Abstract
Endothelial KCa2.3 and KCa3.1 channels contribute to the regulation of myogenic tone in resistance arteries by Ca2+-mobilizing, vasodilatory hormones. To define further the functional role of these channels in distinct vascular beds, we have examined the vasodilatory actions of the KCa channel activator SKA-31 in myogenically active, rat cremaster and middle cerebral arteries. Vessels pressurized to 70 mmHg constricted by 80–100 microns (i.e. 25–45% of maximal diameter). SKA-31 (10 μM) inhibited myogenic tone by 80% in cremaster and ~65% in middle cerebral arteries, with IC50 values of ~2 μM in both vessels. These vasodilatory effects were largely prevented by the KCa2.3 blocker UCL1684 and the KCa3.1 blocker TRAM-34 and abolished by endothelial denudation. Pre-incubation with NG nitro L-arginine methyl ester (L-NAME, 0.1 mM) did not affect the inhibitory response to SKA-31, but attenuated the ACh-evoked dilation by ~45%. Penitrem-A, a blocker of BKCa channels, did not alter SKA-31 evoked vasodilation, but did reduce the inhibition of myogenic tone by ACh, the BKCa channel activator NS1619 and sodium nitroprusside. Collectively, these data demonstrate that SKA-31 produces robust inhibition of myogenic tone in resistance arteries isolated from distinct vascular beds in an endothelium-dependent manner.
Keywords: Endothelium, myogenic tone, resistance artery, calcium-activated K+ channel, vasodilation
1. Introduction
Endothelial small- and intermediate-conductance, Ca2+-activated K+ channels, also known as SKCa and IKCa, or KCa2.3 and KCa3.1 channels, respectively, play an important role in the regulation of vascular function. They are critically involved in the endothelium-dependent dilation of resistance arteries, as the opening of these channels in response to Ca2+-mobilizing agonists (e.g. acetylcholine and bradykinin) generates a robust endothelial hyperpolarizing signal that transfers to the adjacent vascular smooth muscle 1,2. Functionally, this hyperpolarization reduces the steady-state activity of voltage-gated Ca2+ channels in arteries exhibiting pressure-induced myogenic tone, thereby decreasing external Ca2+ entry and contractile behavior. Pharmacological inhibition or genetic deficiency of endothelial KCa2.3 and KCa3.1 channels impairs stimulated NO production in endothelial cells 3,4, agonist-evoked vasodilation of isolated, myogenically active arteries 5,6 and increases arterial tone and blood pressure in rodents 7. Small molecule activators of endothelial KCa channels, in contrast, increase arterial diameter and agonist-evoked NO synthesis 8,9.
The goal of the present study was to examine the effectiveness of a recently developed, second generation KCa channel activator (i.e. SKA-31) 10 to inhibit myogenic tone in small resistance arteries isolated from two distinct vascular beds (i.e. rat skeletal muscle and cerebral circulations). As differences may exist amongst vascular beds in the functional and/or structural heterogeneity of the endothelial layer, as well as in the composition/organization of the vascular wall itself 11,12, it is conceivable that such differences may impact the actions of an endothelium-dependent, vasodilatory agent acting via KCa channel activation. Using quantitative arterial pressure myography, we have observed that SKA-31 produces robust and comparable inhibition of developed tone in cannulated and pressurized (i.e. 70 mmHg) rat cremaster and middle cerebral arteries. Moreover, these inhibitory effects in both vessel types were largely prevented by selective inhibitors of KCa2.3 and KCa3.1 channels, but unaffected by the eNOS inhibitor L-NAME or the pharmacologic blocker of BKCa channels, penitrem-A. Collectively, these observations demonstrate that endothelial KCa channel activators act as effective vasodilators of small resistance arteries in both peripheral and central vascular beds and support the concept that this class of compounds may have broad effectiveness in the vasculature 13–15.
2. Materials and methods
2.1 Vessel isolation and arterial pressure myography
The experimental protocols used in the present study were approved by the University of Calgary Animal Care Committee, and conform to the guidelines for the care and use of laboratory animals established by the Canadian Council on Animal Care and the NIH. Male Sprague-Dawley rats (225–250 g body weight) were obtained from Charles River Laboratories and housed under standard conditions with continuous access to food and water. Rats were injected intraperioneally with sodium pentobarbital (50 mg/kg) to induce surgical anesthesia (i.e. stage 3, loss of blink reflex), and cremaster muscles were then surgically removed and placed in a cooled (4 °C) dissection chamber containing MOPS-based dissection buffer (in mM) (140 NaCl, 4.7 KCl, 2 CaCl2, 1 MgSO4, 1 NaH2PO4, 2 pyruvate, 0.02 EDTA, 5 glucose, 3 MOPS and 0.5% w/v albumin); pH was adjusted to 7.4 with 1 N NaOH 8,16. Euthanasia was completed by an overdose administration of sodium pentobarbital. A total of 42 animals were utilized to obtain the cremaster arteries described in this study. To isolate middle cerebral arteries, anesthetized animals were first sacrificed by decapitation, and the excised brain was similarly placed in a cooled dissection chamber. Thirty-five animals in total were used to isolate 55 middle cerebral arteries. Following isolation, arteries were cannulated on glass pipettes fitted in a pressure myography chamber (Living Systems, Burlington, VT). The vessel lumen was filled with Kreb’s buffer (115 NaCl, 5 mM KCl, 25 mM NaHCO3, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM KH2PO4 and 10 mM D-glucose) containing 1% bovine serum albumin, pH was adjusted to 7.4. The cannulated vessel/chamber apparatus was placed on the stage of an inverted microscope, and the vessel was superfused with Kreb’s buffer at a constant flow of 6–7 ml/min using a peristaltic pump and suction line. Bath solution was maintained at ~34°C for cremaster and 37°C for cerebral vessels. Following 5–10 min of equilibration, the intra-luminal pressure of cannulated vessels was increased in a step-wise manner under no-flow conditions and then maintained at 70 mmHg; vessels typically developed myogenic tone within 20–30 minutes. Continuous video measurement of the intraluminal vessel diameter was carried out using a diameter tracking system (IonOptix, Milton, MA). Drug-containing solutions were added to the bath via the perfusion pump. For endothelial denudation, an air bubble (0.5–1 ml) was slowly passed through the lumen of a vessel following cannulation of one end of the vessel. Physiological saline was then gently passed through the lumen to flush out any cellular debris. The vessel was then fully cannulated and allowed to equilibrate as described above prior to the application of intraluminal pressure and the development of myogenic tone.
Drug induced-changes in vessel internal diameter were calculated and are presented as a percentage of the developed myogenic tone relative to the maximal passive intraluminal diameter determined in an external saline solution containing zero added Ca2+ and 2 mM external EGTA according to the following equation 8:
Where Ddrug = intraluminal diameter in the presence of either SKA-31 or ACh, Dbasal = intraluminal diameter under basal myogenic tone at 70 mmHg and Dpass = maximal intraluminal diameter at 70 mmHg in bath solution containing 2 mM EGTA with no added CaCl2.
Maximal inhibitory responses and IC50 values for SKA-31 evoked inhibition of myogenic tone in cremaster and middle cerebral arteries were determined from fitting the data presented in Figure 1C to the Hill equation as follows:
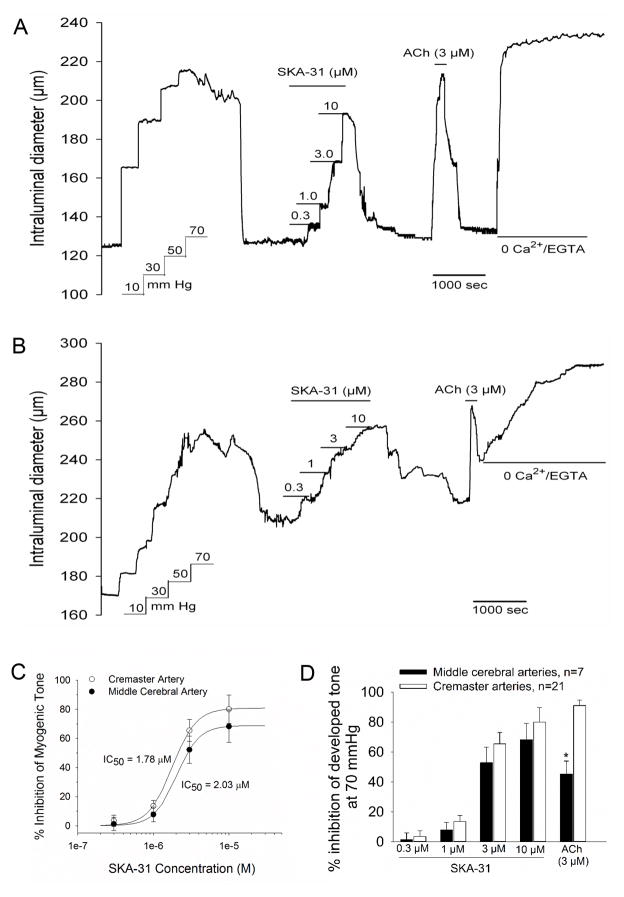
Figure 1.
SKA-31 inhibits myogenic tone in pressurized cremaster and middle cerebral arteries. In single cannulated cremaster (panel A) and middle cerebral (panel B) arteries, step-wise increases in intraluminal pressure resulted in the generation of spontaneous myogenic tone and active vasoconstriction. Serial additions of SKA-31 (as indicated by the horizontal bars above the tracing) produced a concentration-dependent inhibition of myogenic tone that was reversible upon SKA-31 washout from the bath. Addition of a near-maximal concentration of acetylcholine (ACh, 3 μM) also produced a rapid and reversible inhibition of myogenic tone. Exposure of the vessel to Kreb’s buffer containing 2 mM EGTA and no added CaCl2 was performed at the end of the protocol to obtain the maximal passive vessel diameter at 70 mmHg. In panel C, the concentration-response data for SKA-31 evoked inhibition of active tone were fit with a Hill equation (see Materials and Methods), yielding calculated maximal levels of inhibition and IC50 values for SKA-31 action in cremaster arteries (max inhibition = 80.8 %, IC50 = 1.78 ± 0.14 μM, n = 21) and middle cerebral arteries (max inhibition = 64.5%, IC50 = 2.05 ± 0.08 μM, n = 7). The histogram in panel D displays quantification of SKA-31 and ACh-evoked inhibition of myogenic tone in cremaster and middle cerebral arteries, as described under Methods and Materials. Data are presented as mean ± SEM for 21 cremaster and 7 middle cerebral arteries. No difference in the % inhibition of myogenic by SKA-31 at a given concentration was noted between cremaster and cerebral arteries. The asterisk (*) indicates a statistically significant difference compared with the ACh-induced inhibition observed in cremaster arteries, P < 0.05.
Where Rmax = the calculated maximal extent of drug-induced inhibition, IC50 is the calculated drug concentration producing half-maximal inhibition, [drug] are the SKA-31 concentrations used experimentally and N = the calculated Hill coefficient. In the case of ACh, we set a threshold inhibitory response of 10%; any vessels in which administration of ACh, either 0.5 μM or 3 μM, did not produce at least 10% inhibition of myogenic tone were not included in the analysis.
2.2 Reagents
Acetylcholine chloride, DMSO (dimethyl sulfoxide), L-NAME (NG-Nitro-L-arginine methyl ester), apamin, pinacidil and all required chemicals to prepare physiological solutions were purchased from Sigma-Aldrich (Oakville, ON, Canada). NS1619 and penitrem-A were obtained from Santa Cruz Biotechnology (Dallas, TX). Euthanyl (sodium pentobarbital, 250 mg/ml) was purchased from Bimeda-MTC Animal Health Inc., Cambridge, ON, Canada. SKA-31 (naphtho[1,2-d]thiazole-2-ylamine) and TRAM-34 (1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole) were synthesized as previously described 10,17.
SKA-31, UCL1684 and TRAM-34 were were all prepared as stock solutions (5 mM or higher) in DMSO and then diluted directly into the external bath solution. The final concentration of DMSO reaching the tissue was typically 0.05% (vol/vol) or less. In control experiments, we examined the potential effect of a higher concentration of DMSO (0.2% final) on myogenic tone development and responsiveness to acetylcholine and SKA-31 in cremaster and middle cerebral arteries (n = 2 for each). In both types of arteries, 0.2% DMSO had no effect on vessel function or drug-evoked vasodilation (data not shown).
2.3 Statistical Analyses
Responses to either SKA-31 or acetylcholine at a given concentration were compared between cremaster and middle cerebral arteries using a Student’s t-test. Concentration-dependent responses to SKA-31 in one artery type or the other were analyzed using one-way ANOVA and a Tukey’s post-hoc test. A calculated P value of less than 0.05 was taken to indicate a statistically significant difference. All data are reported as mean ± SEM of the indicated number of vessels examined.
3. Results
3.1 SKA-31 effectively inhibits myogenic tone in isolated cremaster and middle cerebral arteries
In single cannulated rat cremaster or middle cerebral arteries, step-wise increases in intraluminal pressure to a steady-state level of 70 mmHg initially produced increases in vessel diameter, followed by the spontaneous development of myogenic tone (Figs 1A and B). The absolute extent of myogenic constriction at 70 mmHg averaged 106.3 ± 18.5 μm and 82.6 ± 7.2 μm in cremaster and middle cerebral arteries, respectively. Following development of steady-state myogenic constriction, serial addition of the KCa channel activator SKA-31 10 at increasing concentrations to the bath resulted in a progressive inhibition of myogenic tone in both artery types that was reversible upon washout of SKA-31. In an effort to “benchmark” the observed SKA-31 evoked responses, we exposed arteries to a near maximal concentration of acetylcholine (ACh, 3 μM), an established endothelium-dependent vasodilator. Brief exposure to ACh produced a robust and reversible inhibition of myogenic tone in both vessel types. Exposure of the vessels to Kreb’s buffer containing 2 mM EGTA and no added CaCl2 at the end of each experiment was utilized to determine the maximal passive intraluminal diameter in each vessel type; these values averaged 264.5 ± 21.8 μm (n = 21) and 293.8 ± 16.1 μm (n = 7) for cremaster and middle cerebral arteries, respectively, in this series of experiments. Quantification of the SKA-31 and ACh-induced inhibition of myogenic tone in cremaster and cerebral arteries is provided in Figure 1C. We did not observe a difference between cremaster (CM) and cerebral (CER) arteries with respect to the IC50 values for SKA-31 evoked inhibition of myogenic tone (CM = 1.78 ± 0.12 μM; CER = 2.03 ± 0.07 μM) (Fig. 1C) or the maximal extent of inhibition by 10 μM SKA-31 (CM = 80.0 ± 9.7%; CER = 68.20 ± 10.8%) (Fig. 1D). For both cremaster and cerebral arteries, the magnitudes of inhibitory responses to SKA-31 at 3 and 10 μM concentrations were significantly larger than those observed at concentrations of 0.3 and 1 μM (P < 0.05).
3.2 Pharmacologic blockade of endothelial KCa2.3 and KCa3.1 channels prevents the inhibition of myogenic tone by SKA-31
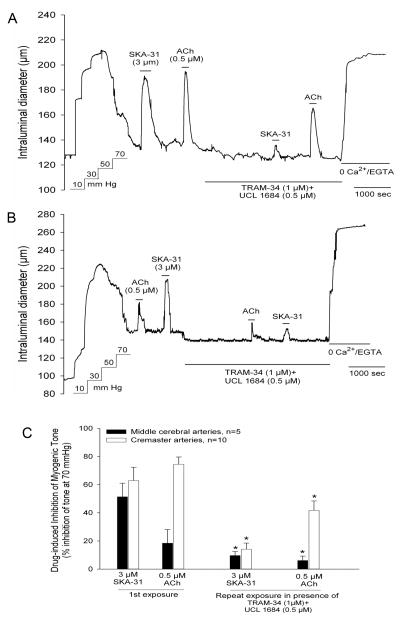
To establish the contribution of specific KCa channels to the SKA-31 and ACh-induced inhibition of myogenic constriction in cremaster and middle cerebral arteries, we examined the effects of these vasodilators in the presence of the KCa2.3 blocker UCL1684 and the KCa3.1 blocker TRAM-34 18. As a small molecule, UCL1684 would be expected to access the endothelial layer more effectively upon bath application compared with the larger, peptidergic KCa2 channel blocker apamin. In a myogenically active cremaster artery, acute exposure to either 3 μM SKA-31 or 0.5 μM ACh produced comparable inhibition of myogenic tone that completely reversed upon washout of each agent from the bath. However, in the presence of 0.5 μM UCL1684 + 1 μM TRAM-34, the SKA-31 and ACh-evoked inhibitory responses were significantly blunted compared with the initial control responses (Figure 2A) and we observed similar effects of UCL1684 + TRAM-34 treatment in middle cerebral arteries (Fig. 2B). The impact of endothelial KCa2.3 and KCa3.1 channel blockade on SKA-31 and ACh-induced inhibition or myogenic tone is quantified in Figure 2C. Interestingly, exposure to UCL1684 + TRAM-34 produced a modest, but significant vasoconstriction in both cremaster (8.1 ± 3.3 μm, n = 10) and middle cerebral (13.9 ± 2.9 μm, n = 5) arteries, as evidenced by a reproducible decrease in intraluminal diameter. This observation implies that the basal KCa2.3 and KCa3.1 activities oppose the development of pressure-induced myogenic tone generated under baseline conditions.
Figure 2.
SKA-31 and ACh-induced inhibition of myogenic tone are largely prevented in the presence of UCL1684 + TRAM-34, blockers of KCa2.3 and KCa3.1 channels, respectively. Panel A shows a representative tracing of vasodilatory responses evoked by SKA-31 and ACh in a cremaster artery in the absence and presence of 0.5 μM UCL1684 + 1 μM TRAM-34. Following drug washout, the vessel was exposed to Kreb’s solution containing 2 mM EGTA and no added CaCl2 to determine the maximal passive diameter. The addition of individual agents to the vessel chamber is indicated by the horizontal bars placed above and below the tracing. Panel B shows a parallel experiment carried out using a middle cerebral artery. Note that the observed inhibitory responses to ACh and SKA-31 were unaffected by the order of drug application during the experiment. The histogram in panel C quantifies the effects of UCL1684 + TRAM-34 treatment on the SKA-31 and ACh-induced inhibition of myogenic tone in cremaster and middle cerebral arteries. The asterisk (*) indicates a statistically significant difference in magnitude compared with the control response observed in the absence of KCa channel blockers, as determined by one-way ANOVA and a Tukey’s post-hoc test, P < 0.05.
3.3 Disruption of the endothelium prevents the vasodilatory effect of SKA-31
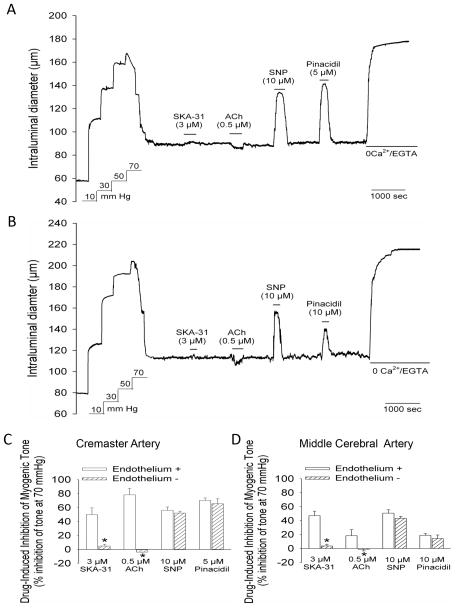
To investigate the requirement of a functionally intact endothelium for the inhibition of myogenic tone by SKA-31, cremaster and middle cerebral arteries were pretreated by the passage of an air bubble through the vessel lumen to physically disrupt endothelial integrity. As displayed in Figures 3A and 3B, this manipulation did not interfere with the normal development of myogenic tone, but completely prevented the vasodilatory action of SKA-31. As expected, ACh-induced vasodilation was also abolished by endothelial denudation in both types of arteries. In contrast, disruption of the endothelium did not prevent relaxation of myogenically active arteries by either a nitrovasodilator (i.e. sodium nitroprusside, SNP) or the KATP channel activator pinacidil, agents that are known to act directly on vascular smooth muscle to cause relaxation. The observed lower efficacy of pinacidil to evoke vasodilation in middle cerebral arteries is consistent with an earlier report 19. Quantification of the magnitude of vasodilation evoked by SKA-31, ACh, SNP and pinacidil in endothelium intact and denuded arteries is provided in Figure 3C and D.
Figure 3.
Endothelium denudation prevents SKA-31 and ACh-evoked vasodilation in cremaster and middle cerebral arteries. Panel A shows a representative tracing depicting the vasodilatory responses to 3 μM SKA-31, 0.5 μM ACh, 10 μM SNP and 5 μM pinacidil in a cremaster artery in which an air bubble had been passed through the vessel lumen prior to the development of myogenic tone. The representative tracing in panel B shows vasodilatory responses to the same vasodilators in a middle cerebral artery in which the endothelium had been disrupted by the introduction of an air bubble. The histograms in panels C and D depict the magnitude of dilator-evoked inhibition of myogenic tone in cremaster (panel C) and middle cerebral arteries (panel D) with intact endothelium (+) or physically disrupted endothelium (-). Vasodilatory responses to SKA-31, ACh, SNP and pinacidil in endothelium-intact vessels were derived from other experiments reported in this study; for intact cremaster arteries, n = 6–14, for intact middle cerebral arteries, n = 4–7. For both endothelium-denuded cremaster and middle cerebral arteries, average values were calculated from 4 vessels of each type. Data are expressed as means ± SEM. The asterisk (*) indicates a statistically significant difference in the magnitude of inhibition compared with the intact endothelium condition, as determined by an unpaired Student’s t-test (P < 0.05).
3.4 The inhibition of myogenic tone by SKA-31 is not affected by the eNOS inhibitor L-NAME
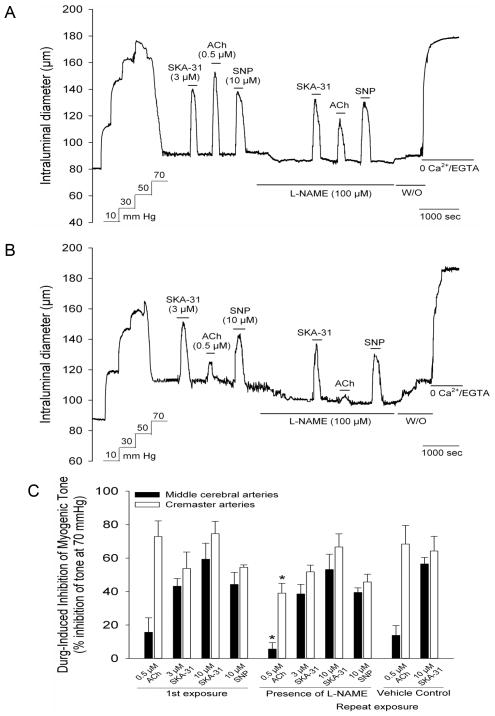
Having established the essential role of the endothelium, we next examined the potential contribution of endothelial nitric oxide synthase (eNOS) activity to the vasodilatory actions of SKA-31 in these two vessel types, and utilized ACh-evoked vasodilation as a positive control for eNOS participation. Inhibition of myogenic tone in response to 3 and 10 μM SKA-31 and 0.5 μM ACh was first recorded in cremaster and cerebral arteries under control conditions and then following treatment with the established eNOS inhibitor L-nitro-arginine methyl ester (L-NAME, 0.1 mM) 20. As illustrated in Figure 4A, 3 μM SKA-31 produced comparable inhibition of myogenic tone in a cremaster artery in both the absence and presence of L-NAME. In contrast, L-NAME treatment significantly blunted the ACh-induced inhibition of myogenic constriction in the same vessel. As expected, inhibition of myogenic by sodium nitroprusside (SNP, 10 μM) was unaffected by L-NAME treatment, which is consistent with its direct vasodilatory actions on vascular smooth muscle 21. Similar responses to SKA-31, ACh and SNP were observed in middle cerebral arteries (Fig. 4B). The histogram in Figure 4C quantifies the extent of SKA-31, ACh and SNP-induced inhibition of myogenic tone in the absence and presence of L-NAME treatment, along with time control data for cremaster and cerebral artery responsiveness following repeated exposures to SKA-31 and ACh (right hand columns of the histogram). Collectively, these data demonstrate that SKA-31 induced inhibition of myogenic tone in cremaster and middle cerebral arteries was unaffected by L-NAME treatment, in contrast to the significant reduction in the ACh-evoked response by L-NAME in both vessel types. Interestingly, we observed that exposure of myogenically active arteries to L-NAME alone produced a modest and reversible constriction of intraluminal diameter, which averaged 12.3 ± 4.1 μm and 8.7 ± 3.3 μm for cremaster (n = 10) and cerebral arteries (n = 8), respectively.
Figure 4.
SKA-31 evoked inhibition of myogenic tone is not blunted in the presence of the NO synthase inhibitor L-NAME. Following development of stable myogenic tone, brief exposure of a cannulated cremaster artery to 3 μM SKA-31, 0.5 μM ACh and 10 μM SNP produced robust and reversible inhibition of developed tone (panel A). Re-exposure to SKA-31, ACh and SNP was then carried out in the presence of 100 μM L-NAME, as indicated by the horizontal bar beneath the tracing. Washout (W/O) of L-NAME from the bath is also marked by a horizonatal bar. Panel B displays the same experimental protocol applied to a cannulated middle cerebral artery. Quantification of the effect of L-NAME treatment on the SKA-31, ACh and SNP-evoked inhibition of myogenic tone in cremaster and middle cerebral arteries is shown in panel C. The left-hand columns display the control dilatory responses to SKA-31, ACh and SNP, whereas the middle columns describe responses to repeated exposures to these dilators in the presence 100 μM L-NAME (n = 3–10 for cremaster, n = 3–8 for middle cerebral arteries). The right-hand columns display the responses to SKA-31 and ACh in vessels treated with the saline vehicle used for L-NAME suspension (n = 4 for both vessel types). The asterisk (*) indicates a statistically significant difference in the magnitude of inhibition compared with the initial drug-evoked response, as determined by one-way ANOVA and a Tukey’s post-hoc test (P < 0.05).
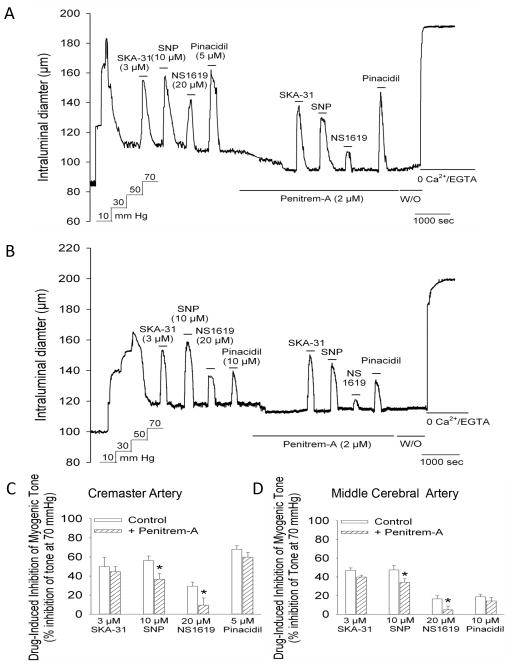
3.5 The inhibition of myogenic tone by SKA-31 is not altered by a blocker of BKCa channels
To further establish the selective activation of KCa2.3 and KCa3.1 channels by SKA-31 in myogenically active vessels, cremaster and middle cerebral arteries were treated with 2 μM penitrem-A, a highly selective pharmacologic blocker of large conductance, Ca2+-activated K+ (BKCa) channels 22. As shown in Figure 5, the extent of inhibition of myogenic tone by SKA-31 was unaltered in the presence of penitrem-A and we also observed no effect of this treatment on the vasodilatory action of the KATP channel activator pinacidil. However, inhibition of myogenic tone by 20 μM NS1619, a pharmacologic activator of BKCa channels 23,24, was largely prevented in the presence of penitrem-A and we further observed a significant reduction in the magnitude of SNP-mediated vasodilation in cremaster and cerebral arteries. The histograms in Figures 5C and 5D quantify the effect of penitrem-A on the magnitude of inhibition induced by SKA-31, NS1619, pinacidil and SNP in cremaster and middle cerebral arteries, respectively. Exposure to penitrem-A alone also produced a modest, yet significant decrease in intraluminal diameter in cremaster (14.6 ± 1.5 μm, n = 6) and cerebral arteries (9.1 ± 3.1 μm, n = 4), in agreement with the presence of active BKCa channels in myogenically contracted arteries 25–27.
Figure 5.
The BKCa channel blocker penitrem-A does not impair the SKA-31-induced inhibition of myogenic tone. The representative tracing in panel A shows vasodilatory responses to 3 μM SKA-31, 10 μM SNP, 20 μM NS1619 and 5 μM pinacidil in a myogenically active cremaster artery under control conditions and following exposure to 2 μM penitrem-A, as indicated by the horizontal bar beneath the tracing. The washout (W/O) of penitrem-A is also marked by a horizontal bar. The tracing in panel B depicts the effect of penitrem-A treatment on the inhibition of myogenic tone by the same set of agents in a middle cerebral artery. The summary data presented in panels C and D display the degree of inhibition of developed myogenic tone by SKA-31, SNP, NS1619 and pinacidil in the absence and presence of penitrem-A treatment in cremaster (panel C, n = 6) and middle cerebral arteries (panel D, n = 4). Data are expressed as means ± SEM. The asterisk (*) indicates a statistically significant difference in the magnitude of inhibition compared with control condition, as determined by an unpaired Student’s t-test.
4. Discussion
The results of this study demonstrate that the second generation KCa2/3.1 channel activator SKA-31 effectively inhibits myogenic tone with similar potency in isolated, pressurized resistance arteries from two distinct vascular beds (i.e. skeletal muscle and cerebral circulations). Functionally, SKA-31-induced inhibition of myogenic tone in both vessel types was largely prevented by UCL1684 and TRAM-34, selective blockers of KCa2.x and KCa3.1 channel, respectively (Figure 2), but was unaffected by penitrem-A, a blocker of BKCa channels (Figure 5). Furthermore, endothelial denudation abolished the vasodilatory action of SKA-31 in cremaster and cerebral arteries (Figure 3), thereby establishing the endothelium as the exclusive site of action for SKA-31 and ruling out any direct vasodilatory effect of SKA-31 on vascular smooth muscle. Collectively, these observations are in agreement with the reported expression of both KCa2.3 and KCa3.1 channels in vascular endothelium 2,28–30 and the lack of vasodilatory action of KCa activators in resistance arteries lacking functional endothelium 8 and mice with genetic knockout of KCa2.3 and KCa3.1 channels 6,31. The observed insensitivity of SKA-31-evoked vasodilation to the eNOS inhibitor L-NAME in both cremaster and middle cerebral arteries further indicates that eNOS activity per se is not required for the direct vasodilatory actions of SKA-31 in small resistance arteries. This observation is supported by our recent data obtained in the intact coronary circulation of rat heart, where acute increases in coronary flow evoked by SKA-31 were unaffected by L-NAME treatment 32 and is further consistent with data in which the vasodilatory action of NS309, a related KCa2/3.1 channel activator, was unaffected by L-NAME in cremaster 8 and small mesenteric arteries 33. In addition, endothelial KCa channel activators are reported to have significant vasodilatory effects in eNOS knockout mice 32,34. Taken together, these data support a mechanism in which KCa channel activators cause vascular relaxation primarily by inducing endothelium-dependent hyperpolarization of the surrounding smooth muscle, which is thought to involve electrical charge transfer via myo-endothelial connections and the K+-evoked activation of smooth muscle inward rectifier K+ channels 2,35. Smooth muscle hyperpolarization will decrease Ca2+ entry via voltage-gated, L-type Ca2+ channels, thereby promoting de-activation of the smooth muscle contractile machinery and relaxation.
Mechanistically, the independence of KCa channel activator-evoked relaxation from NO synthesis may be explained by the fact that these activators on their own do not elevate cytosolic free [Ca2+], which is an important stimulus for increasing Ca2+-dependent eNOS activity 36. Although KCa channel activators indirectly increase the electrical driving force for Ca2+ entry, as a result of endothelial membrane hyperpolarization, a meaningful increase in cytosolic free [Ca2+] via enhanced Ca2+ influx will only occur in the presence of open Ca2+ entry channels at the endothelial cell surface (e.g. TRPC, Orai, etc.). In non-excitable cells, opening of Ca2+ entry channels at the plasma membrane typically follows agonist-stimulated release of ER-stored Ca2+ (i.e. SOCE) 37. As Ca2+ store release is not triggered by KCa channel activators, these agents alone do not elevate cytosolic free Ca2+, as we have previously reported 8. The fact that SKA-31 mediated inhibition of myogenic tone is preserved in the absence of NO signalling 32,34 further predicts that this vasodilatory mechanism will be minimally impacted under conditions of reduced NO bioavailability (e.g. endothelial dysfunction, atherosclerosis), which is observed in isolated hearts from type 2 diabetic rats 38. Our observations that L-NAME treatment alone produced a modest, yet significant decrease in basal luminal diameter in both cremaster and cerebral arteries (Figure 4) suggest that active NO synthesis may be occurring under basal conditions in our pressurized arteries; alternatively, L-NAME may be acting directly on vascular smooth muscle to increase contractile tone, as previously described 39.
In cremaster arteries, inhibition of myogenic tone by the classical endothelium-dependent vasodilatory hormone ACh was significantly blunted by selective blockers of endothelial KCa2.3 and KCa3.1 channels (i.e. UCL1684 and TRAM-34, respectively) (Figure 2C) and by treatment with the eNOS inhibitor L-NAME (Figure 4C). These observations are thus in agreement with previous studies implicating both KCa channel activity and eNOS stimulation in the endothelium-dependent vasodilation evoked by ACh and other Ca2+-mobilizing hormones 4,5,40–44. In middle cerebral arteries, pharmacologic blockade of either KCa2/3.1 channels or eNOS also produced quantifiable decreases in ACh-induced vasodilation (Figs 2C and 4D), although we did observe considerable experimental variation in the magnitude of ACh-evoked dilation at moderate (0.5 μM, Figs. 2C and 4D) and high (3 μM, Fig. 1D) concentrations of ACh. Out of a total of 21 middle cerebral arteries examined, a near-maximal concentration of ACh (i.e. 3 μM) inhibited myogenic tone by less than 10% in 14 vessels; note that these 14 “ACh-unresponsive” arteries were omitted from all data analysis (e.g. Figures 1C and 1D). Similar inconsistency in the evoked inhibition of contractile tone in middle cerebral arteries by ACh and other endothelium-dependent vasodilatory hormones has been described previously by other investigators 45–48. Interestingly, in the same 14 “unresponsive” cerebral vessels omitted from analysis, 10 μM SKA-31 was found to inhibit myogenic tone by 58.0 ± 2.5% (mean ± SE, range of response = 35.5 – 76.4%); this degree of inhibition by SKA-31 is not statistically different than that observed for the 7 “ACh-responsive” cerebral arteries reported in Figure 1D. These observations thus exclude the possibility that endothelial damage or dysfunction impaired ACh responsiveness in these 14 cerebral vessels. Although the reason(s) for this variability in ACh action remains unclear, it is possible that differences in the structural properties of middle cerebral compared with cremaster arteries (e.g. extent and composition of the extracellular matrix) 12 may contribute to this variability in hormonal sensitivity.
5. Conclusion
The results of our study demonstrate that direct activation of endothelial KCa2.3 and KCa3.1 channels by SKA-31 is capable of producing robust and reversible inhibition of developed myogenic tone in small resistance arteries from skeletal muscle and cerebral circulations. The observed efficacy with which KCa channel activators inhibit myogenic tone in a variety of arteries/vascular beds under normal and pathological conditions 35 indicates that the cellular mechanism(s) underlying this response is both widespread and resilient. Pharmacologic manipulation of endothelial KCa channels may thus have beneficial effects on vascular function under conditions in which endothelial NO bioavailability is compromised and may provide therapeutic benefit in the treatment of cardiovascular-related disorders 15,49.
Acknowledgments
This work was supported by research funding awarded to A.P. Braun (Canadian Institutes of Health Research MOP 97901), to H. Wulff (National Institutes of Health NS072585) and to M.A. Hill (National Institutes of Health RO1 HL092241).
Abbreviations
- ACh
acetylcholine
- BKCa
large conductance, calcium-activated K+ channel
- DMSO
dimethyl sulfoxide
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid
- eNOS
endothelial nitric oxide synthase
- KCa
calcium-activated K+ channel
- L-NAME
NG nitro L-arginine methyl ester
- NO
nitric oxide
- SOCE
store-operated calcium entry
- SKA-31
naphtho[1,2-d]thiazole-2-ylamine
- TRAM-34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- TRPC
transient receptor potential channel
- UCL1684
6,12,19,20,25,26-Hexahydro-5,27: 13,18:21,24-trietheno-11,7-metheno-7H-dibenzo [b,n] [1,5,12,16]tetraazacyclotricosine-5,13-diium dibromide
Footnotes
Disclosure on Potential Conflicts of Interest
The authors confirm that no conflicts of interest exist with respect to the work carried out under this project and the results described in this manuscript.
References
- 1.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: Bringing the Concepts Together. TIPS. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 2.Edwards G, Félétou M, Weston AH. Endothelium-Derived Hyperpolarising Factors and Associated Pathways: a Synopsis. Pflügers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 3.Sheng J-Z, Braun AP. Small- and Intermediate-Conductance Ca2+-Activated K+ Channels Directly Control Agonist-Evoked Nitric Oxide Synthesis in Human Vascular Endothelial Cells. Am J Physiol Cell Physiol. 2007;293:C458–C467. doi: 10.1152/ajpcell.00036.2007. [DOI] [PubMed] [Google Scholar]
- 4.Stankevicius E, Lopez-Valverde V, Rivera L, Hughes AD, Mulvany MJ, Simonsen U. Combination of Ca2+-Activated K+ Channel Blockers Inhibits Acetylcholine-Evoked Nitric Oxide Release in Rat Superior Mesenteric Artery. Br J Pharmacol. 2006;149:560–572. doi: 10.1038/sj.bjp.0706886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doughty JM, Plane F, Langton PD. Charybdotoxin and Apamin Block EDHF in Rat Mesenteric Artery If Selectively Applied to Endothelium. Am J Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- 6.Brähler S, Kaistha A, Schmidt VJ, Wölfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Köhler R. Genetic Deficit of SK3 and IK1 Channels Disrupts the Endothelium-Derived Hyperpolarizing Factor Vasodilator Pathway and Causes Hypertension. Circ. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 7.Köhler R, Ruth P. Endothelial Dysfunction and Blood Pressure Alterations in K+ Channel Transgenic Mice. Pflügers Arch. 2010;459:969–976. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- 8.Sheng J-Z, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa Channels Enhance Agonist-Evoked Endothelial Nitric Oxide Synthesis and Arteriolar Dilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalsgaard T, Kroigaard C, Misfeldt M, Simonsen U. Openers of Small Conductance Calcium-Activated Potassium Channels Selectively Enhance NO-Mediated Bradykinin Vasodilatation in Porcine Retinal Arterioles. Br J Pharmacol. 2010;160:1496–1508. doi: 10.1111/j.1476-5381.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Köhler R, Wulff H. Naphthol[1,2-d]Thiazol-2-Ylamine (SKA-31), a New Activator of KCa2 and KCa3.1 Potassium Channels, Potentiates the Endothelium-Derived Hyperpolarizing Factor Response and Lowers Blood Pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorin E, Shreeve SM. Heterogeneity of Vascular Endothelial Cells in Normal and Disease States. Pharmacology and Therapeutics. 1998;78:155–166. doi: 10.1016/s0163-7258(98)00005-9. [DOI] [PubMed] [Google Scholar]
- 12.Clifford PS, Ella SR, Stupica AJ, Nourian Z, Li M, Martinez-Lemus LA, Dora KA, Yang Y, Davis MJ, Pohl U, Meininger GA, Hill MA. Spatial Distribution and Mechanical Function of Elastin in Resistance Arteries: A Role in Bearing Longitudinal Stress. Artherioscler Thromb Vasc Biol. 2011;31:2889–2896. doi: 10.1161/ATVBAHA.111.236570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Félétou M. Calcium-Activated Potassium Channels and Endothelial Dysfunction: Therapeutic Options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalsgaard T, Kroigaard C, Simonsen U. Calcium-Activated Potassium Channels - a Therapeutic Target for Modulating Nitric Oxide in Cardiovascular Disease? Expert Opin Ther Targets. 2010;14:825–837. doi: 10.1517/14728222.2010.500616. [DOI] [PubMed] [Google Scholar]
- 15.Wulff H, Köhler R. Endothelial Small-Conductance and Intermediate-Conductance KCa Channels: An Update on Their Pharmacology and Usefulness As Cardiovascular Targets. J Cardiovasc Pharmacol. 2013;61:102–112. doi: 10.1097/FJC.0b013e318279ba20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium Measurement in Isolated Arterioles During Myogenic and Agonist Stimulation. Am J Physiol Heart Circ Physiol. 1991;261:H950–H959. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- 17.Wulff H, Miller MJ, Hänsel W, Grissmer S, Cahalan MD, Chandy KG. Design of a Potent and Selective Inhibitor of the Intermediate-Conductance Ca2+-Activated K+ Channel, IKCa1: A Potential Immunosuppressant. Proc Natl Acad Sci USA. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels and Their Therapeutic Indications. Curr Med Chem. 2007;14:1437–1457. doi: 10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- 19.McCarron JG, Quayle JM, Halpern W, Nelson MT. Cromakalim and Pinacidil Dilate Small Mesenteric Arteries but Not Small Cerebral Arteries. Am J Physiol Heart Circ Physiol. 1991;261:H287–H291. doi: 10.1152/ajpheart.1991.261.2.H287. [DOI] [PubMed] [Google Scholar]
- 20.Griffith OW, Gross SS. Inhibitors of nitric oxide synthases. In: Feelisch M, Stamler JS, editors. Methods in Nitric Oxide Research. John Wiley and Sons Ltd; 1996. [Google Scholar]
- 21.McDonald LJ, Murad F. Nitric Oxide and CGMP Signaling. Adv Pharmacol. 1995;34:263–275. doi: 10.1016/s1054-3589(08)61091-1. [DOI] [PubMed] [Google Scholar]
- 22.Asano S, Bratz IN, Berwick ZC, Fancher IS, Tune JD, Dick GM. Penitrem A As a Tool for Understanding the Role of Large Conductance Ca2+/Voltage-Sensitive K+ Channels in Vascular Function. J Pharmacol Exp Ther. 2012;342:453–460. doi: 10.1124/jpet.111.191072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olesen SP, Munch E, Moldt P, Drejer J. Selective Activation of Ca(2+)-Dependent K+ Channels by a Novel Benzimidazolone. Eur J Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 24.Nardi A, Olesen SP. BK Channel Modulators: A Comprehensive Overview. Curr Med Chem. 2008;15:1126–1146. doi: 10.2174/092986708784221412. [DOI] [PubMed] [Google Scholar]
- 25.Brayden JE, Nelson MT. Regulation of Arterial Tone by Activation of Calcium-Dependent Potassium Channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 26.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of Arterial Smooth Muscle by Calcium Sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 27.Kotecha N, Hill MA. Myogenic Contraction in Rat Skeletal Muscle Arterioles: Smooth Muscle Membrane Potential and Ca2+ Signaling. Am J Physiol Heart Circ Physiol. 2005;289:H1326–H1334. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- 28.Köhler R, Degenhardt C, Kühn M, Runkel N, Paul M, Hoyer J. Expression and Function of Endothelial Ca2+-Activated K+ Channels in Human Mesenteric Artery. A Single-Cell Reverse Transcriptase-Polymerase Chain Reaction and Electrophysiological Study in Situ. Circ Res. 2000;87:496–503. doi: 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- 29.Burnham MP, Bychkov R, Félétou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an Apamin-Sensitive Small-Conductance Ca2+-Activated K+ Channel in Porcine Coronary Artery Endothelium: Relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Félétou M, Vanhoutte PM. Characterization of a Charybdotoxin-Sensitive Intermediate Conductance Ca2+-Activated K+ Channel in Porcine Coronary Endothelium: Relevance to EDHF. Br J Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köhler R, Hoyer J. The Endothelium-Derived Hyperpolarizing Factor: Insights From Genetic Animal Models. Kidney Int. 2007;72:145–150. doi: 10.1038/sj.ki.5002303. [DOI] [PubMed] [Google Scholar]
- 32.Mishra RC, Belke D, Wulff H, Braun AP. SKA-31, a Novel Activator of SKCa and IKCa Channels, Increases Coronary Flow in Male and Female Rat Hearts. Cardiovasc Res. 2013;97:339–348. doi: 10.1093/cvr/cvs326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stankevicius E, Dalsgaard T, Kroigaard C, Beck L, Boedtkjer E, Misfeldt M, Nielsen G, Schjorring O, Hughes AD, Simonsen U. Opening of Small and Intermediate Calcium-Activated Potassium Channels Induces Relaxation Mainly Mediated by Nitric-Oxide Release in Large Arteries and Endothelium-Derived Hyperpolarizing Factor in Small Arteries From Rat. J Pharmacol Exp Ther. 2011;339:842–850. doi: 10.1124/jpet.111.179242. [DOI] [PubMed] [Google Scholar]
- 34.Hasenau AL, Nielsen G, Morisseau C, Hammock D, Wulff H, Köhler R. Improvement of Endothelium-Dependent Vasodilations by SKA-31 and SKA-20, Activators of Small- and Intermediate-Conductance Ca2+-Activated K+ Channels. Acta Physiol Scand. 2011;203:117–126. doi: 10.1111/j.1748-1716.2010.02240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grgic I, Kaistha A, Hoyer J, Köhler R. Endothelial Ca2+-Activated K+ Channels in Normal and Impaired EDHF-Dilator Responses - Relevance to Cardiovascular Pathologies and Drug Discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming I, Busse R. Molecular Mechanisms Involved in the Regulation of the Endothelial Nitric Oxide Synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 37.Parekh AB, Putney JW., Jr Store-Operated Calcium Channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 38.Mishra RC, Wulff H, Cole WC, Braun AP. A Pharmacologic Activator of Endothelial KCa Channels Enhances Coronary Flow in the Hearts of Type Diabetic Rats. J Mol Cell Cardiol. 2014;72:364–373. doi: 10.1016/j.yjmcc.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Murphy TV, Kotecha N, Hill MA. Endothelium-Independent Constriction of Isolated, Pressurized Arterioles by Nω-Nitro-L-Arginine Methyl Ester (L-NAME) Br J Pharmacol. 2007;151:602–609. doi: 10.1038/sj.bjp.0707262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and Intermediate-Conductance Calcium-Activated K+ Channels Provide Different Facets of Endothelium-Dependent Hyperpolarization in Rat Mesenteric Artery. J Physiol (Lond) 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for the Involvement of Both IKCa and SKCa Channels in Hyperpolarizing Responses of the Rat Middle Cerebral Artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- 42.Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Köhler R. Impaired Endothelium-Derived Hyperpolarizing Factor-Mediated Dilations and Increased Blood Pressure in Mice Deficient of the Intermediate-Conductance Ca2+-Activated K+ Channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 43.Potocnik SJ, McSherry IN, Ding H, Murphy TV, Kotecha N, Dora KA, Yuill KH, Sandow SL, Triggle CR, Hill MA. Endothelium-Dependent Vasodilation in Myogenically-Active Mouse Skeletal Muscle Arterioles: Role of EDH and K+ Channels. Microcirculation. 2009;16:377–390. doi: 10.1080/10739680902804042. [DOI] [PubMed] [Google Scholar]
- 44.Busse R, Fleming I, Hecker M. Signal Transduction in Endothelium-Dependent Vasodilatation. Eur Heart J. 1993;14 (suppl 1):2–9. [PubMed] [Google Scholar]
- 45.Dacey RG, Jr, Bassett JE. Cholinergic Vasodilation of Intracerebral Arterioles in Rats. Am J Physiol Heart Circ Physiol. 1987;253:H1253–H1260. doi: 10.1152/ajpheart.1987.253.5.H1253. [DOI] [PubMed] [Google Scholar]
- 46.Nakagomi T, Hongo K, Kassell NF, Sasaki T, Lehman RM, Ogawa H, Vollmer DG, Torner JC. Pharmacological Comparison of Endothelium-Dependent Relaxation in Isolated Cerebral and Extracerebral Arteries. J Neurosurg. 1988;69:580–587. doi: 10.3171/jns.1988.69.4.0580. [DOI] [PubMed] [Google Scholar]
- 47.Lagaud GJ, Skarsgard PL, Laher I, Van Breeman C. Heterogeneity of Endothelium-Dependent Vasodilation in Pressurized Cerebra and Small Mesenteric Resistance Arteries of the Rat. J Pharmacol Exp Ther. 1999;290:832–839. [PubMed] [Google Scholar]
- 48.Toda N, Kawakami M, Yamazaki M, Okamura T. Comparison of Endothelium-Dependent Responses of Monkey Cerebral and Temporal Arteries. Br J Pharmacol. 1991;102:805–810. doi: 10.1111/j.1476-5381.1991.tb12256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Köhler R, Kaistha BP, Wulff H. Vascular KCa-Channels As Therapeutic Targets in Hypertension and Restenosis. Expert Opin Ther Targets. 2010;14:143–155. doi: 10.1517/14728220903540257. [DOI] [PMC free article] [PubMed] [Google Scholar]