Abstract
Objectives
Quadriceps weakness persists after anterior cruciate ligament reconstruction. Muscle atrophy and activation failure may contribute. This study examined the roles of atrophy and activation failure in quadriceps weakness after anterior cruciate ligament reconstruction.
Design
Case series.
Methods
Twenty patients six months post-anterior cruciate ligament reconstruction participated. Atrophy was determined as peak quadriceps cross sectional area from magnetic resonance images. Quadriceps activation was quantified via the central activation ratio, while muscle strength was measured isometrically. All testing was performed bilaterally. Hierarchical linear regression and one-way ANOVAs were performed to examine the relation of muscle strength with activation and atrophy.
Results
Cross sectional area (R2=0.307; p=0.011) explained more of the variance in quadriceps strength than central activation ratio (R2<0.001; p=0.987). Strength and cross sectional area were lower in the injured (strength: 2.03±0.51Nm/kg; cross sectional area: 68.81±17.80cm2) versus uninjured limb (strength: 2.89±0.81Nm/kg; cross sectional area: 81.10±21.58cm2; p<0.001). There were no side-to-side differences in central activation ratio; however, quadriceps activation failure was present bilaterally (injured: 0.87±0.12; uninjured: 0.85±0.14; p=0.571).
Conclusions
Quadriceps cross sectional area was strongly related to muscle strength six months after anterior cruciate ligament reconstruction and substantial injured versus uninjured limb deficits were demonstrated for strength and cross sectional area. Patients may benefit from exercises aimed at improving quadriceps cross sectional area post-operatively.
Keywords: anterior cruciate ligament, muscle, strength, MRI, central activation ratio
Introduction
Quadriceps weakness is nearly ubiquitous following anterior cruciate ligament (ACL) injury and reconstruction (ACLr).1 Strength deficits upwards of 30% in the reconstructed compared to the contralateral limb have been reported six months post-operatively,2 a time when patients often return to full activity. The presence of quadriceps weakness may be hazardous to the patient. The quadriceps are important to lower limb control during dynamic activity and quadriceps weakness could alter movement strategies potentiating re-injury.3 To optimally prepare patients to return to full activity, complete quadriceps function must be restored. However, before quadriceps strength deficits can be effectively countered, a deeper understanding of why quadriceps weakness persists throughout rehabilitation is needed.
Quadriceps disuse atrophy occurs following knee joint immobilization and may contribute to quadriceps weakness after ACL injury and reconstruction.4 Konishi and colleagues5 previously reported an approximately 7% deficit in total quadriceps volume in the reconstructed versus contralateral limb in patients 6-12 months following ACL reconstruction. Deficits of 3% in volume6 and cross sectional area7 (CSA) remained 12-18 months post-operatively. Similar magnitudes of quadriceps atrophy were reported by Lorentzon et al.8 in people with ACL deficiency, though no relation between atrophy and strength was identified. The authors concluded that muscle atrophy alone did not cause quadriceps weakness suggesting, instead, that incomplete volitional muscle activation may contribute.8
Quadriceps activation failure (QAF) is a common consequence of ACL injury and reconstruction. QAF is often associated with joint damage, effusion, and pain.4 These factors alter the afferent signal sent to the central nervous system, which leads to an inhibitory signal transmitted to the quadriceps α-motoneuron pool and a decrease in voluntary muscle activity.4 Previous reports of QAF following ACLr suggest deficits upwards of 15% present two years post-operatively.1 In patients undergoing total knee arthroplasty, a population with similar magnitude quadriceps dysfunction to those following ACL injury9, QAF accounted for nearly twice the quadriceps strength deficit as muscle atrophy in the acute postoperative period.9 Elucidating how quadriceps muscle atrophy and QAF contribute to lingering weakness when patients return to full activity seems imperative. Thus, the purpose of this study was to determine if quadriceps atrophy and QAF contribute to persistent knee extension strength deficits in patients when they were cleared to return to full activity after ACL reconstruction. We hypothesized that persistent quadriceps weakness would result from a combination of QAF (measured by central activation ratio [CAR]) and muscle atrophy (measured through cross sectional area [CSA]) and that QAF would more strongly predict quadriceps weakness than would muscle atrophy. We further hypothesized that greater quadriceps weakness, QAF, and CSA would be present in the injured compared with the uninjured limb.
Methods
Twenty-two patients were recruited for participation; one was excluded after secondary screening revealed she did not fulfill all of the inclusion criteria. Another individual reported for magnetic resonance imaging (MRI) testing but failed to report for CAR assessment. He could not be reached for follow-up and was excluded from analysis, leaving 20 patients (7 males, 13 females; age: 20.65±5.17years; height: 1.72±0.08m; mass: 74.47±14.49kg) who underwent patellar tendon autograft ACL reconstruction. Patients received physician clearance for return to full activity prior to enrollment.
Patients reported for testing on two occasions (212.89±31.62 days post-ACL reconstruction), with quadriceps muscle atrophy, in the form of muscle CSA measured at one session and strength and CAR measured at the other (12.89±16.63 days between testing sessions). All measures were assessed bilaterally.
Potential patients were excluded if they had: 1) a history of lower extremity surgery other than their recent ACLr; 2) current pain in either knee; 3) a partial or complete meniscectomy with their ACLr; 4) other ligamentous damage concurrent with their ACL injury; or 5) a known heart condition. Pregnant females were also excluded. This study was approved by the medical school institutional review board. Patients rated their knee symptoms and function using the 2000 International Knee Documentation Committee (IKDC) subjective form. IKDC scores can range from 0-100, with lower scores indicating worse symptoms and functional impairments. The Tegner scale was utilized to determine physical activity levels. The IKDC and Tegner scales were completed when patients reported for strength and QAF testing.
To assess quadriceps muscle atrophy, patients lay supine in a MRI scanner (Philips Achieva 3T Quasar Dual, Philips Electronics, Andover, MA, USA) for simultaneous, bilateral thigh scans. The proton density-weighted images had the following parameters: repetition time 200-3000ms, echo time 35ms, slice thickness 6mm, gap between slices 6mm, matrix 364×180, and field of view 480×281mm.
Peak CSA for each of the quadriceps muscles, and total quadriceps peak CSA, was evaluated. The contours of each muscle were traced in every axial image in which the muscle appeared using ImageJ software (version 142q, National Institutes of Health, Bethesda, MD, USA) and an Intuos4 pen tablet (Wacom Technology Corporation, Vancouver, WA, USA). The sum of each muscle's CSA yielded the total CSA for each slice. The slice with the greatest total CSA was used for statistical analysis.9 All atrophy measurements were performed by a single investigator with high intrarater reliability (intraclass correlation coefficient of 0.988).
Joint effusion was measured while patients lay supine and the superior pole of the patella was located by palpation. A mark was placed 1cm proximal to the superior pole and the circumference of the knee at this location was obtained with a cloth tape measure.10
Quadriceps strength was assessed using knee extension maximal voluntary isometric contractions (MVICs) while patients were seated on an isokinetic dynamometer (Biodex System 3, Biodex Medical Systems, Shirley, NY, USA) with the hip flexed to 85° and the knee flexed to 90°. For MVIC testing, patients were instructed to kick out as hard as they could while watching a computer monitor running a custom-written Labview (version 8.5, National Instruments, Austin, TX, USA) program that displayed their real-time torque output. After completion of the first MVIC, the program displayed a solid line reflecting the patient's peak torque value from the initial trial and a dashed line set 10% above the peak torque recorded during the initial trial (Figure 1). For subsequent trials, patients were encouraged to reach the target torque value (dashed line). If patients increased their torque during any ensuing trial, the height of the solid and dashed lines was adjusted accordingly. A minimum of three knee extension MVICs were performed, with at least 2 minutes of rest in between repetitions, until no improvements in torque were observed by an investigator. Once peak torque was reached, the highest torque value from all recorded repetitions was noted and used as a threshold for QAF assessment.
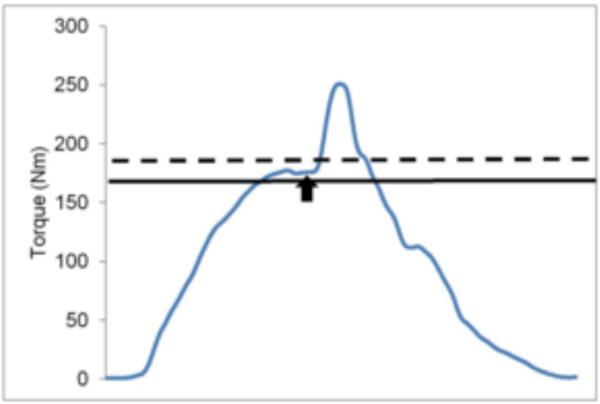
Figure 1.
Screenshot from strength and QAF testing. The blue line represents the patient's real-time torque output. The solid black line corresponds to the patient's peak value from the MVIC trials and also serves as a threshold for QAF testing. Real-time torque output must cross threshold for the electrical stimulus to be delivered. The dotted line represents the patient's target value, which was set 10% above maximal strength. The black arrow corresponds to delivery of the electrical stimulus. QAF= central activation failure. MVIC= maximal voluntary isometric contraction.
For QAF testing, self-adhesive, stimulating electrodes (Dura-Stick II [5×9cm] Chattanooga Group, Hixson, TN, USA) were applied proximally over the rectus femoris and distally over the vastus medialis. At the beginning of QAF testing, the peak torque value from the MVIC trials was input into the custom-written program. The program utilized this threshold (peak torque) value to determine whether or not to trigger the electrical stimulator (S88 and SIU8T, GRASS Technologies, West Warwick, RI, USA) to deliver a stimulus (100ms-long train, 600μs pulse duration, 100pps deliver rate, 130V maximal voltage). Similar to MVIC testing, patients were instructed to generate enough torque to reach the dashed target line displayed on the screen. The program delivered the stimulus once a patient's torque value reached threshold and then fell 1 Nm below their peak torque for the current trial. If a patient failed to reach the solid threshold line, the program would not deliver the stimuli and the patient would be given two minutes of rest before the trial was repeated. The dashed target line was set so as to be unreachable for the patient; however, if a patient did reach the target value, the maximal strength value (solid threshold line) was reset and QAF testing was reinitiated. Three repetitions of QAF testing were performed with 2 minutes of rest between repetitions.
Maximal strength was determined from these three repetitions. The largest of the peak torque values (Nm) generated immediately prior to delivery of the electrical stimulus was utilized to quantify maximal strength (Nm). From this same trial yielding maximal strength, QAF was quantified via the central activation ratio (CAR). To determine the CAR, the patient's peak torque generated immediately prior to delivery of the electrical stimulus was divided by the peak torque generated as a result of the electrical stimulus.9
A limb symmetry index (LSI) was calculated for strength, CAR, and CSA as operative limb/non-operative limb. Hierarchical linear regression analysis examined the association between quadriceps CAR and CSA with knee extension MVIC in the ACLr limb. Collinearity of CAR and CSA was evaluated using collinearity statistics in SPSS. One-way ANOVAs determined if quadriceps CAR, CSA, MVIC, and effusion differed between limbs. The alpha level was set a priori at p≤0.05. Statistical analyses were performed using IBM SPSS Statistics 19 (IBM, Armonk, NY, USA).
Results
The IKDC scores were 75.99±15.86. Tegner scores were 5.90±2.10. Peak quadriceps strength differed between limbs, with the injured (148.39±37.91Nm) being significantly weaker than the uninjured limb (212.98±62.57Nm; p<0.001; LSI 0.70). Similarly, the injured limb (68.81±17.80cm2) had a smaller CSA than the uninjured limb (81.10±21.58cm2; p<0.001; LSI 0.85; Figure 3). Quadriceps CAR did not differ between the injured (0.87±0.12) and uninjured limbs (0.85±0.14; p=0.571; LSI 1.02). Joint effusion was significantly greater in the injured (42.83±7.16cm) compared to the uninjured limb (40.19±3.59cm; p=0.041).
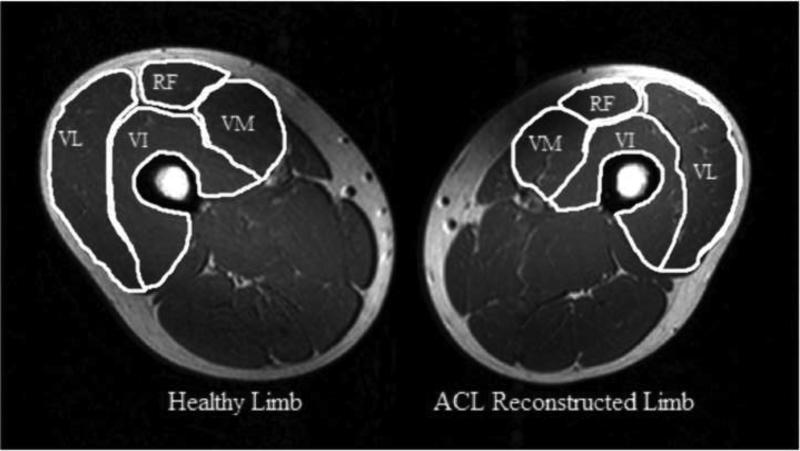
Figure 3.
Representative magnetic resonance image demonstrating quadriceps atrophy. Images were obtained from proton density-weighted images with the following parameters: repetition time 200-3000ms, echo time 35ms, slice thickness 6mm, gap between slices 6mm, matrix 364×180, and field of view 480×281mm. VL= vastus lateralis. RF= rectus femoris. VM= vastus medialis. VI= vastus intermedius.
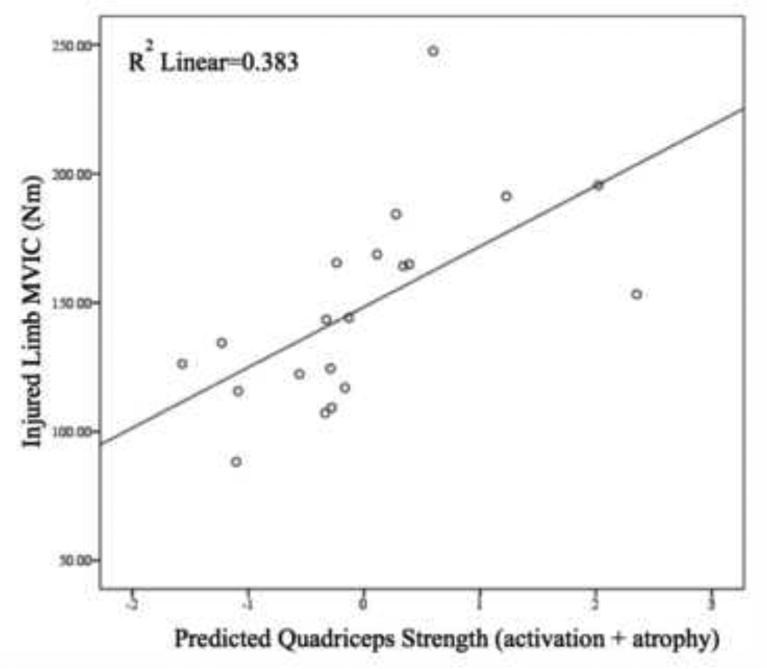
When CAR and CSA were entered in to the statistical model together, regression analyses demonstrated a significant relation between these variables and the peak MVIC following ACL reconstruction (R2=0.383; p=0.016; Figure 2). The variance in quadriceps strength was significantly accounted for by muscle CSA (R2=0.307; p=0.011). The CAR did not significantly contribute to the variance in quadriceps strength (R2<0.001; p=0.987). CAR and CSA were not collinear.
Figure 2.
Results of hierarchical linear regression analysis. MVIC= maximal voluntary isometric contraction.
Discussion
Quadriceps weakness presents frequently after ACL injury and reconstruction. While the precise cause of quadriceps weakness is unknown, peripheral and central mechanisms have been implicated. The present study sought to determine the contributions of muscle CSA and CAR to lingering quadriceps strength deficits following ACL reconstruction. When examined together, quadriceps CAR and CSA were associated with knee extension MVIC following ACLr. Previous studies9,11 demonstrated quadriceps muscle CSA and CAR contributed to strength loss in non-copers following ACL injury (2 months post-injury) and in patients 1 month after total knee arthroplasty, respectively. Emphasizing restoration of muscle CSA and voluntary activation early after injury/surgery seems necessary for strength gains to occur.
CAR did not significantly explain the variance in knee extension strength. Recent evidence suggests the relation between atrophy and CAR and muscle strength may be mediated by time since injury/surgery. Previous work12,13 examined muscle CSA and CAR in relation to quadriceps strength following total knee arthroplasty. As patients reached and surpassed the one-year post-operative time point, quadriceps MVIC became less associated with CAR and more associated with muscle CSA. Similarly, Krishnan and Williams14 recently demonstrated that chronic quadriceps strength deficits in patients 2-15 years following ACL reconstruction are not significantly associated with muscle activation. Thus, it seems possible that while CAR is significantly associated with quadriceps strength early after ACL injury or surgery (i.e., 1-3 months), the same may not be true when patients are returning to activity (i.e., 6 months post-operatively). This finding is likely due to improvements in quadriceps activation over time, while deficits in muscle atrophy linger long-term.12
Muscle atrophy, as measured through quadriceps CSA, was significantly related to knee extension strength in our patients an average of 7 months after ACL reconstruction (212.89±31.62 days postoperatively). It has been suggested that voluntary muscle activation is important in explaining quadriceps strength deficits after ACL reconstruction due to altered afferent transmission to supraspinal structures and reduced spinal reflexive excitability;8 however, the present results suggest that muscle atrophy may be more important than previously believed. While it has been suggested that activation may need to be improved before hypertrophy can take place,13 it seems that greater emphasis needs to be placed on achieving muscle hypertrophy throughout rehabilitation in order to restore muscle CSA and alleviate quadriceps dysfunction prior to athletes returning to activity.
Together, CAR and CSA explained 38% of the variance in knee extension strength in our patients, raising the question of what explains the other 62%. One possible contributing factor is muscle morphology. If patients demonstrated selective Type II fiber atrophy, greater strength deficits could have resulted than in the presence of selective Type I fiber atrophy. Previous investigations of muscle morphology following ACL injury have demonstrated that selective Type I15 or Type II16 atrophy may occur. Additional studies, however, demonstrated that both fiber types atrophy.8 Further research to clarify the influence of ACL injury on muscle morphology and muscle morphology's role in quadriceps strength deficits seems necessary. Muscle architecture may also influence quadriceps strength deficits. Fiber pennation angle may influence muscle strength and this angle may be influenced by training or detraining. In fact, strength training sufficient to increase muscle CSA increases fiber pennation angle such that the force generating capacity of the muscle improves.17 However, detraining has the opposite effect.18 We did not assess muscle morphology or architecture and, thus, cannot determine if these influenced the present outcomes. Additional factors contributing to quadriceps strength deficits may include patellar tendon stiffness; however, recent research19 suggests that patellar tendon stiffness may not be directly related to quadriceps strength.
Knee joint effusion and pain may additionally contribute to the variance in quadriceps strength. Previous investigators have demonstrated that 60mL of saline is capable of inducing a 13% reduction in quadriceps strength.20 Though we did not measure the volume of effusion, our patients did present with side-to-side differences in knee joint effusion. Pain may also be related to strength deficits,20 though pain levels reported by our patients were low-to-moderate (median 1.5, range 0-6, from IKDC questionnaire). Thus, it seems unlikely that knee pain contributed substantially to our results.
The injured limb peak quadriceps CSA was smaller than that in the uninjured limb. Compared to previous reports of healthy adults, our patients demonstrated smaller injured and uninjured limb quadriceps CSA21 suggesting a bilateral deficit that is not sufficiently countered through rehabilitation. Further, when compared to patients three months after ACL injury,11 our patients demonstrate greater quadriceps atrophy. It is likely that ACLr results in additional atrophy compared to that generated by the injury itself, thus resulting in greater magnitudes of quadriceps atrophy in patients undergoing reconstruction than in ACL-deficient patients. Greater post-operative atrophy has been demonstrated in the hamstrings musculature following ACL injury and subsequent semitendinosus/gracilis autograft reconstruction22 and in the quadriceps following total knee arthroplasty.9 Similar outcomes could be expected in the quadriceps following patellar tendon autograft ACLr, though longitudinal studies are needed to confirm this hypothesis.
Possibly contributing to the quadriceps strength deficits observed presently is that all of our patients underwent ACLr using a patellar tendon autograft. Incomplete healing of the patellar tendon is present six months post-operatively, as indicated by increased tendon thickness and width23 and a visually present tendon defect on MRI.24 Yet, whether or not this influences strength is inconclusive. Shelbourne and colleagues demonstrated that pre-operative patellar tendon width may influence the recovery of postoperative quadriceps strength early after surgery.25 However, by six months post-operatively, patellar tendon width no longer influences isokinetic quadriceps strength.26 Future studies may benefit from incorporation of patellar tendon CSA to clarify the relation between patellar tendon width and quadriceps strength.
Substantial quadriceps strength deficits were present in our patients. The average injured limb quadriceps strength was approximately 70% of that in the uninjured limb, which is consistent with previous reports six months post-operatively.27 This large of a strength deficit, however, may be confounded by the presence of bilateral QAF, which may lead to an underestimation of weakness in the injured limb. All patients completed standard outpatient rehabilitation at a single orthopedic clinic, emphasizing restoration of quadriceps strength beginning during the first post-operative week and lasting for approximately four months post-operatively. Strength training consisted of open and closed kinetic chain exercises. It seems based on these results that traditional rehabilitation does not sufficiently restore quadriceps strength by the time patients are released to full activity. Quadriceps muscle contraction is vital to execution of various dynamic movement strategies. Further, the quadriceps are important energy absorbers during weight bearing, dysfunction of which has potential implications for joint degeneration. Thus, more complete restoration of quadriceps strength following ACL reconstruction seems imperative.
Substantial deficits in quadriceps central activation were present bilaterally in our patients. In fact, our patients demonstrated deficits of 13% in the injured and 15% in the uninjured limb, which is well below the 5% or smaller deficit that is considered healthy.28 The magnitude of QAF in those with ACL injury varies, with some studies reporting greater29 and others reporting smaller11 levels of QAF than those demonstrated presently. Bilateral QAF has also been observed following ACL reconstruction, with Urbach and colleagues reporting 15% and 16% quadriceps central activation deficits in the reconstructed and contralateral limbs, respectively, upwards of two years post-operatively.1 Together, these results suggest that central drive to the quadriceps is impaired bilaterally and that the impairment lingers six months after ACLr.
This study is not without limitations. The burst superimposition technique allows for an estimation of maximal torque production and voluntary activation without regard to descending drive to the motoneuron pool. What actually contributes to the voluntary activation assessed with this measurement technique is unknown.30 It is possible that had we utilized a different assessment technique, such as the Hoffman reflex or V-wave, our results could have been different. Future studies may benefit from incorporation of these measurement techniques so a more complete understanding of central and descending drive to the motoneuron pool can be achieved.
Conclusion
CSA is related to muscle strength at return to full activity following ACLr. Substantial side-to-side deficits were demonstrated for strength and CSA, while bilateral deficits in QAF were present. The presence of these deficits suggests that current post-operative rehabilitation efforts may insufficiently restore muscle size, activation, and strength. Continued emphasis needs to be placed on establishing the optimal methods by which to remove atrophy and QAF during post-operative rehabilitation.
Practical Implications.
Patients demonstrate side-to-side strength deficits and bilateral QAF when they return to full activity following ACLr and post-operative rehabilitation.
Rehabilitation following ACLr should emphasize improving quadriceps voluntary activation bilaterally.
Continued emphasis on improving quadriceps strength after surgery is necessary.
Acknowledgements
The authors would like to thank Mark Villwock, MS and Lindsey Lepley, PhD, ATC for their assistance with patient recruitment and data collection. The project described was supported by Grant Number 1 K08 AR05315201A2 from NIAMS/NIH and a doctoral dissertation grant from the National Athletic Trainers’ Association Research and Education Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urbach D, Nebelung W, Weiler HT, et al. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc. 1999 Dec;31(12):1691–1696. doi: 10.1097/00005768-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Bryant AL, Kelly J, Hohmann E. Neuromuscular adaptations and correlates of knee functionality following ACL reconstruction. J Orthop Res. 2008 Jan;26(1):126–135. doi: 10.1002/jor.20472. [DOI] [PubMed] [Google Scholar]
- 3.Keays SL, Bullock-Saxton JE, Newcombe P, et al. The relationship between knee strength and functional stability before and after anterior cruciate ligament reconstruction. J Orthop Res. 2003 Mar;21(2):231–237. doi: 10.1016/S0736-0266(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 4.Young A, Stokes M, Iles JF. Effects of joint pathology on muscle. Clin Orthop Relat Res. 1987 Jun;(219):21–27. [PubMed] [Google Scholar]
- 5.Konishi Y, Ikeda K, Nishino A, et al. Relationship between quadriceps femoris muscle volume and muscle torque after anterior cruciate ligament repair. Scand J Med Sci Sports. 2007 Dec;17(6):656–661. doi: 10.1111/j.1600-0838.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 6.Konishi Y, Oda T, Tsukazaki S, et al. Relationship between quadriceps femoris muscle volume and muscle torque at least 18 months after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2012 Dec;22(6):791–796. doi: 10.1111/j.1600-0838.2011.01332.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindstrom M, Strandberg S, Wredmark T, et al. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013 Aug;23(4):431–442. doi: 10.1111/j.1600-0838.2011.01417.x. [DOI] [PubMed] [Google Scholar]
- 8.Lorentzon R, Elmqvist LG, Sjostrom M, et al. Thigh musculature in relation to chronic anterior cruciate ligament tear: muscle size, morphology, and mechanical output before reconstruction. Am J Sports Med. 1989 May-Jun;17(3):423–429. doi: 10.1177/036354658901700318. [DOI] [PubMed] [Google Scholar]
- 9.Mizner RL, Petterson SC, Stevens JE, et al. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. The Journal of bone and joint surgery. 2005 May;87(5):1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirwan JR, Byron MA, Winfield J, et al. Circumferential measurements in the assessment of synovitis of the knee. Rheumatol Rehabil. 1979 May;18(2):78–84. doi: 10.1093/rheumatology/18.2.78. [DOI] [PubMed] [Google Scholar]
- 11.Williams GN, Buchanan TS, Barrance PJ, et al. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005 Mar;33(3):402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 12.Meier WA, Marcus RL, Dibble LE, et al. The long-term contribution of muscle activation and muscle size to quadriceps weakness following total knee arthroplasty. J Geriatr Phys Ther. 2009;32(2):35–38. [PubMed] [Google Scholar]
- 13.Petterson S, Barrance P, Marmon A, et al. Time Course of Quad Strength, Area and Activation After Knee Arthroplasty and Strength Training. Med Sci Sports Exerc. 2011;43(2):225–231. doi: 10.1249/MSS.0b013e3181eb639a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29(5):633–640. doi: 10.1002/jor.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edstrom L. Selective atrophy of red muscle fibres in the quadriceps in long-standing knee-joint dysfunction. Injuries to the anterior cruciate ligament. J Neurol Sci. 1970 Dec;11(6):551–558. doi: 10.1016/0022-510x(70)90105-x. [DOI] [PubMed] [Google Scholar]
- 16.Lopresti C, Kirkendall DT, Street GM, et al. Quadriceps Insufficiency following Repair of the Anterior Cruciate Ligament*. J Orthop Sports Phys Ther. 1988;9(7):245–249. doi: 10.2519/jospt.1988.9.7.245. [DOI] [PubMed] [Google Scholar]
- 17.Aagaard P, Andersen JL, Dyhre-Poulsen P, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001 Jul 15;534(Pt. 2):613–623. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suetta C, Hvid LG, Justesen L, et al. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009 Oct;107(4):1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 19.Seynnes OR, Erskine RM, Maganaris CN, et al. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J Appl Physiol. 2009 Aug;107(2):523–530. doi: 10.1152/japplphysiol.00213.2009. [DOI] [PubMed] [Google Scholar]
- 20.Palmieri-Smith RM, Villwock M, Downie B, et al. Pain and effusion and quadriceps activation and strength. J Athl Train. 2013 Mar-Apr;48(2):186–191. doi: 10.4085/1062-6050-48.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tate CM, Williams GN, Barrance PJ, et al. Lower extremity muscle morphology in young athletes: an MRI-based analysis. Med Sci Sports Exerc. 2006 Jan;38(1):122–128. doi: 10.1249/01.mss.0000179400.67734.01. [DOI] [PubMed] [Google Scholar]
- 22.Williams GN, Snyder-Mackler L, Barrance PJ, et al. Muscle and tendon morphology after reconstruction of the anterior cruciate ligament with autologous semitendinosus-gracilis graft. The Journal of bone and joint surgery. 2004 Sep;86-A(9):1936–1946. doi: 10.2106/00004623-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Svensson M, Kartus J, Ejerhed L, et al. Does the patellar tendon normalize after harvesting its central third?: a prospective long-term MRI study. Am J Sports Med. 2004 Jan-Feb;32(1):34–38. doi: 10.1177/0363546503258935. [DOI] [PubMed] [Google Scholar]
- 24.Bernicker JP, Haddad JL, Lintner DM, et al. Patellar tendon defect during the first year after anterior cruciate ligament reconstruction: appearance on serial magnetic resonance imaging. Arthroscopy. 1998 Nov-Dec;14(8):804–809. doi: 10.1016/s0749-8063(98)70014-3. [DOI] [PubMed] [Google Scholar]
- 25.Shelbourne KD, Johnson BC. Effects of patellar tendon width and preoperative quadriceps strength on strength return after anterior cruciate ligament reconstruction with ipsilateral bone-patellar tendon-bone autograft. Am J Sports Med. 2004 Sep;32(6):1474–1478. doi: 10.1177/0363546503262171. [DOI] [PubMed] [Google Scholar]
- 26.Shelbourne KD, Rubinstein RA, Jr., VanMeter CD, et al. Correlation of remaining patellar tendon width with quadriceps strength after autogenous bone-patellar tendon-bone anterior cruciate ligament reconstruction. Am J Sports Med. 1994 Nov-Dec;22(6):774–777. doi: 10.1177/036354659402200608. discussion 777-778. [DOI] [PubMed] [Google Scholar]
- 27.Keays SL, Bullock-Saxton J, Keays AC. Strength and function before and after anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2000 Apr;(373):174–183. doi: 10.1097/00003086-200004000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Stackhouse SK, Dean JC, Lee SC, et al. Measurement of central activation failure of the quadriceps femoris in healthy adults. Muscle Nerve. 2000 Nov;23(11):1706–1712. doi: 10.1002/1097-4598(200011)23:11<1706::aid-mus6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Snyder-Mackler L, De Luca PF, Williams PR, et al. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. The Journal of bone and joint surgery. 1994 Apr;76(4):555–560. doi: 10.2106/00004623-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Taylor JL. Point: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009 Jul;107(1):354–355. doi: 10.1152/japplphysiol.91220.2008. [DOI] [PubMed] [Google Scholar]