Abstract
Background
HIV-1 viral load (VL) testing is recommended to monitor antiretroviral therapy (ART) but not universally available. We examined monitoring of first-line and switching to second-line ART in sub-Saharan Africa, 2004–2013.
Methods
Adult HIV-1 infected patients starting combination ART in 16 countries were included. Switching was defined as a change from a non-nucleoside reverse-transcriptase inhibitor (NNRTI)-based regimen to a protease inhibitor (PI)-based regimen, with a change of ≥1 NRTI. Virological and immunological failures were defined per World Health Organization criteria. We calculated cumulative probabilities of switching and hazard ratios with 95% confidence intervals (CI) comparing routine VL monitoring, targeted VL monitoring, CD4 cell monitoring and clinical monitoring, adjusted for programme and individual characteristics.
Findings
Of 297,825 eligible patients, 10,352 patients (3·5%) switched during 782,412 person-years of follow-up. Compared to CD4 monitoring hazard ratios for switching were 3·15 (95% CI 2·92–3·40) for routine VL, 1·21 (1·13–1·30) for targeted VL and 0·49 (0·43–0·56) for clinical monitoring. Overall 58.0% of patients with confirmed virological and 19·3% of patients with confirmed immunological failure switched within 2 years. Among patients who switched the percentage with evidence of treatment failure based on a single CD4 or VL measurement ranged from 32·1% with clinical to 84.3% with targeted VL monitoring. Median CD4 counts at switching were 215 cells/µl under routine VL monitoring but lower with other monitoring (114–133 cells/µl).
Interpretation
Overall few patients switched to second-line ART and switching occurred late in the absence of routine viral load monitoring. Switching was more common and occurred earlier with targeted or routine viral load testing.
Introduction
The scale-up of antiretroviral therapy (ART) continues and is an important part of the Millennium Development Goal to halt and reverse the AIDS epidemic.1 The aim is to achieve the 90-90-90 treatment targets by 2020: 90% of all people living with HIV know their HIV status, 90% of all people with HIV receive sustained ART and 90% of all people on ART have viral suppression.2 The number of patients who experience treatment failure and who need second-line therapy has also increased.3,4,5 Second-line ART is generally the last treatment option in these settings, and about three times as expensive as first-line ART.6
The goal of monitoring patients on ART is to maximize the durability of first-line regimens. In industrialized countries plasma HIV 1-RNA viral load (VL) and CD4 positive T cell counts (CD4 counts) are regularly measured.7 The decision to switch a patient to second-line ART is based on evidence of virological treatment failure and genotypic or phenotypic resistance testing. Although the World Health Organization (WHO) recommends that VL is monitored routinely, access to VL tests is limited in many settings. Decisions about switching patients to second-line ART are therefore based on clinical and CD4 criteria for treatment failure,8 however sensitivity and positive predictive value of these criteria for virological failure are poor.9,10,11 Patients with suppressed viral replication may thus unnecessarily be switched to second-line ART whereas patients failing first-line therapy may be switched late, or not switched at all.3,5,12
In an analysis of treatment programmes in Africa, Asia and Latin America we found that switching to second-line regimens tended to occur earlier and at higher CD4 cell counts in ART programmes with VL monitoring compared with programmes using CD4 monitoring.13 In the present study we examined data from 32 treatment programmes in sub-Saharan Africa to investigate rates of switching to second-line ART, switching without evidence of treatment failure, and failure not followed by switching, in patients monitored with routine or targeted VL measurements, CD4 cell counts or clinical criteria.
Methods
Study design
The International epidemiological Database to Evaluate AIDS Africa (IeDEA) is a multiregional collaboration of HIV cohort studies. We included ART programmes that participate in the East, Southern, and West African regions of IeDEA.14 Data were collected during routine baseline and follow-up clinical visits, and included socio-demographic data, date of ART start, type of ART and, where available, CD4 counts and VL at enrolment and follow-up. Individual-level data were de-identified and transferred to regional data centers. Site-level data were collected using a site survey that captured data on type and setting of clinics. All research in IeDEA is overseen by Institutional Review Boards (IRBs) or Ethics Committees in the countries where data are collected, and by Ethics Committees with oversight over the analytical teams.14
Participants
HIV-1 infected patients aged ≥16 years, initiating ART between 2004 and 2013, were eligible. All ART-naïve patients with a known date of ART start who initiated first-line ART with a WHO-recommended regimen that contained at least two NRTIs (nucleoside reverse transcriptase inhibitors) and one NNRTI (non-nucleoside reverse transcriptase inhibitors) were included. We excluded patients who switched to second-line ART during their first 6 months on ART or switched back to first-line ART within 6 months of initiating second-line ART.
Outcome
The outcome was switching to second-line ART, defined as a change from the NNRTI-based regimen to a protease inhibitors (PI)-based regimen, in addition to a change of at least one NRTI, but excluding changes from lamivudine (3TC) to emtricitabine (FTC) or vice-versa. 3TC and FTC are both analogues of cytidine, with cross-resistance between the two. A change to a boosted PI and integrase inhibitor, lopinavir/ritonavir (LPV/r) and raltegravir (RAL) was also counted as a switch to second-line ART. Patients were considered to be at risk of switching due to virologic failure after 6 months on first-line ART because viral load is expected to be suppressed at 6 months. Substitutions of single drugs did not count as switching to second-line ART.
Procedures
Monitoring strategies were defined for each programme and calendar year based on the number of measurements per 100 person-years. Routine VL monitoring was assumed if at least 75 VL tests had been done; CD4 monitoring if at least 75 CD4 counts had been done; and targeted VL monitoring if in addition to CD4 monitoring at least five but less than 75 VL measurements were done. A site was considered to use clinical monitoring if it did not meet the criteria for any other monitoring strategy. The threshold of 75 VL measurements was chosen because all South African programmes, which routinely monitor VL,15 met this criterion.
CD4 count at ART initiation was defined as the value closest to the date of ART-initiation within 3 months prior and 1 month after. The window for VL was 6 month prior and 7 days after ART initiation. Values at initiation of second-line ART were defined in the same way. Confirmed virological failure was defined as two consecutive VL values >1,000 copies/ml within 12 months on first-line ART. Confirmed immunologic failure was defined as two consecutive CD4 counts within 12 months on first-line ART meeting WHO 2006 criteria:16 i) CD4 count below 100 cells/ µl after six months of therapy, ii) a return to, or a fall below, the pre-therapy CD4 baseline after six months of therapy or iii) a 50% decline from the on-treatment peak CD4 value. Failures based on a single (unconfirmed) value were also considered. WHO criteria were used to define clinical stage.17 A patient was considered lost to follow up if the time between the last visit and the administrative closure of the database was >1 year.
Statistical Analysis
We used Cox regression models stratified by region (East Africa, West Africa, Southern Africa) to identify determinants of switching and calculate cumulative incidence functions of switching and virological failure.18,19 We considered age, sex, baseline CD4 cell count, baseline WHO clinical stage, year of ART start, type of clinic, location (urban/rural), and time-updated monitoring strategy. We tested the proportional-hazards assumption on the basis of Schoenfeld residuals and inspected log-log plots. We used Kaplan-Meier methods to estimate the probability of switching after failure. We used weighted nonlinear least-squares regression to fit a logistic function to the rate of switching and the number of VL tests per 100 person-years in a given calendar year.
We multiply imputed missing CD4 cell counts and WHO clinical stage at start of ART by chained equations, based on the clinic, monitoring strategy, level of care (health centre/district hospital/regional or university hospital), location (rural/urban), gender, age, calendar year of ART initiation, and on whether or not the patient died. In a sensitivity analysis we ran the Cox regression model after excluding patients with missing data (complete case analysis). All analyses were done in Stata version 13 (Stata Corporation, College Station, Texas, USA).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Characteristics of ART Programmes
Thirty-two ART programmes from 16 African countries were included: 6 from IeDEA East Africa, 14 from IeDEA Southern Africa and 12 from IeDEA West Africa (supplementary Table S1). The East and West Africa programmes were mostly urban; the Southern Africa region included several rural programmes. The number of patients ranged from <50 in the Centre National de Transfusion Sanguine in Ivory Coast, to >120,000 patients at CIDRZ in Zambia. In South Africa VL was routinely monitored throughout, the Rakai programme in Uganda introduced routine VL monitoring in 2005 and the Queen Elisabeth Hospital in Malawi in 2013. AMPATH in Kenya, 22 out of 97 facilities of CIDRZ and most West African programmes (CHU Ouédraogo, CIRBA, CePReF, Gabriel Touré, Point G, SMIT, UATH) used targeted VL at some time. FACES, TUMBI, IDI in East Africa and Solidarmed Mozambique, Newlands in Zimbabwe, most CIDRZ facilities and some West African programmes used CD4 monitoring in most years. Clinical monitoring dominated at Queen Elisabeth Hospital in Malawi until 2013, the Solidarmed programmes in Lesotho and Zimbabwe, the Masaka Regional Hospital in Uganda, and at Tokoin in Togo (supplementary Table S1).
Patient Characteristics
A total of 577,826 patients were recorded in the IeDEA databases; 297,825 patients (51·5%) were eligible for the present analysis and followed up during 782,412 person-years. Median age at ART initiation was 36 years, and 62·7% (186,819) of included patients were female (Table 1). Having less than 6 months follow-up, not starting ART, age <16 years at start of ART, and previous exposure to antiretroviral drugs were common reasons for exclusion (supplementary Figure S1).
Table 1.
Patient characteristics at initiation of first-line antiretroviral therapy.
| Monitoring Strategy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical | CD4 count | Targeted viral load | Routine viral load | |||||
| Number of patients (row %) | 39685 | (13·3%) | 91080 | (30·6%) | 110296 | (37·0%) | 56764 | (19·1%) |
| Median age (years, IQR) | 36 | (30–43) | 36 | (30–42) | 36 | (30–43) | 36 | (30–42) |
| Gender | ||||||||
| Male | 15126 | (38·1%) | 33525 | (36·8%) | 39998 | (36·3%) | 22357 | (39·4%) |
| Female | 24559 | (61·9%) | 57555 | (63·2%) | 70298 | (63·7%) | 34407 | (60·6%) |
| Year of ART initiation | ||||||||
| 2004–2007 | 17787 | (44·8%) | 38073 | (41·8%) | 34130 | (30·9%) | 25159 | (44·3%) |
| 2008–2010 | 15958 | (40·2%) | 45886 | (50·4%) | 59904 | (54·3%) | 21562 | (38·0%) |
| 2011–2013 | 5940 | (15·0%) | 7121 | (7·8%) | 16262 | (14·7%) | 10043 | (17·7%) |
| WHO stage | ||||||||
| I | 3051 | (8·7%) | 16739 | (20·3%) | 26900 | (25·6%) | 14763 | (32·6%) |
| II | 7408 | (21·1%) | 19312 | (23·4%) | 22744 | (21·6%) | 5731 | (12·7%) |
| III | 20725 | (59·1%) | 38446 | (46·6%) | 47563 | (45·2%) | 18141 | (40·1%) |
| IV | 3892 | (11·1%) | 7917 | (9·6%) | 7986 | (7·6%) | 6598 | (14·6%) |
| Missing | 4609 | (11·6%) | 8666 | (9·5%) | 5103 | (4·6%) | 11531 | (20·3%) |
| CD4 cell count (cells/µL) | ||||||||
| <50 | 3122 | (14·8%) | 11552 | (16·0%) | 13134 | (14·9%) | 8980 | (21·1%) |
| 50–99 | 3469 | (16·4%) | 12145 | (16·8%) | 13938 | (15·8%) | 7613 | (17·9%) |
| 100–249 | 9731 | (46·1%) | 34227 | (47·3%) | 41313 | (46·8%) | 21320 | (50·2%) |
| 250–349 | 3486 | (16·5%) | 10036 | (13·9%) | 12578 | (14·3%) | 3284 | (7·7%) |
| >=350 | 1298 | (6·1%) | 4331 | (6·0%) | 7255 | (8·2%) | 1315 | (3·1%) |
| Median (IQR) | 155 | (80–240) | 149 | (77–226) | 157 | (82–237) | 131 | (60–197) |
| Not measured | 18579 | (46·8%) | 18789 | (20·6%) | 22078 | (20·0%) | 14252 | (25·1%) |
| Viral load (log10 copies/ml) | ||||||||
| Median IQR | 4.9 | (2.6–5.4) | 4.5 | (3–5.6) | 5.1 | (4.2–5.6) | 4.76 | (4.1–5.3) |
| Not measured | 39638 | (99·9%) | 91008 | (99·9%) | 108605 | (98·5%) | 37160 | (65·5%) |
| First-line ART* | ||||||||
| NVP/D4T/3TC | 28614 | (72·1%) | 32479 | (35·7%) | 33984 | (30·8%) | 9805 | (17·3%) |
| EFV/XTC/TDF | 870 | (2·2%) | 17657 | (19·4%) | 25199 | (22·8%) | 12430 | (21·9%) |
| NVP/AZT/3TC | 5608 | (14·1%) | 17875 | (19·6%) | 21353 | (19·4%) | 4006 | (7·1%) |
| EFV/D4T/3TC | 1726 | (4·3%) | 4366 | (4·8%) | 5569 | (5·0%) | 23699 | (41·8%) |
Patients were classified according to the monitoring strategy used in their ART programme during the first year of antiretroviral therapy.
Data are numbers of patients (column %) unless otherwise stated.
IQR: Interquartile range; ART: Antiretroviral therapy; 3TC: lamivudine; D4T: stavudine; NVP: nevirapine; AZT: zidovudine; EFV: efavirenz; XTC: 3TC or FTC (Emtricitabine)
The four most common regimens are shown
During their first year on ART 110,296 patients (37·0%) were under targeted viral load monitoring, 91,080 (30·6%) were under CD4 monitoring, 56,764 (19·1%) were under routine VL monitoring, and 13·3% (39,685) were monitored clinically. The majority of patients (50·8%) started ART in WHO clinical stage 3 or 4. Over a third of patients started a nevirapine/stavudine/lamivudine (NVP/D4T/3TC) first-line regimen. Other common regimens were efavirenz/tenofovir plus lamivudine or emtricitabine (EFV/TDF/XTC), nevirapine/zidovudine/lamivudine (NVP/AZT/3TC) and efavirenz/stavudine/lamivudine (EFV/D4T/3TC) (Table 1). Among patients monitored clinically, baseline CD4 count was missing in almost half of patients, whereas about 20% of counts were missing in other patients. The median CD4 cell count at start of ART was around 150 cells/µl for all monitoring strategies except for routine VL monitoring (131 cells/µl).
Switching to Second-line ART
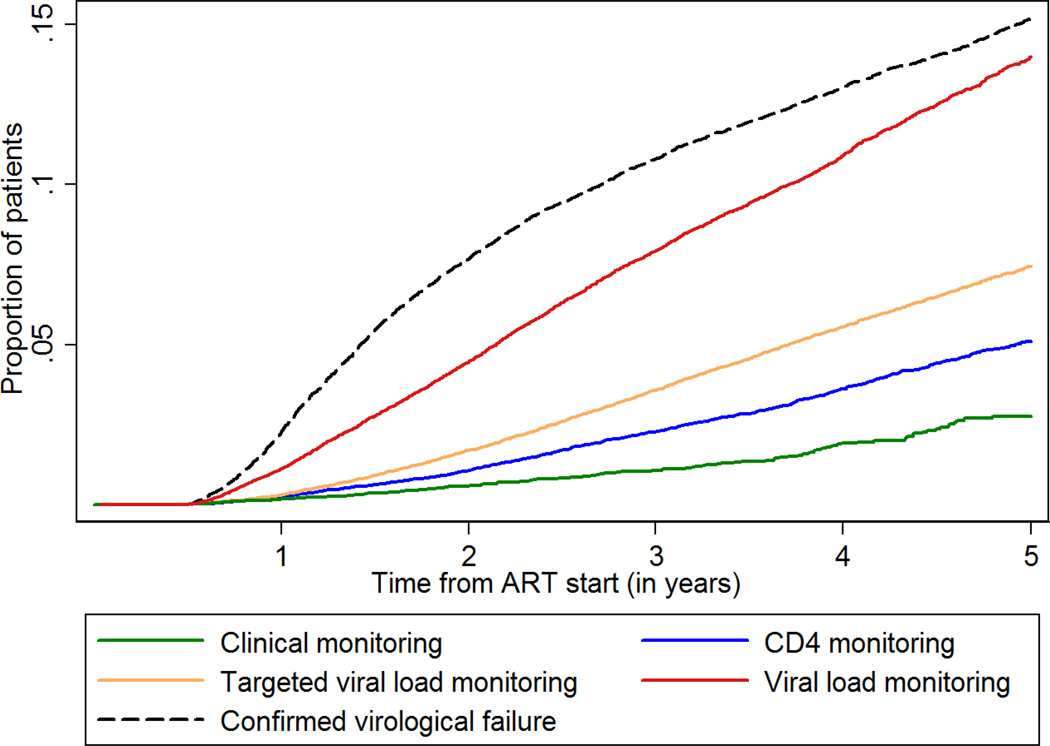
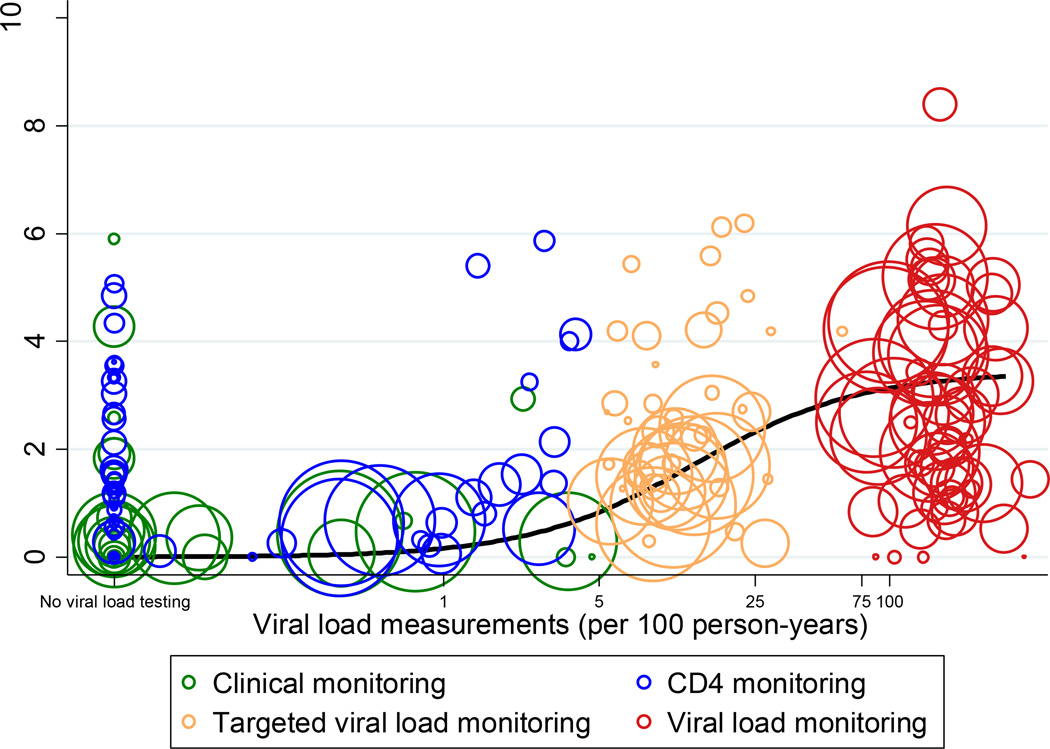
Overall 10,352 patients (3·5%) switched, for a rate of 1·63 per 100 person-years (95% CI 1·60–1·66). The cumulative probability of switching at five years was 7·9% overall, and 2·7% with clinical, 5·1% with immunological, 7·4% with targeted VL and 14·0% with routine VL monitoring (Figure 1). Heterogeneity between clinics and calendar years was large (Figure 2). Switching rates ranged from no switches in some sites and calendar years with clinical or CD4 monitoring to 8 per 100 person-years in one site and year with routine VL monitoring. Targeted VL monitoring typically consisted of 5 to 25 tests per 100 person-years. Switching rates increased with more viral load testing, but plateaued above 75 tests per 100 person-years (Figure 2).
Figure 1. Cumulative probability of confirmed virological failure during routine viral load monitoring and switching to second-line antiretroviral therapy according to monitoring strategy.
Confirmed virological failure was defined as two values above 1,000 copies/ml within one year. Routine VL monitoring was assumed if at least 75 VL tests had been done per 100 person-years. CD4 monitoring was assumed if at least 75 CD4 counts had been done per 100 person-years. Targeted VL monitoring was assumed if in addition to CD4 monitoring at least 5 but less than 75 VL measurements were done per 100 person-years. A site was considered to use clinical monitoring if it did not meet the criteria for any other monitoring strategy.
Figure 2. Viral load testing and switching to second-line antiretroviral therapy.
Bubble plot of rates of viral load testing and switching to second-line ART according to monitoring strategy. Each bubble represents the estimate for one treatment programme and calendar year. The size of the circles is proportional to the number of person-years in the respective year and programme. The black line shows the fit from regression model. Routine VL monitoring was assumed if at least 75 VL tests had been done per 100 person-years. CD4 monitoring was assumed if at least 75 CD4 counts had been done per 100 person-years. Targeted VL monitoring was assumed if in addition to CD4 monitoring at least 5 but less than 75 VL measurements were done per 100 person-years. A site was considered to use clinical monitoring if it did not meet the criteria for any other monitoring strategy.
Compared to sites with CD4 monitoring adjusted hazard ratios for switching to second-line ART were 3·15 (95%-CI 2·92–3·40) for sites with routing VL monitoring, 1·21 (95% CI 1·13–1·30) for sites with targeted VL testing, and 0·49 (0·43–0·56) with immunological monitoring (Table 2). Switching was also more common in urban compared to rural clinics, and in regional and University and district hospitals compared to health centres. Switching rates were higher in men than in women, in younger patients and in patients who started ART with lower CD4 counts compared to older patients and patients with higher counts (Table 2). In the complete case analysis the association with the monitoring strategy was slightly stronger (supplementary Table S2).
Table 2.
Univariable and multivariable analysis of predictors of switching to second-line antiretroviral therapy.
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI) | P | |
| Monitoring Strategy | <0·0001 | <0·0001 | ||
| Clinical | 0·51 (0·45–0·58) | 0·49 (0·43–0·56) | ||
| CD4 cell count | 1·00 | 1·00 | ||
| Targeted viral load | 1·55 (1·46–1·64) | 1·21 (1·13–1·30) | ||
| Routine viral load | 3·29 (3·10–3·49) | 3·15 (2·92–3·40) | ||
| Age at ART start | <0·0001 | <0·0001 | ||
| >30 years | 1·00 | 1·00 | ||
| ≤30 years | 1·30 (1·25–1·36) | 1·30 (1·25–1·36) | ||
| CD4 at ART start | <0·0001 | <0·0001 | ||
| ≥350 | 1·00 | 1·00 | ||
| 250–349 | 0·68 (0·60–0·76) | 0·77 (0·69–0·87) | ||
| 100–249 | 0·75 (0·69–0·82) | 0·78 (0·72–0·85) | ||
| 50–99 | 1·00 (0·92–1·09) | 1·07 (0·98–1·17) | ||
| <50 | 1·57 (1·44–1·72) | 1·55 (1·43–1·69) | ||
| Rural/Urban | <0·0001 | <0·0001 | ||
| Rural | 1·00 | 1·00 | ||
| Urban | 2·89 (2·70–3·10) | 1·50 (1·37–1·64) | ||
| Gender | 0·0126 | 0·0020 | ||
| Female | 1·00 | 1·00 | ||
| Male | 1·05 (1·01–1·09) | 1·07 (1·02–1·11) | ||
| Type of clinic | <0·0001 | <0·0001 | ||
| Health Center | 1·00 | 1·00 | ||
| District Hospital | 1·11 (1·06–1·16) | 1·11 (1·05–1·17) | ||
| Regional/University Hospital | 0·96 (0·92–1·01) | 1·14 (1·08–1·21) | ||
| WHO stage at ART start | <0·0001 | 0·0013 | ||
| 1 | 1·00 | 1·00 | ||
| 2 | 0·74 (0·69–0·79) | 0·98 (0·92–1·05) | ||
| 3 | 0·84 (0·79–0·89) | 1·02 (0·96–1·09) | ||
| 4 | 1·12 (1·04–1·21) | 1·14 (1·06–1·23) | ||
| Year of ART initiation | 0·2233 | |||
| 2004–2007 | 1·00 | <0·0001 | 1·00 | |
| 2008–2010 | 0·90 (0·86–0·94) | 0·96 (0·92–1·01) | ||
| 2011–2013 | 1·09 (0·96–1·22) | 1·02 (0·91–1·15) | ||
Results from Cox models stratified by IeDEA region. The multivariable analysis was adjusted for all variables in the table. Missing CD4 cell counts or WHO clinical stages were imputed.
Routine VL monitoring was assumed if at least 75 VL tests had been done per 100 person-years. CD4 monitoring was assumed if at least 75 CD4 counts had been done per 100 person-years. Targeted VL monitoring was assumed if in addition to CD4 monitoring at least 5 but less than 75 VL measurements were done per 100 person-years. A site was considered to use clinical monitoring if it did not meet the criteria for any other monitoring strategy.
ART: Antiretroviral therapy; CI: Confidence Interval; HR: Hazards Ratio
CD4 cell counts at the time of switching were missing in 4,642 (44·8%) of patients (Table S3). In those with measurements the median (IQR) count was 215 cells/µl (117–335) in routine VL sites, 133 cells/µl (60–236) in targeted VL sites, 125 cells/µl (53–230) at CD4 sites and 114 cells/µl (36–212) in clinical monitoring sites. In sites with targeted VL, CD4 or clinical monitoring, the most common second-line regimen was ritonavir-boosted lopinavir/tenofovir plus emtricitabine or lamivudine (LPVr/TDF/XTC). In sites with routine VL monitoring boosted lopinavir/zidovudine/didanosine (LPVr/AZT/DDI), and more recently, LPVr/XTC/TDF or LPVr/XTC/AZT, were frequently used. Thirty-nine patients switched to a combination of LPV/r and RAL. Common second-line regimens and first- to second-line regimen sequences are shown in supplementary Table S4.
Switching and Failure
The rate of confirmed virological failure in patients under routine VL monitoring was 4·23 per 100 person-years at risk, and the cumulative probability at 5 years was 15·1% (Figure 1). In clinical monitoring sites a few patients had CD4 counts or VL tests, and in CD4 sites few patients were additionally tested for VL (Table 3). Overall, 58·0% of patients with confirmed virological failure, 19·3% of patients with confirmed immunological failure and 16·7% of patients with evidence of virological or immunological failure based on a single (unconfirmed) measurements switched. Switching was more likely in patients with confirmed failure, and less likely with clinical than with laboratory monitoring (Table 3).
Table 3.
Switching among patients with treatment failure under different monitoring strategies.
| Monitoring Strategy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical | CD4 cell count | Targeted viral load | Routine viral load | |||||
| No. of patients with failure |
Percent switched to second-line ART at 2 years (95% CI) |
No. of patients with failure |
Percent switched to second-line ART at 2 years (95% CI) |
No. of patients with failure |
Percent switched to second-line ART at 2 years (95% CI) |
No. of patients with failure |
Percent switched to second-line ART at 2 years (95% CI) |
|
| Virological failure | ||||||||
| Single value | 98 | 32·7% (22·4–46·3%) |
465 | 57·5% (52·2–63·1%) |
6952 | 55·8% (54·3–57·3%) |
11346 | 36·5% (35·3–37·6%) |
| Confirmed | 13 | 0% | 56 | 62·2% (48·7–75·7%) |
1279 | 65·5% (62–68·9%) |
5102 | 56·1% (54·4–57·8%) |
| Immunological failure | ||||||||
| Single value | 1587 | 6·2% (4·9–7·8%) |
14261 | 9·9% (9·3–10·5%) |
27074 | 12·5% (12–13%) |
7694 | 19·5% (18·4–20·7%) |
| Confirmed | 285 | 9·2% (5·9–14%) |
3656 | 17·2% (15·7–18·7%) |
9452 | 19·4% (18·4–20·4%) |
2499 | 23·1% (21·1–25·2%) |
| Any evidence of treatment failure | 1636 | 6·7% (5·4–8·3%) |
14381 | 10·1% (9·5–10·7%) |
29532 | 15% (14·5–15·6%) |
14866 | 27·7% (26·8–28·7%) |
ART: Antiretroviral therapy; CI: Confidence Interval
Estimates of the proportion who switched to second line ART up to 2 years after treatment failure from Kaplan-Meier life-table analyses.
Patients were classified according to the monitoring strategy used in their ART programme at the time of treatment failure. Routine VL monitoring was assumed if at least 75 VL tests had been done per 100 person-years. CD4 monitoring was assumed if at least 75 CD4 counts had been done per 100 person-years. Targeted VL monitoring was assumed if in addition to CD4 monitoring at least 5 but less than 75 VL measurements were done per 100 person-years. A site was considered to use clinical monitoring if it did not meet the criteria for any other monitoring strategy.
Immunologic failure was defined according to the World Health Organization (WHO)16 as CD4 count below 100 cells/ µl after six months of therapy, a return to, or a fall below the pre-therapy CD4 baseline after six months of therapy, or a 50% decline from the on-treatment peak CD4 value. Virological failure was defined as a viral load value >1,000 copies/ml.
Among patients who switched to second-line ART evidence of failure based on single (unconfirmed) measurements before switching was present in 32·1% of patient during clinical monitoring but in 84·3% with targeted VL monitoring. The prevalence of confirmed virological failure was lower: 57.1% with routine VL and 15.2% with targeted viral load monitoring. The prevalence of confirmed immunological failure was 26.1% with routine CD4 monitoring and 34.2% with targeted VL monitoring but only 11.0% with routine VL monitoring (Table 4).
Table 4.
Prevalence of first-line antiretroviral treatment failure among patients who switched to second-line ART under different monitoring strategies.
| Monitoring strategy at time of switching |
No. of patients switching to second-line ART |
No. (%) with confirmed virological failure |
No. (%) with confirmed immunological failure |
No. (%) with single value virological failure |
No. (%) with single value immunological failure |
Any evidence of failure* |
|---|---|---|---|---|---|---|
| Clinical monitoring | 268 | 3 (1·1%) | 22 (8·2%) | 22 (8·2%) | 76 (26·4%) | 86 (32·1%) |
| CD4 monitoring | 1565 | 39 (2·5%) | 409 (26·1%) | 196 (12·5%) | 870 (55·6%) | 910 (58·2%) |
| Targeted VL monitoring | 4452 | 676 (15·2%) | 1522 (34·2%) | 3110 (69·9%) | 2892 (65·0%) | 3754 (84·3%) |
| Routine VL monitoring | 4067 | 2321 (57·1%) | 448 (11·0%) | 3157 (77·6%) | 1143 (28·1%) | 3223 (79·3%) |
| Total | 10352 | 3039 (29·4%) | 2401 (23·2%) | 6485 (62·6%) | 4981 (48·1%) | 7973 (77·0%) |
Data are numbers of patients (%). Routine VL monitoring was assumed if at least 75 VL tests had been done per 100 person-years. CD4 monitoring was assumed if at least 75 CD4 counts had been done per 100 person-years. Targeted VL monitoring was assumed if in addition to CD4 monitoring at least 5 but less than 75 VL measurements were done per 100 person-years. A site was considered to use clinical monitoring if it did not meet the criteria for any other monitoring strategy.
Virological or immunological failure, based on a single value.
ART: Antiretroviral therapy; CI: Confidence Interval.
Discussion
In this cohort study of almost 300,000 HIV-positive patients starting ART in 16 countries in Sub-Saharan Africa, we found that about 1·6 in every 100 patients switched to second-line ART each year, and that overall 7·9% of patients were on second-line ART after 5 years. The rate of switching varied greatly between treatment programmes and monitoring strategies. Compared to CD4 monitoring, switching rates were about 3 times higher under routine VL monitoring, slightly higher in programmes using targeted VL and only about half in programmes using clinical monitoring. As expected, switching under targeted VL monitoring was generally based on a single detectable value, and most patients switched under clinical monitoring did not have laboratory evidence of failure. In the absence of routine VL monitoring switching occurred later, at lower CD4 cell counts. Finally, many patients with confirmed treatment failure were not switched to second-line ART.
In settings where VL testing is not routinely available, the first priority should be to confirm failure in patients in whom treatment failure is suspected based on clinical or immunologic criteria.20 Targeted VL testing of selected patients based on CD4 count criteria is promising in this situation. With targeted VL testing the accuracy of identifying treatment failure is greater than with clinical or CD4 criteria, and compared to routine VL monitoring, the number of patients tested and costs are reduced.21 However, the higher rate of switching under routine VL monitoring observed in our study makes it likely that under targeted VL virological failures were missed. The number of failures missed will have been even greater in patients monitored immunologically or clinically. Of note, among patients with evidence of virological failure, those who had targeted VL testing rather than routine VL testing were more likely to be switched to second-line ART, probably reflecting a high degree of suspicion, coupled with the intention to switch in case of detectable viral load.
The proportion switching among those with confirmed virological failure ranged widely, depending on the monitoring strategy. ART providers may choose not to change regimens in patients with suspected non-adherence. The ANRS 12110 trial showed that in one third of all failing patients, virological failure occurred due to non-adherence and not due to resistance to NRTIs and/or NNRTIs.22 It is likely that in the routine programmes included in our study adherence problems were more common than in clinical trials, which may explain the lower proportion of patients with virological failure switched to second-line ART. The lack of resistance testing is a challenge to optimal patient care, and limited our ability to gauge whether patients with treatment failure needed second-line ART.
The low switching rates among patients with confirmed immunological failure under routine CD4 monitoring suggest that immunological criteria are not trusted by care givers, or too complex to interpret. Clinicians working in resource-limited settings may be reluctant to switch patients to the last available option, particularly if patients have not developed complications. Interestingly, even in the ANRS 12110 trial only 13 (39%) of the 33 patients with virological failure switched to second-line ART.22 Unnecessary switches were also an issue: even under routine VL monitoring only about 60% of patients who were switched had confirmed virological failure.
Previous studies from resource-limited settings reported rates of switching to second-line ART ranging from 0·6 to 3.3 per 100 person-years but the number of patients switching was small, precluding detailed analyses of predictors of switching.23,24,25 A multi-cohort analysis from Mozambique, Malawi and Guinea-Conakry also found that patients with low baseline CD4 and patients who started treatment in earlier calendar years had higher probability of switching.26 The DART trial in Uganda and Zimbabwe compared three-monthly CD4 count monitoring and clinical monitoring and found higher switching rates in the CD4 monitoring arm.27 Similarly, the ANRS 12110 trial in Cameroon reported that patients in whom VL and CD4 cell counts were measured every 6 months had higher rates of switching than patients assigned to clinical monitoring.22 In contrast, a trial from Thailand, which compared CD4 monitoring with VL monitoring, found similar rates of switching.28 Our study extends these findings not only by comparing clinical, immunological and virological monitoring in a very large African multi-cohort study, but also by assessing the importance of different levels of VL testing, including targeted VL testing. We defined a programme’s monitoring strategy based on the number of laboratory tests actually performed, and considered changes in monitoring strategies during follow-up. We included data from 32 different programmes in 16 African countries, from urban and rural health centres, district hospitals, and tertiary care referral hospitals in East, Southern and West Africa. Our results are thus likely to be relevant for many African settings.
We could not identify treatment failure in patients under clinical monitoring since not all sites systematically collect data on opportunistic infections and diagnostic capabilities are limited in many treatment sites. The programmes participating in IeDEA may not be representative of the national ART programme in the different countries. Our study included smaller rural sites, but urban hospitals dominated. Since rates of switching are lower in rural health centres than in urban referral hospitals our study might overestimate switching rates. The CD4 cell count at baseline was missing in half of patients who were monitored clinically, and also missing in some patients monitored with CD4 counts or VL. We did not examine the cost-effectiveness of routine and targeted viral load monitoring compared to other monitoring strategies. In previous studies we found that VL monitoring may be cost-effective, particularly when assuming that it can improve adherence to first-line ART, and that the gap between costs of first-line and second-line ART can be decreased.29,30
In conclusion, in this large study of routine care settings in sub-Saharan Africa we found wide variations in patterns of switching to second-line ART. The observational nature of the routine data analysed, and the lack of data on adherence and drug resistance mean that the true incidence of appropriate and inappropriate switching, and the prevalence of undetected virological failure remains unknown in many patients and treatment programmes. Our results are nevertheless compatible with the notion that expanding access to VL monitoring, as recommended by WHO, will prevent inappropriate switches, enable early failure detection and preserve second-line treatment options in Africa.
Supplementary Material
Panel: Research in Context.
Literature review
We searched PubMed from Jan 1, 2003 to Dec 1, 2014 for studies published in any language that compared switching to second-line ART between different monitoring strategies, using the search strategy “(switch*[tiab] OR second-line[tiab]) AND (viral load monitoring[tiab] OR CD4 monitoring[tiab] OR clinical monitoring[tiab])”. We identified three randomized controlled trials (RCTs), several cohort studies and two relevant systematic reviews. The reviews concluded that while the evidence base was limited, the low rate of switching to second-line ART was of concern, and that laboratory monitoring with VL or CD4 counts might improve clinical outcomes.
Added value of this study
This is the first large-scale cohort analysis from sub-Saharan Africa to examine rates of switching to second-line ART, switching without evidence of treatment failure, and failure not followed by switching, in patients monitored with routine or targeted VL measurements, CD4 cell counts or clinical criteria. Overall 8% of patients were on second-line ART after 5 years. The rate of switching varied greatly between treatment programmes and monitoring strategies. Compared to CD4 monitoring, switching rates were about 3 times higher under routine VL monitoring, slightly higher in programmes using targeted VL and only about half in programmes using clinical monitoring. The proportion switching among those with evidence of treatment failure ranged from 32% with clinical to 84% with virological monitoring. Median CD4 counts at switching were higher with routine VL monitoring than with other monitoring.
Implications of all the available evidence
In the absence of regular VL monitoring the true incidence of appropriate and inappropriate switching, and the prevalence of undetected virological failure remains unknown in many patients and treatment programmes. Taken together the data from the present study and previous RCTs and observational studies support the notion that expanding access to VL monitoring, as recommended by WHO, and the judicious use of targeted VL monitoring, will prevent inappropriate switches, enable early failure detection and preserve second-line treatment options in Africa. Pragmatic RCTs with long follow up are needed to confirm or exclude a benefit of VL monitoring on clinical outcomes.
Acknowledgements
We thank all patients who contributed data to this study.
This work was supported by the National Institute of Allergy and Infectious Diseases (5U01-AI069924, U01AI069911, U01AI069919). SJR was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases and OK by the Swiss National Science Foundation (Ambizione/Prosper grant 32333B_131629).
Appendix
The members of the IeDEA regional study groups are as follows:
IeDEA Southern Africa
Participating sites: Frank Tanser, Africa Centre for Health and Population Studies, University of Kwazulu-Natal, Somkhele, South Africa; Christopher Hoffmann, Aurum Institute for Health Research, Johannesburg, South Africa; Benjamin Chi, Centre for Infectious Disease Research in Zambia, Lusaka, Zambia; Denise Naniche, Centro de Investigação em Saúde de Manhiça, Manhiça, Mozambique; Robin Wood, Desmond Tutu HIV Centre (Gugulethu and Masiphumelele clinics), Cape Town, South Africa; Kathryn Stinson, Khayelitsha ART Programme and Médecins Sans Frontières, Cape Town, South Africa; Geoffrey Fatti, Kheth’Impilo Programme, South Africa; Sam Phiri, Lighthouse Trust Clinic, Lilongwe, Malawi; Janet Giddy, McCord Hospital, Durban, South Africa; Cleophas Chimbetete, Newlands Clinic, Harare, Zimbabwe; Kennedy Malisita, Queen Elizabeth Hospital, Blantyre, Malawi; Brian Eley, Red Cross War Memorial Children’s Hospital and Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa; Michael Hobbins, SolidarMed SMART Programme, Pemba Region, Mozambique; Kamelia Kamenova, SolidarMed SMART Programme, Masvingo, Zimbabwe; Olatunbosun Faturiyele, SolidarMed SMART Programme, Maseru, Lesotho; Matthew Fox, Themba Lethu Clinic, Johannesburg, South Africa; Hans Prozesky, Tygerberg Academic Hospital, Stellenbosch, South Africa; Karl Technau, Empilweni Clinic, Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa; Shobna Sawry, Harriet Shezi Children’s Clinic, Chris Hani Baragwanath Hospital, Soweto, South Africa.
Regional Data Centres: Matthias Egger (Principal Investigator), Julia Bohlius, Nello Blaser, Janne Estill, Olivia Keiser, Gilles Wandeler, Luisa Salazar-Vizcaya, Andreas Haas, Marie Ballif, Eliane Rohner, Natascha Wyss, Zofia Baranczuk, Kelly Goodwin, Cam Ha Dao Ostinelli, Institute of Social and Preventative Medicine, University of Bern, Switzerland; Mary-Ann Davies (Principal Investigator), Andrew Boulle, Lucy Campbell, Morna Cornell, Leigh Johnson, Nicola Maxwell, Landon Myer, Michael Schomaker, Mireille Porter, Centre for Infectious Disease Epidemiology and Research, University of Cape Town, South Africa.
IeDEA East Africa
Participating sites: Samuel Ayaya, Lameck Diero, Edwin Sang, Elyne Rotich, AMPATH, Eldoret, Kenya; Elizabeth Bukusi, Medical Research Institute, Kisumu, Kenya; Geoffrey R. Somi, National AIDS Control Program, Dar es Salaam, Tanzania; Rita Lyamuya, Morogoro Regional Hospital, Morogoro, Tanzania; Edward Lugina, Ocean Road Cancer Institute, Dar es Salaam, Tanzania; Mark Urassa, Denna Michael Mkwasa, Kapella Ngonyani, National Institute for Medical Research, Dar es Salaam, Tanzania; Andrew Kambugu, Tumbi Regional Hospital, Kibaha, Tanzania; Philippa Easterbrook, Marion Achieng-Kariuki, Infectious Diseases Institute, Kampala, Uganda; Fred Nalugoda, Rakai Health Sciences Program, Kalisizo, Uganda; John Ssali, Masaka Regional Referral Hospital, Masaka, Uganda; Mwebesa Bosco Bwana, Winnie Muyindike, Mbarara University of Science and Technology, Mbarara, Uganda.
Regional Data Centre: Constantin T. Yiannoutsos (Principle Investigator), Beverly S. Musick, Yee Yee H. Kuhn, Indiana University, Indianapolis, Indiana, United States of America.
IeDEA West Africa
Participating sites: Djimon Marcel Zannou, CNHU Hubert Maga, Cotonou, Benin; Joseph Drabo, CHU Yalgado, Ouagadougou, Burkina Faso; Adrien Bruno Sawadogo, CHU Souro Sanou, Bobo Dioulasso, Burkina Faso; Eugène Messou, ACONDA-CePReF, Abidjan, Cote d’Ivoire; Clarisse Amani Bosse, ACONDA-MTCT-Plus, Abidjan, Cote d’Ivoire; Henri Chenal, CIRBA, Abidjan, Cote d’Ivoire; Albert Minga, CMSDS/CNTS, Abidjan, Cote d’Ivoire; Aristophane Koffi Tanon, SMIT, CHU de Treichville, Abidjan, Cote d’Ivoire, Serge Olivier Koule, USAC, CHU de Treichville, Abidjan, Cote d’Ivoire; Christian Wejse, Bandim Health Project, Bissau, Guinea-Bissau; David Leuenberger, Jean Hebelamou, Centre Medical Macenta, Macenta, Guinea; Moussa Y Maïga, CH Gabriel Toure, Bamako, Mali; Hamar Alassane Traore, Daouda Minta, CH Point G, Bamako, Mali; Vivian Kwaghe, UATH, Abuja, Nigeria; Festus Igbinoba, National Hospital Abuja, Nigeria; Okwara Benson, Clément Adebamowo, UBTH, Benin City, Nigeria; Moussa Seydi, SMIT, CHU Fann, Dakar, Senegal; Akessiwe Patassi, CHU Tokoin/Sylvanus Olympio, Lome, Togo.
Regional Data Centres: François Dabis (Principal Investigator), Elise Arrivé, Nathalie de Rekeneire, Antoine Jaquet, Valériane Leroy, Charlotte Lewden & Annie Sasco, Bordeaux, France; Emmanuel Bissagnene (Co-Principal Investigator), Patrick Coffie & Didier Ekouevi (Abidjan, Cote d’Ivoire); Man Charurat, UMB/IHV, Abuja, Nigeria.
Footnotes
Contributors
ME obtained funding for the project and wrote the first draft of the study protocol. All authors contributed to the final version of the protocol. ADH performed statistical analyses, with interpretation of results by ME, OK, GW and ADH. ADH wrote the first draft of the paper, which was revised by ME. All authors commented on earlier drafts of the paper. EB, SB and ADH were responsible for data management at the regional IeDEA data centres. All investigators assisted in implementation, fieldwork, or data collection at study sites. All authors reviewed and approved the final version for submission.
Declaration of interests
We declare that we have no conflicts of interest.
References
- 1.World Health Organization. HIV/AIDS. Data and statistics. [accessed April 8, 2015];2015 http://www.who.int/hiv/data/en/
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90. An ambitious treatment target to help end the AIDS epidemic. Geneva: 2014. [accessed April 5, 2015]. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [Google Scholar]
- 3.Harries AD, Zachariah R, van Oosterhout JJ, et al. Diagnosis and management of antiretroviral-therapy failure in resource-limited settings in sub-Saharan Africa: challenges and perspectives. Lancet Infect Dis. 2010;10:60–65. doi: 10.1016/S1473-3099(09)70321-4. [DOI] [PubMed] [Google Scholar]
- 4.Fox MP, Cutsem G Van, Giddy J, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr. 2012;60:428–437. doi: 10.1097/QAI.0b013e3182557785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keiser O, Tweya H, Braitstein P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010;15:251–258. doi: 10.1111/j.1365-3156.2009.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antiretroviral (ARV) Ceiling Price List. New York, N.Y.: 2013. [accessed July 29, 2014]. http://www.clintonhealthaccess.org/news-and-information/ARV-Ceiling-Price-List-May-2013. [Google Scholar]
- 7.Keiser O, Orrell C, Egger M, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoSMed. 2008;5:e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Geneva: World Health Organization; 2010. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. [PubMed] [Google Scholar]
- 9.Keiser O, MacPhail P, Boulle A, et al. Accuracy of WHO CD4 cell count criteria for virological failure of antiretroviral therapy. Trop Med Int Heal. 2009;14:1220–1225. doi: 10.1111/j.1365-3156.2009.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. AIDS. 2008;22:1971–1977. doi: 10.1097/QAD.0b013e32830e4cd8. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds SJ, Nakigozi G, Newell K, et al. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS. 2009;23:697–700. doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigaloff KC, Hamers RL, Wallis CL, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58:23–31. doi: 10.1097/QAI.0b013e318227fc34. [DOI] [PubMed] [Google Scholar]
- 13.Keiser O, Tweya H, Boulle A, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23:1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–1264. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Health D of. The South African Antiretroviral Treatment Guidelines 2013. [accessed Nov 19, 2014];Pretoria. 2013 http://www.sahivsoc.org/upload/documents/2013 ART Treatment Guidelines Final 25 March 2013 corrected.pdf. [Google Scholar]
- 16.World Health O. Antiretroviral therapy for HIV Infection in adults and adolescents in resource-limited settings: towards universal access. Recommendations for a public health approach. 2006 http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf.
- 17.World Health Organization. Geneva: World Health Organization; 2007. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. [Google Scholar]
- 18.Gutierrez RG. Parametric frailty and shared frailty survival models. Stata J. 2002;2:22–44. [Google Scholar]
- 19.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3:35–44. doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Recommendations for a public health approach. Geneva: World Health Organization; 2014. March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [PubMed] [Google Scholar]
- 21.Lynen L, An S, Koole O, et al. An algorithm to optimize viral load testing in HIV-positive patients with suspected first-line antiretroviral therapy failure in Cambodia. J Acquir Immune Defic Syndr. 2009;52:40–48. doi: 10.1097/QAI.0b013e3181af6705. [DOI] [PubMed] [Google Scholar]
- 22.Laurent C, Kouanfack C, Laborde-Balen G, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. Lancet Infect Dis. 2011;11:825–833. doi: 10.1016/S1473-3099(11)70168-2. [DOI] [PubMed] [Google Scholar]
- 23.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. AntivirTher. 2007;12:83–88. [PubMed] [Google Scholar]
- 24.Landier J, Akonde A, Pizzocolo C, et al. Switch to second-line ART in West African routine care: incidence and reasons for switching. AIDS Care. 2011;23:75–78. doi: 10.1080/09540121.2010.498867. [DOI] [PubMed] [Google Scholar]
- 25.Auld AF, Mbofana F, Shiraishi RW, et al. Four-year treatment outcomes of adult patients enrolled in Mozambique’s rapidly expanding antiretroviral therapy program. PLoS One. 2011;6:e18453. doi: 10.1371/journal.pone.0018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palombi L, Marazzi MC, Guidotti G, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral-treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis An Off Publ Infect Dis Soc Am. 2009;48:115–122. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 27.Mugyenyi P, Walker AS, Hakim J, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375:123–131. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jourdain G, Le Cœur S, Ngo-Giang-Huong N, et al. Switching HIV treatment in adults based on CD4 count versus viral load monitoring: a randomized, non-inferiority trial in Thailand. PLoS Med. 2013;10:e1001494. doi: 10.1371/journal.pmed.1001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estill J, Salazar-Vizcaya L, Blaser N, Egger M, Keiser O. The Cost-Effectiveness of Monitoring Strategies for Antiretroviral Therapy of HIV Infected Patients in Resource-Limited Settings: Software Tool. PLoS One. 2015;10:e0119299. doi: 10.1371/journal.pone.0119299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estill J, Egger M, Blaser N, et al. Cost-effectiveness of point-of-care viral load monitoring of ART in resource-limited settings: Mathematical modelling study. AIDS. 2013;27:1483–1492. doi: 10.1097/QAD.0b013e328360a4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.