013 | 0.055 ±0.010 | P = 0.4182 F(2,7) = 0.9899 |
| B8 | 0.061 ±0.017 | 0.037 ±0.015 | 0.047 ±0.008 | P = 0.5549 F(2,7) = 0.6415 |
| B9 | 0.063 ±0.017 | 0.040 ±0.021 | 0.051 ±0.005 | P = 0.6168 F(2,7) = 0.5181 |
| B10 | 0.078 ±0.027 | 0.040 ±0.017 | 0.052 ±0.009 | P = 0.4671 F(2,7) = 0.8502 |
| B11 | 0.114 ±0.036 | 0.037 ±0.013 | 0.056 ±0.005 | P = 0.1550 F(2,7) = 2.462 |
| Sample | 30 Minutes (ng/μl) | *60 Minutes (ng/μl) | 360 Minutes (ng/μl) | |
|---|---|---|---|---|
| CSF | 0.24 ±0.07 | 0.17 ±0.09 | 0.16 ±0.02 | P = 0.6882 F(2,7) = 0.3944 |
| Blood | 1.4 ±0.3 | 1.0 ±0.5 | 1.0 ±0.1 | P = 0.3698 F(2,7) = 1.150 |
All data were analyzed by a one-way ANOVA with Bonferroni’s post hoc test. All data are presented as the mean ± SEM normalized DNSP-11 levels (ng or μl/mg wet sample weight).
Denotes the time point used for intranasal administration prior to 6-OHDA infusion.
Figure 8. Tracking of a one-time 125I-labeled DNSP-11 dose 60 minutes after intranasal administration.
Normal F344 rats were given a one-time intranasal dose of 125I-labeled DNSP-11, at 60 minutes blood (500 μl) and cerebrospinal fluid (100–120 μl) were collected from each rat and processed by gamma counting (n=3) and autoradiography (n=1). (A) Representative sagittal brain section (0.5 mm thick), exposed for 21 days on a GE phosphor screen. A qualitative increase in radioactive signal was found in the olfactory bulbs 60 minutes post intranasal administration and diffusely throughout the brain. (B) Data are shown as the normalized DNSP-11 concentrations (ng or μl/mg of wet sample weight) as analyzed by gamma counting at 60 minutes. (C) Blood and CSF; the visible increase in radioactive signal found in the olfactory bulbs at 60 minutes is consistent with autoradiography. Data are presented as the normalized DNSP-11 concentrations (ng or μl/mg). Rats treated with vehicle only were found to have CPM lower then background levels < 50 (data not pictured). * Denotes the olfactory bulb of a representative sagittal section of the midbrain after autoradiography analysis.
4. Discussion
A major challenge in treating neurodegenerative disorders such as PD with neurotrophic molecules has been the delivery of large compounds across the blood brain barrier (BBB) to the CNS [2]. There has been the need for the development of smaller molecular weight compounds capable of affecting the nigrostriatal system and that can be used in less-invasive delivery methods such as intranasal administration [9, 30]. As previously discussed, our team has shown that DNSP-11 while having similar in vitro and in vivo actions as GDNF, is also distinct in its ability to protect against cell-induced cytotoxicity in dopaminergic neurons using a mechanism that does not involve binding to the GFRα-1 receptor [18, 19]. To the best of our knowledge, this is the first demonstration to show that repeated intranasal administration of DNSP-11; (1) alters DA, DOPAC, HVA, and DA turnover in the striatum and SN of normal rats, (2) exhibits protective and/or restorative effects in a unilateral 6-OHDA striatal lesion model of PD, and (3) can be traced after a one-time 125I–labeled DNSP-11 intranasal dose to CSF and targeted regions of the CNS (striatum and SN).
To determine efficacy, our dose response study examined the repeated intranasal administration of various doses of DNSP-11 while using light isoflurane anesthesia. We showed that repeated administration of 300 μg of DNSP-11 is capable of stimulating DA turnover in both the striatum and SN in normal rats [43]. These results are similar to neurochemical findings in normal rats after a single intranigral injection of DNSP-11 [18, 19]. Using the selected 300 μg DNSP-11 dose, we then examined its ability to either protect and/or restore function in the nigrostriatal system after a unilateral 6-OHDA lesion to the dorsolateral striatum, which mimics the later stages of PD [22]. We show that repeated intranasal administration of DNSP-11 was able to significantly decrease d-amphetamine induced rotation at 2 weeks (p < 0.05) by 55% and was trending on significance by 35% at week 4 (p > 0.05). Interestingly, in a separate small group of rats (data not shown), we observed that d-amphetamine-induced rotations also showed a decreased after 6 weeks of DNSP-11 administration as compared to vehicle (data not shown). The observed decrease in d-amphetamine-induced rotation suggests either sparing and/or improvement of the remaining dopaminergic terminals found in the lesioned striatal hemisphere [25]. These results are similar to our previously published findings that indicated DNSP-11 decreased apomorphine-induced rotation by 50% in a unilateral MFB lesion rat model 4 weeks post infusion following a single direct intranigral infusion [18]. In addition, we note that we switched to d-amphetamine-induced rotation measures in these studies due to the use of the Kirik and co-authors unilateral 6-OHDA lesion model [22], expecting more of a partial lesion of the DA neuronal system. However, our HPLC-EC studies demonstrate that we achieved, on average, extensive (> 98%) DA lesions of the nigrostriatal pathway, supporting that perhaps low dose apomorphine-induced rotation would have likely been a better test for the extensive DA lesions and possible DA receptor supersensitivity that we produced [25]. Additional studies in other models may help better characterize the potential of intranasal DNSP-11 treatments to damaged DA neurons.
At week 4 of d-amphetamine testing, an attrition rate was observed in both the vehicle (n = 2) and DNSP-11 (n = 4) treated groups. The d-amphetamine dose (2.5 mg/kg, i.p.) used in this study is not known to cause such an attrition rate, even at higher doses (5.0 mg/kg, i.p.) [25]. However, Datla and co-authors have shown that isoflurane, when used as an anesthetic during 6-OHDA infusion, increased both TH+ dopaminergic cell loss in the SNpc and striatal DA depletion as measured by HPLC-EC compared to other anesthetics [34]. Furthermore, the concomitant repeated daily doses of isoflurane used in our study for intranasal delivery, in addition to the 6-OHDA lesion, may have been a contributing factor to the observed attrition. Therefore, a “build-up toxicological-effect” is thought to be at play in our studies. We caution to other investigators that repeated use of isoflurane anesthesia may not be the optimum anesthetic to use for repeated intranasal administration in rats.
As our present unilateral 6-OHDA lesion model targeted the dorsolateral striatum [22], neurochemical results after repeated intranasal administration of DNSP-11 parallel previous intranigral infusion findings in that DNSP-11 was capable of decreasing DA turnover by 35% (p < 0.01) in the ipsilateral striatum compared to vehicle [18, 19, 35]. Moreover, while the sparing of TH+ neurons in the SN was not evident in the rostal region (p > 0.05), the caudal and middle sectionsdid show either a trend or statistically significant (p < 0.05) sparing of TH+ neurons, respectively (Fig. 7)..
The intranasal administration of DNSP-11 allowed for bilateral administration of DNSP-11 as compared to prior studies, where it was administered unilaterally by direct injection into the CNS (18, 19). In contrast to the intranasal delivery of DNSP-11 in normal rats, we saw no evidence for a similar effect on the unlesioned side in the unilateral 6-OHDA-lesioned rats. This is in contrast to the contralateral effects of GDNF that we observed in aged and MPTP-treated nonhuman primates from unilateral intracranial administration [2, 7]. While puzzling this may relate to the effects of the extensive unilateral 6-OHDA lesion on the nigrostriatal pathway and the fact that we did not examine other neuronal systems that could be affected by the bilateral administration of DNSP-11. Future studies will investigate the effects of DNSP-11 in other model systems, such as movement impaired aged rats, which exhibit bilateral dopamine neuron deficits.
To better understand DNSP-11’s route of transport after intranasal administration, we examined a one-time intranasal dose of 125I-labeled DNSP-11 in normal rats as a function of time. Numerous studies in rodents have demonstrated the rapid up-take of radiolabeled compounds after intranasal administration which has been contributed to (1) CSF found in the perineural spaces surrounding the olfactory sensory neurons (OSNs) and/or (2) perivascular delivery to the CNS powered by bulk flow through the propulsion of arterial blood and diffusion [12, 14, 16, 33, 36–40]. Paralleling previous tracer studies in rodents, we demonstrate the rapid up-take of our tracer in CSF and CNS in as early as 30 minutes and persisting for at least 360 minutes after a one-time intranasal dose; with visually higher signal of tracer found in the olfactory bulbs at 60 minutes and in the most caudal section of the brainstem at 30 minutes (p > 0.05). These findings are again consistent with other intranasal studies that implicate the rapid transport of different compounds by the OSNs to the olfactory bulbs and trigeminal nerves [11]. While the olfactory system is highly vascularized by facial arteries and/or through the transport by the lymphatic system [11, 12], it is likely that DNSP-11 would not be delivered systemically to the CNS due to a half-life of less than 12 minutes in rat plasma (data not shown). Collectively, these data show diffuse signal throughout the CNS at each time point, indicating a rapid delivery mechanism after intranasal administration to the CNS that suggesting a combination of either perivascular and/or perineural transport [33, 41, 42].
In summary, this series of studies provides methodology for intranasal administration of DNSP-11 and indicates (1) the efficacy of repeated intranasally administered DNSP-11 using light isoflurane anesthesia and (2) its ability to affect the nigrostriatal system in both normal and 6-OHDA lesioned rodents. Using a determined bioactive dose (300 μg), repeated intranasal administration of DNSP-11 was able to regulate DA turnover after a lesion to the dorsolateral striatum, and area equivalent to that of the human putamen, which sees the greatest loss of DA in PD [22]. We also observed increased sparing of TH+ neurons in specific regions of the SNpc, which saw some of the highest losses of TH+ neurons in our rat model system. Furthermore, our 125I–labeled study indicates sufficient biodistribution throughout the CNS and in targeted regions of interest (striatum & SN). Collectively, this series of experiments supports further investigation of intranasally administered DNSP-11, in other PD model systems due to its effects on the nigrostriatal system following repeated administration [18–20, 43, 44].
Supplementary Material
Highlights.
DNSP-11 was delivered intranasally in sedated normal rats and 6-OHDA lesioned rats
Repeated DNSP-11 delivery affects dopamine turnover in normal and lesioned rats
Intranasal DNSP-11 administration increases TH+ neurons in 6-OHDA lesioned rats
Repeated DNSP-11 delivery reduced drug-induced rotation in unilateral lesioned rats
DNSP-11 is traced throughout the brain and CSF after a single intranasal dose
Acknowledgments
The authors thank Matt Hazzard and Tom Dolan of the University of Kentucky Academic Technology Group for helping with the medical illustrations used in Figure 1. The authors also thank April Evans and Ryan Weeks of the University of Kentucky Department of Anatomy & Neurobiology for assistance with intranasal dosing and Dr. Anders Anderson of the University of Kentucky Magnetic Resonance Imaging and Spectroscopy Center for his assistance with the autoradiography. The project described was supported by funds from: NINDS (NS039787: all; NS060924: W.A.C.; NS075694: L.H.B.); National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1RR033173 (G.A.G., L.H.B); NIA (AG013494: D.M.G., G.A.G.; T32-AG000242: J.T-C., M.J.S.); Kentucky INBRE Pilot (NCRR 5P20RR016481-12, NIGMS 8 P20 GM103436-12: L.H.B); Endowed Chair Funds (D.M.G.); Dupree Trust (G.A.G); Estate of Laura C. Miller (L.H.B); PhRMA Foundation (L.H.B.); Columbus Foundation (L.H.B.); and the University of Kentucky College of Medicine Start-up Funds (L.H.B.).
Abbreviations
- TH – positive neurons
tyrosine hydroxylase-immunoreactive
- STS
Staurosporine
- 6-OHDA
6-hydroxydopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- 5-HT
5-hydroxytryptamine
- 5-HIAA
5-hydroxyindoleacetic acid
- DA
dopamine
- DNSP-11
dopamine neuron stimulating peptide-11
- GDNF
glial cell line-derived neurotrophic factor
- GFR
GDNF-family receptor
- HVA
homovanillic acid
- PD
Parkinson’s disease
- SN
substantia nigra
- SNpc
substantia nigra pars compacta
- SNpr
substantia nigra pars reticulata
- TH
tyrosine hydroxylase
- OSNs
olfactory sensory neurons
- OB
olfactory bulbs
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and other funding sources.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peterson AL, Nutt JG. Treatment of Parkinson’s Disease with Trophic Factors. Neurotherapeutics. 2008;5(2):270–280. doi: 10.1016/j.nurt.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gash DM, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380(6571):252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 3.Lin L, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 4.Gill SS, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9(5):589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 5.Chapman C, et al. Intranasal Treatment of Central Nervous System Dysfunction in Humans. Pharmaceutical Research. 2013;30(10):2475–2484. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slevin JT, et al. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line—derived neurotrophic factor. Journal of Neurosurgery. 2005;102(2):216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 7.Grondin R, et al. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. 2002;125:2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- 8.Slevin JT, et al. Unilateral intraputaminal glial cell line–derived neurotrophic factor in patients with Parkinson disease: response to 1 year each of treatment and withdrawal. Neurosurgical Focus. 2006;20(5):1–7. doi: 10.3171/foc.2006.20.5.2. [DOI] [PubMed] [Google Scholar]
- 9.Lang AE, et al. Randomized controlled trial of intraputamenal glial cell line–derived neurotrophic factor infusion in Parkinson disease. Annals of Neurology. 2006;59(3):459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore MF, et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Experimental Neurology. 2006;202(2):497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Thorne RG, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Thorne R, Frey W., II Delivery of Neurotrophic Factors to the Central Nervous System. Clinical Pharmacokinetics. 2001;40(12):907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ross TM, et al. Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. Journal of Neuroimmunology. 2004;151(1–2):66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 15.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. J Pharm Sci. 2009;98(7):2501–15. doi: 10.1002/jps.21604. [DOI] [PubMed] [Google Scholar]
- 16.Dhuria SV, Hanson LR, Frey WH., 2nd Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J Pharmacol Exp Ther. 2009;328(1):312–20. doi: 10.1124/jpet.108.145565. [DOI] [PubMed] [Google Scholar]
- 17.Migliore MM, et al. Neurotrophic and neuroprotective efficacy of intranasal GDNF in a rat model of Parkinson’s disease. Neuroscience. 2014;274(0):11–23. doi: 10.1016/j.neuroscience.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Bradley LH, et al. Dopamine Neuron Stimulating Actions of a GDNF Propeptide. PLoS ONE. 2010;5(3):e9752. doi: 10.1371/journal.pone.0009752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuqua JL, et al. Dynamic changes in dopamine neuron function after DNSP-11 treatment: Effects in vivo and increased ERK 1/2 phosphorylation in vitro. Peptides. 2014;54(0):1–8. doi: 10.1016/j.peptides.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelps KA, et al. Evaluation of the physical and in vitro protective activity of three synthetic peptides derived from the pro- and mature GDNF sequence. Neuropeptides. 2011;45(3):213–218. doi: 10.1016/j.npep.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Immonen T, et al. A proGDNF-related peptide BEP increases synaptic excitation in rat hippocampus. Experimental Neurology. 2008;210(2):793–796. doi: 10.1016/j.expneurol.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Kirik D, Rosenblad C, Björklund A. Characterization of Behavioral and Neurodegenerative Changes Following Partial Lesions of the Nigrostriatal Dopamine System Induced by Intrastriatal 6-Hydroxydopamine in the Rat. Experimental Neurology. 1998;152(2):259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- 23.Lundblad M, et al. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. European Journal of Neuroscience. 2002;15(1):120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 24.Cass WA, et al. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Research. 2003;984(1–2):133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- 25.Hudson JL, et al. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Research. 1993;626(1–2):167–174. doi: 10.1016/0006-8993(93)90576-9. [DOI] [PubMed] [Google Scholar]
- 26.Hoffer BJ, et al. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neuroscience Letters. 1994;182(1):107–111. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 27.Hall M, Hoffer B, Gerhardt G. Rapid and sensitive determination of catecholamines in small tissue sample by HPLC coupled with dual electrode coulometric electrochemical detection. LCGC. 1989;7:258–65. [Google Scholar]
- 28.Kearns CM, Gash DM. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Research. 1995;672(1–2):104–111. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- 29.Ai Y, et al. Intraputamenal infusion of GDNF in aged rhesus monkeys: Distribution and dopaminergic effects. The Journal of Comparative Neurology. 2003;461(2):250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- 30.Watson GPC. The rat brain in stereotaxic coordinates. New York: Academic; Academic Press; 1986. [Google Scholar]
- 31.Nirogi R, et al. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. Journal of Neuroscience Methods. 2009;178(1):116–119. doi: 10.1016/j.jneumeth.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Schwarting R, Huston J. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Progress in Neurobiology. 1996;49(3):215–266. doi: 10.1016/s0301-0082(96)00015-9. [DOI] [PubMed] [Google Scholar]
- 33.Gozes I, et al. Activity-Dependent Neurotrophic Factor: Intranasal Administration of Femtomolar-Acting Peptides Improve Performance in a Water Maze. Journal of Pharmacology and Experimental Therapeutics. 2000;293(3):1091–1098. [PubMed] [Google Scholar]
- 34.Datla K, Zbarsky V, Dexter D. Effects of anaesthetics on the loss of nigrostriatal dopaminergic neurons by 6-hydroxydopamine in rats. Journal of Neural Transmission. 2006;113(5):583–591. doi: 10.1007/s00702-005-0353-x. [DOI] [PubMed] [Google Scholar]
- 35.Zigmond MJ, et al. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends in Neurosciences. 1990;13 (7):290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- 36.Wolak DJ, Thorne RG. Diffusion of macromolecules in the brain: implications for drug delivery. Molecular pharmaceutics. 2013;10(5):1492–1504. doi: 10.1021/mp300495e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadaczek P, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14(1):69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danielyan L, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88 (6):315–24. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Gregory TF, et al. A method for microscopic studies of cerebral angioarchitecture and vascular- parenchymal relationships, based on the demonstration of ‘paravascular’ fluid pathways in the mammalian central nervous system. Journal of Neuroscience Methods. 1985;14(1):5–14. doi: 10.1016/0165-0270(85)90110-4. [DOI] [PubMed] [Google Scholar]
- 40.Syková E, Nicholson C. Diffusion in Brain Extracellular Space. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhuria SV, Hanson LR, Frey WH. Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 2009;328(1):312–320. doi: 10.1124/jpet.108.145565. [DOI] [PubMed] [Google Scholar]
- 42.Laukova M, et al. Early Intervention With Intranasal NPY Prevents Single Prolonged Stress-Triggered Impairments in Hypothalamus and Ventral Hippocampus in Male Rats. Endocrinology. 2014;155(10):3920–33. doi: 10.1210/en.2014-1192. [DOI] [PubMed] [Google Scholar]
- 43.Sonne JH. Anatomy and Neurobiology. University of Kentucky College of Medicine; 2013. Effects of Intranasally Administered DNSP-11 on the Central Dopamine System of Normal and Parinsonian Fischer 344 Rats. [Google Scholar]
- 44.Turchan-Cholewo J. DNSP-11 protection against complex I toxins in dopaminergic neurons. Submitted. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.