Abstract
Phytogenic feed additives are plant-derived products used in poultry feeding to improve overall performance of broilers. In this study, 588 one day-old Cobb 500 chicks were fed one of four diets and housed on either dirty or clean litter for 3wks. Treatments included: Group I: commercial diet with no additive and housed on clean litter; Group II: commercial diet with no additive and housed on dirty litter; Group III: commercial diet with a 0.05% inclusion of the anitobiotic, BMD (bacitracin methylene disalicylate); Group IV: commercial diet with a 0.05% inclusion of a phytogenic feed additive (PFA). The study was designed around a random block assignment of treatments allocated to groups of twenty-one birds per pen. Blood samples were obtained from chicks at 18 days of age for measurement of leukocyte oxidative activity by a bioluminescence technique. Results of the study showed that chicks in the treatment groups fed the PFA had significantly lower oxidative stress (p<0.02) when compared to the BMD treatment group. Once this was determined, electron spin resonance (ESR) spin trapping was used to detect and measure hydroxyl or superoxide radicals in. Fenton chemistry was utilized for production of hydroxyl radicals and a xanthine/xanthine oxidase reaction for the production of superoxide radicals in the diet and in RAW 264.7 mouse peritoneal monocytes exposed to the diet. Results from the reactions showed that the antibiotic scavenges hydroxyl and superoxide radicals more efficiently than the phytogenic. The results were comparable to those measured in the RAW 264.7 cells.
Keywords: Antibiotics, electron spin resonance, phytogenic feed additive, oxidative stress
INTRODUCTION
Antibiotics have been used at subtherapeutic levels for improvement in growth for approximately 50 years in the United States and other countries. In 1998 some European countries passed regulations banning the use of antibiotic additives in the feed and water of poultry while in 2006 the European Union completely banned the use of antibiotics in livestock and poultry feed or water. This is in light of rising antibiotic resistance attributed to antibiotic runoff from feed facilities and residues found in meat products and similarity to drugs used by humans to treat bacterial infections (Dibner et al., 2005). In the U.S. poultry industry antibiotics are approved by the Food and Drug Administration (FDA) and regulation of usage is closely monitored by both the FDA and USDA.
In poultry, the effects of antibiotics on growth and overall health of the bird is debated. Past research has shown that antibiotic growth promoters have increased feed intake and growth of livestock birds, however, most noticeable is the effect on feed efficiency (Dibner et al., 2005). Studies for this reason, focused on the interaction between antibiotics and the natural microflora of the gut as well as the reduction of pathogens leading to infection within the bird.
Given that antibiotic resistance is a concern for both the poultry industry and issues of human health, research has become focused on finding alternatives to antibiotics. Such alternatives include, but are not limited to, prebiotics, probiotics, enzymes and phytogenic based feed additives (Windisch et al., 2007).
Phytogenic feed additives (PFA), also referred to as phytobiotics or botanicals, are plant derived products that, when combined, can exhibit improvement in performance and health of livestock, due to a reduction in feed intake and improved feed conversion ratio. Physiologically, phytogenies have been reported to decrease ammonia output, oxidative stress and lipid peroxidation in poultry (Windisch et al., 2007) as well as increase overall gut health by supporting symbiotic gut microflora.
Oxidative stress was first termed several years ago to describe a condition in which excess production of oxidants causes and imbalance with the antioxidants of the body (VanDyke, 2002). The sources of oxidants and antioxidants can be both endogenous and exogenous. Endogenous oxidants arise from the formation of reactive oxygen species (ROS) from the mitochondrial electron transport chain as well as enzyme sources and from xenobiotics (Parks and Granger, 1986). Exogenous oxidants can come from the environment, for example, exposure to certain metals or chemicals. ROS can include, but are not limited to, hydroxyl radicals, superoxide radicals, peroxynitrite, or hydrogen peroxide. ROS present the risk of damaging the cellular macromolecules, which includes proteins, RNA, DNA and lipids among others (Aruoma, 1998). Antioxidants are the body’s way of combating the effect that oxidants can have within the body. Exogenous antioxidants are found in the vitamin E family (VanDyke, 2002), whereas, endogenous antioxidants include enzymes such as superoxide dismutase and endproducts of purine breakdown such as uric acid (Simoyi et al., 2002).
Most avian species will live significantly longer than mammals of comparable size even though they have a greater metabolism, a higher core body temperature and blood sugar concentrations two-fold greater than their mammalian counterparts of the same size (Holmes and Austad, 1995). Birds also sustain a much higher oxidative burden due to these physiological characteristics.
Phytogenic feed additives have been reported to exhibit antioxidative capabilities (Windisch et al., 2007). Although the exact mechanism has not been established, it is known that addition of spices such as black pepper (Gulcin, 2005; Singh et al., 2004), cayenne pepper, tumeric and ginger as well as other spices (Windisch et al., 2007; Shobana et al., 2000) referred to as pungent substances, can reduce oxidative stress. The herb, thyme, has also been implicated in the reduction of oxidative stress and DNA damage in quail (Sengul et al., 2008). Antibiotics given at subtherapeutic levels have been shown to be beneficial to poultry. However, oxidative stress is increased in birds given specific antibiotics have shown that oxidative stress can be increased (Sengul et al., 2008). Conflicting data from Kohanski et al. (2007) demonstrates that bactericidal type antibiotics can cause an overproduction of hydroxyl radicals whereas bacteriostatic antibiotics do not exhibit these properties. Thus, it is not entirely clear what effect antibiotics have on oxidative stress in poultry and the mechanism responsible for its beneficial properties.
The purpose of this study was to compare the mechanism by which a phytogenic feed additive (PFA) and a commonly used antibiotic (BMD) can affect oxidative stress, growth and feed intake in broiler chicks. The hypothesis is that the phytogenic feed additive will be equivalent in performance as compared to the antibiotic, thus supporting its use as a possible antibiotic alternative for the poultry industry.
MATERIALS AND METHODS
All experimental protocols (IACUC#12-0201) were approved by the West Virginia University Animal Care and Use Committee.
Study 1
For this study, six hundred Cobb 500 (Cobb × Cobb) day old broilers were generously donated from the Pilgrim’s Pride Hatchery in Moorefield, West Virginia. The chicks were divided into groups, each containing twenty one birds per group. The study was designed in a randomized complete block. There were seven blocks containing four treatments. Each pen contained either clean litter or built-up litter that had been exposed to several generations of organically raised broilers. Organic litter was chosen so that there would be no prior exposure to antibiotics. The four treatments were as follows: Group I with no additive and clean litter, Group II with built-up litter control with no additive, Group III with built-up litter with 0.05% antibiotic addition to the feed and Group IV with built-up litter and a 0.05% phytogenic additive to the feed. The antibiotic used was bacitracin methylene diasalycilate (BMD). BMD is commonly used by the American poultry industry as an additive to the feed. PFA composition was not provided by the donating company. Diets were prepared according to industry standards by Dr. Joseph Moritz. All feed was made at West Virginia University Animal Science Farm in Morgantown. From hatch to day eighteen, chicks were fed a starter crumble diet (Table 1). Chicks were weighed at an age of eighteen days of age as a measurement of total pen weight. Feed bags were weighed at the start of each growth period and weighed again at the end of each period to determine feed intake. Measurement of Oxidative Stress. Oxidative stress was determined by measurement of leukocyte oxidative activity (LOA) as previously described by VanDyke et al. (2002). One milliliter of blood was taken from the chicks at 18 days of age and combined to obtain sample of 4 mL in total of whole blood. Twelve samples from each treatment group were pooled and analyzed to determine oxidative stress. Blood was drawn using heparinized needles and placed into EDTA treated tubes. The blood was immediately placed on ice. Whole blood was treated with 4 mL of Mono-poly resolving agent (ICN Biomedicals Inc. Aurora, Ohio) to separate the leukocytes. Leukocyte layers were drawn and placed in chilled microcentrifuge tubes and put into ice. The cells (100 µL) were treated with PBS (200 or 300 µL), PMA (100 or 0 µL) and LO12 (100 µL) before being read in a luminometer. Samples were run with and without the addition of PMA. Samples were run for 20 min and results calculated by using the ratio of stimulated versus non-stimulated cells. The ratio was averaged for a treatment and reported in the data. SAS software (SAS Institute, 2002) was used to determine any significant differences between the groups. Results were considered significant at p<0.05.
Table 1.
Starter diet fed to chicks 0–18 days of age
| Ingredients | Amount Inclusion (%) |
|---|---|
| Corn | 62.92 |
| SBM | 32.20 |
| Defl. Phosophorous | 1.53 |
| Meat and bone meal | 1.35 |
| SB oil | 0.50 |
| Limestone | 0.50 |
| Methionine | 0.26 |
| NB30001 | 0.25 |
| Salt | 0.24 |
| Lysine | 0.20 |
| Coban2 | 0.08 |
| Threonine | 0.04 |
| BMD3 | 0.05 |
| PFA4 | 0.05 |
| Calculated values | |
| Crude protein (%) | 21.54 |
| Crude fat (%) | 3.04 |
| Calcium (%) | 0.91 |
| Phosphorous(%) | 0.72 |
| Supplied/kg of diet: | Manganese, 0.02% |
| Zinc, 0.02% | Iron, 0.01% |
| Copper, 0.0025% | Iodine, 0.0003% |
| Selenium, 0.00003% | Folic acid, 0.69 mg |
| Choline 386 mg | Riboflavin, 6.61 mg |
| Biotin 0.03 mg | Vitamin B6 1.38 mg |
| Niacin, 27.56 | Pantothenic acid, 6.61 mg |
| Thiamine,2.20 mg | Manadione, 0.83 mg |
| Vitamin B12 0.01 mg | Vitamin E, 16.53 IU |
| Vitamin D3, 2133 IU | Vitamin A 7716 IU |
Active drug ingredient Monensin Sodium 60 gpb (90 gfton inclusion)-Elanco Animal Health, Indianapolis, IN. Aids in the prevention of coccidiosis caused by Eimeria sp
Bacitracin Methylene Disalicylate used as the antibiotic in Treatment 3
Phytogenic feed additive used in Treatment 4
Histology
At the end of the study, adult birds were sacrificed and tissues collected for histological analysis and sent to the West Virginia University Health Sciences Center Department of Pathology for preparation and analysis. Sections of the duodenum, ileum and colon were analyzed for differences in health or morphology of the small intestine of birds in all groups.
Litter analysis
Analysis of the litter was provided by Superior Laboratories (Galloway, Ohio). Samples were taken from four pens in each treatment. Samples were analyzed for Escherichia coli and Salmonella spp. which can provide a microbial challenge to birds housed on litter containing high coliform counts or testing positive for these bacterial species.
Study 2
Electron Spin Resonance(ESR). ESR spin trapping was employed to measure antioxidant activity in the PFA and the antibiotic additive. ESR spin trapping detects short-lived free radical intermediates. The technique involves the addition-type reaction of a radical with a paramagnetic compound (spin trap) which forms a relatively long-lived free radical product (spin adduct). This was measured using ESR (Model ESP 300E, Burker Instruments, Billercia, MA). ESR setting for all measurements were center field 3480±100, frequency 9.8 GHz, power 63.5 mW, gain 1×104, mod. Amp 1G, time constant 40 ms, sweep time 41 s. All spectra were scanned once except cellular samples which were scanned 3 times. Acquisif® software was used to record the data. Samples were analyzed in a flat cell assembly with DMPO.
Generation of hydroxyl radicals was achieved using Fenton chemistry:
Fe2++H2O2→Fe3++·OH+−OH
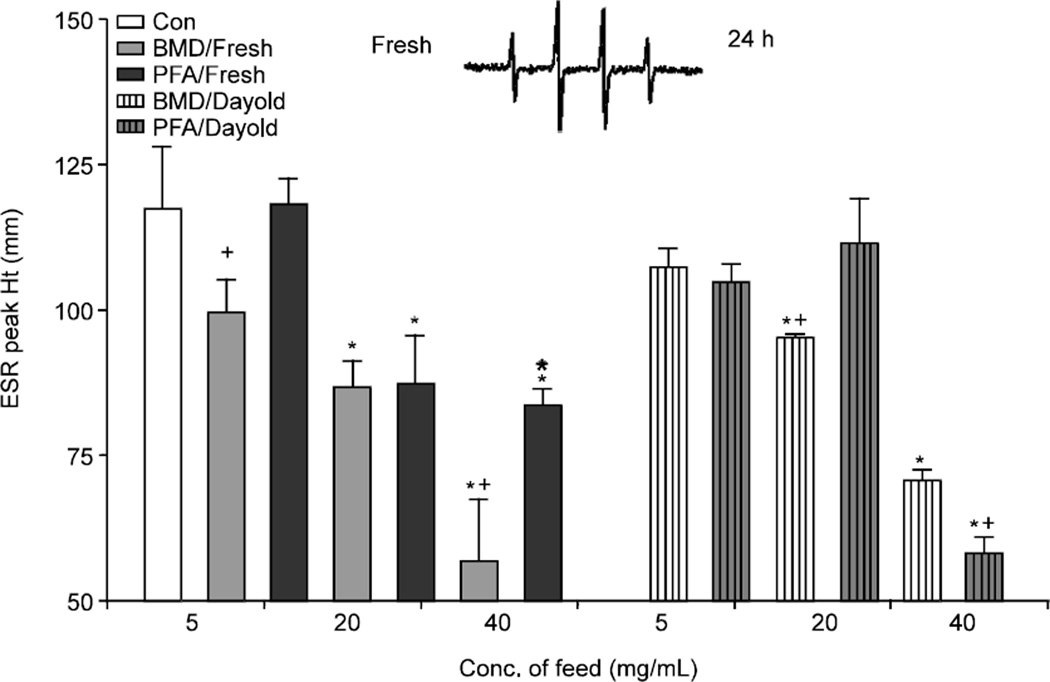
Hydroxyl radicals can be identified by the 1:2:2:1 peak ratio on ESR spectra. 1:2:2:1 ESR peak pattern is indicative of the trapped hydroxyl radical (Fig. 2). Samples were prepared fresh or allowed to remain at room temperature for 24 h and measured at physiological concentrations. The reaction sequence was as follows for the control: DMPO (100 mM)+H2O2 (1 mM)+FeSO4 (0.1 mM). Either fresh or day old preparations of the PFA or the antibiotic additive (BMD) was added to this reaction at their, respective concentrations (5, 20 and 40 mg/mL) to observe differences in peak height which is indicative of the additive’s ability to quench radicals. Samples were run in triplicate for each concentration. These doses were determined by investigating a range of concentrations from 0.5 to 50 mg/mL. The 5 mg/mL concentration showed a maximal change in peak height, while 40mg/ml showed almost all reduction of peak height.
Fig. 2.
Measurement of hydroxyl radical quenching by the phytogenic and antibiotic feed additive in either fresh or 24 h preparations. (*) denotes significance differences when compared to the control and (+) denotes a significant difference when compared to the phytogenic feed additive. Significance is defined as p<0.05. Inset: representative ESR hydroxyl spectra
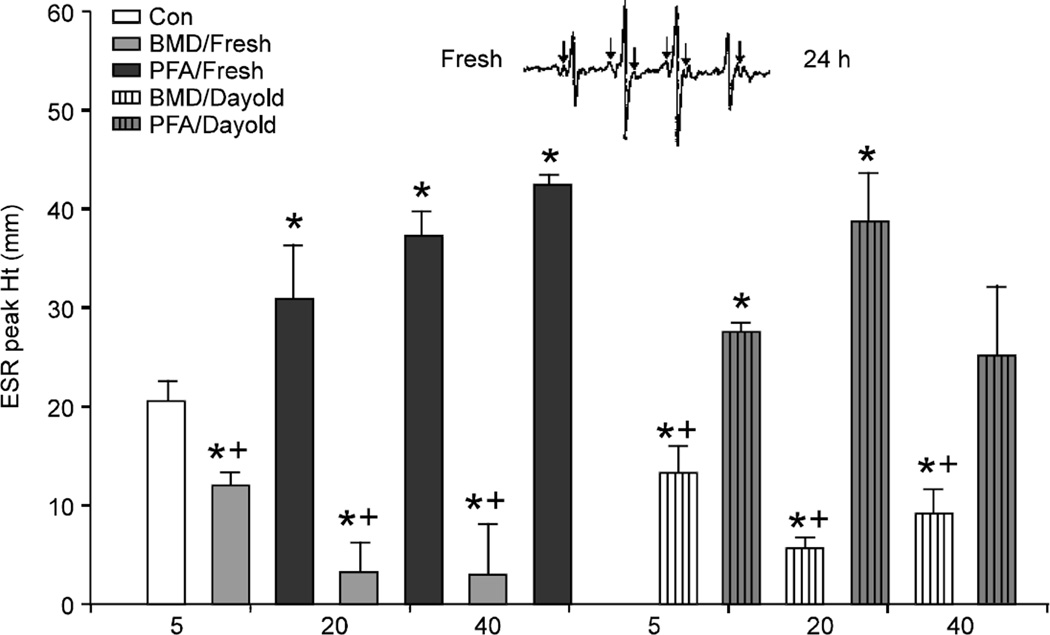
Superoxide radicals were generated using a xanthine/xanthine oxidase reaction. Samples were read 1min after initiation of the reaction sequence. Superoxide radicals are seen as hyperfine splits (Fig. 3) and the ability of the additives to quench these splits is measured.
Fig. 3.
Measurement of superoxide radical quenching by the phytogenic and antibiotic feed additive in either fresh or 24 h preparations. (*) denotes significance differences when compared to the control and (+) denotes a significant difference when compared to the phytogenic feed additive. Significance is defined as p<0.05. Inset: representative ESR superoxide spectra, arrows denote hyperfine splitting
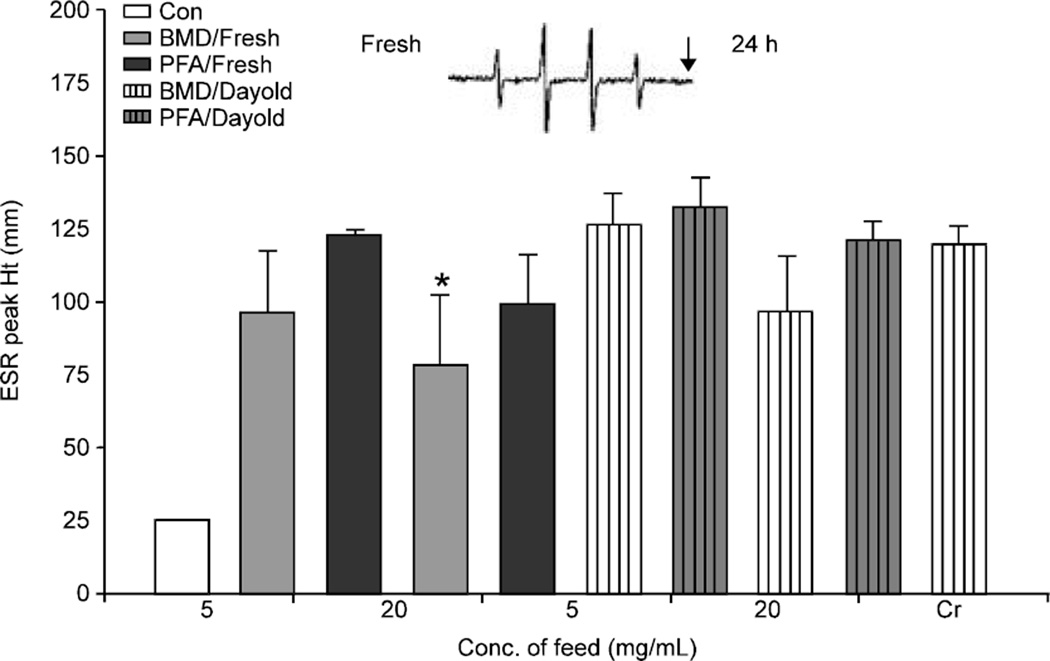
RAW 264.7 mouse peritoneal monocytes were purchased from American Type Culture Collection (Rockville, MD). RAW 264.7 cells are commonly used and have been found to respond to particle exposure in a manner similar to primary alveolar macrophages [46–49]. RAW 264.7 cells were cultured in DMEM with 10% FBS, 2 mM L-glutamine and 50 mg/mL pen/strep at 37°C in a 5% CO2 incubator. Cells were split after confluence approximately every 3 days. Reactants and 100 mM of the spin trap DMPO were mixed in a final volume of 1 mL suspended in PBS. After a 5 min incubation at 37°C the reaction mixture was transferred to a flat cell and ESR measurements were conducted. Experiments were performed at room temperature and under ambient air and are used to measure the reactivity potential of a material. Mouse RAW-264.7 cells were used to model radical quenching capabilities of the additives in vitro. Cells were grown in commercial media and incubated at 37°C until harvested for use. Cells were washed twice with PBS (phosphate buffered saline) before harvest. Normal cell “noise” or radical production was measured by Acquisit® software to provide a baseline. Cr (VI) was added to the cells to induce respiratory burst radical production in the cells. Measurements were taken as reduction or increase in peak height in the presence of the additives as compared to the chromium-treated cells. The reaction sequence is as follows:
RAW 264.7 cells+DMPO+feed additive+5 min+Cr(VI)
ESR settings remained the same as in the previous study.
Statistical analysis was done with SigmaStat software at NIOSH. Samples were run in triplicate and the mean was taken from these results. Results were considered significant at p<0.05.
RESULTS
Study 1
Nutrition parameters
There were no significant differences between groups in feed intake or feed conversion ratio. Birds in Group I showed a significantly (p<0.05) higher live weight gain than birds in Group III and Group IV treatments.
Litter analysis
The litter was analyzed to assess the source of the bacterial challenge to the birds which could lead to oxidative stress (Table 2). Litter analysis showed heightened levels of E.coli in pens starting on dirty litter. Specifically, as compared to Group I with 171,000 per gram of dry solids, Group II had a coliform count of 25 million/g of dry solids, Group III had 6.7 million coliforms per gram of dry solids and Group IV had 149 million/g of dry solids. Group III litter also tested positive for Salmonella spp. (per 25 g of solids).
Table 2.
Microbial Analysis of Litter Samples (Superior Laboratories, Galloway OH)
| Sample | E.Coli* | Salmonella spp.** |
|---|---|---|
| Group I | 171,00 | Negative |
| Group II | 25×106 | Negative |
| Group III | 6.7×106 | Positive |
| Group IV | 149×106 | negative |
Per g of dry solids; MPN method
Per 25 g of sample
Oxidative stress
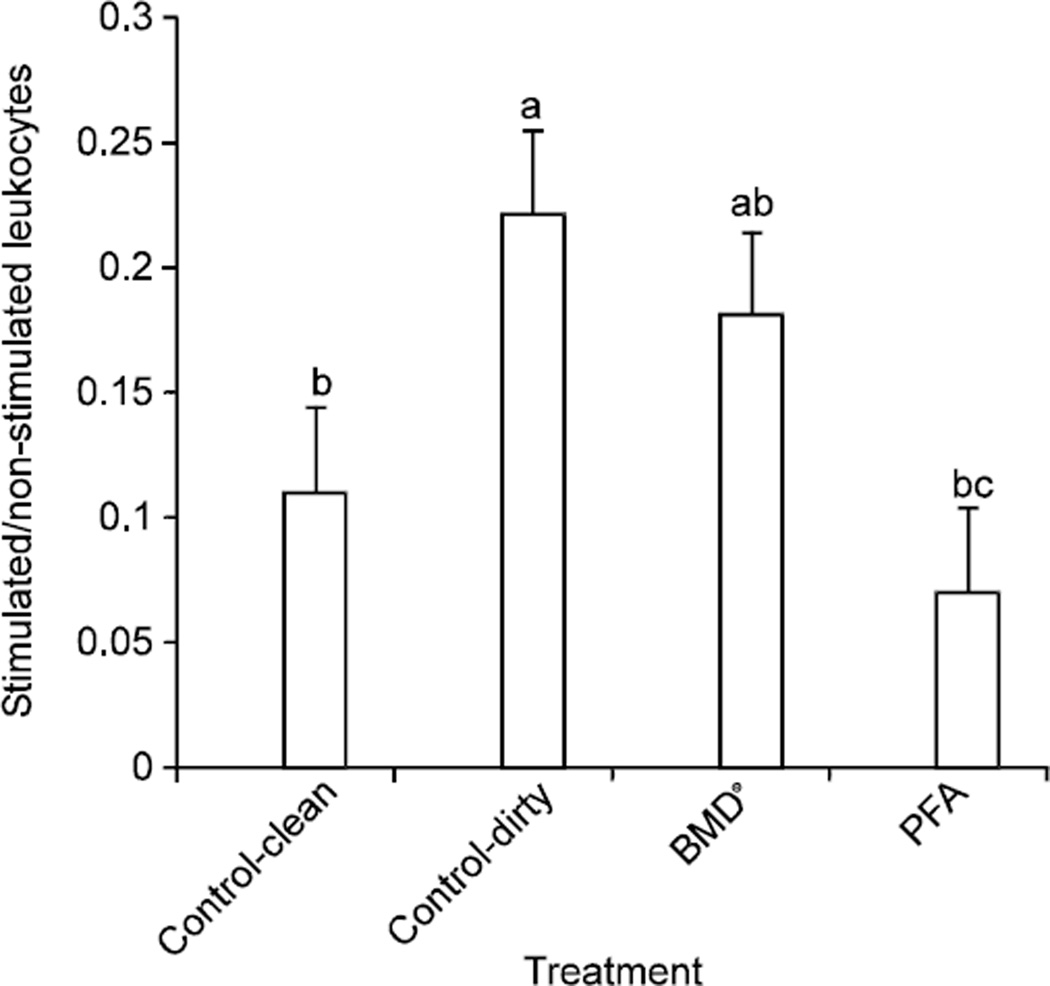
Birds in the PFA treatment showed significantly lower levels of LOA, indicating decreased oxidative stress as compared to birds in the Group II (p<0.02) and Group III (p<0.04) treatments (Fig. 1). Birds in Group II reared on built-up litter had a significant (p<0.05) increase in LOA as compared to bird in the Group I treatment, indicating that oxidative stress was higher in these birds started on dirty litter. There were no significant differences between birds in the Group I treatments as compared to birds in the Group III and Group IV treatments.
Fig. 1.
Oxidative stress measurement in combined whole blood samples of 18 day old chicks. Leukocyte activity was measured. Differences in Letters indicate significant decrease in oxidative stress (p<0.05)
Histology
There were no significant differences in any of the samples concerning the morphology and no abnormalities in the tissue samples were reported by the pathologist.
Study 2
Hydroxyl radical
The ·OH radical forms the DMPO-OH spin adduct which is the spectra seen in Fig. 2 while the superoxide radical forms the DMPO-OOH spin adduct which causes hyperfine splitting of the spectra as indicated by the arrows in Fig. 3. Freshly prepared BMD (5 mg/mL) significantly reduced hydroxyl peaks as compared to the PFA and the control (Fig. 2). Both BMD and PFA reduced hydroxyl peaks when freshly prepared at a concentration of 20 mg/mL. Although, there was no significant difference between the two additives, suggesting that one was not more effective than the other. Fresh preparations at 40 mg/mL of both additives significantly reduced hydroxyl radical peaks. BMD exhibited a more significant reduction of peak height as compared to PFA.
There were no significant differences with BMD and PFA versus the control at 5 mg/mL of day old preparation (Fig. 2). However, day old BMD significantly quenched the hydroxyl radicals at 20 and 40 mg/mL. The day old9 PFA additive also significantly quenched radicals at 40 mg/mL, which was more effective than BMD at this concentration (Fig. 2).
Superoxide radicals
Freshly prepared BMD significantly reduced superoxide peaks in a dose-dependent manner (Fig. 3). Interestingly, the PFA additive did not quench superoxide radicals at any concentration. Rather, PFA significantly increased peak height, indicating that superoxide radicals were being generated in the reaction sequence. This observation was the same for all concentrations of the day old PFA additive. Freshly prepared PFA additive also revealed a dose-dependent increase in ESR peak height as compared to the day-old preparation where an effect was not observed at the highest dose.
RAW 264-7 mouse peritoneal cells
RAW 264.7 cells stimulated by Cr (VI) produced radicals which were then trapped by DMPO to form the DMPO-OH adduct as seen in figure 4. The reduced Cr (V) radical can also be observed as indicated by the arrow confirming that the cells are viable and generating radicals. A fresh preparation of 20 mg/mL of BMD, caused the only significant reduction in peak height. All other concentrations were very similar to the peak height observed in the chromium exposed cells.
Fig. 4.
Measurement of cellular generated radical quenching by the phytogenic and antibiotic feed additive in either fresh or 24 h preparations. (*) denotes significance differences when compared to the control and (+) denotes a significant difference when compared to the phytogenic feed additive. Significance is defined as p<0.05. Inset: representative ESR spectra, arrow denotes Cr(V) radical verifying that cells are viable and generating radicals
DISCUSSION
Birds have the unique physiological capability to handle greater oxidative burdens over longer lifetimes than mammals of comparable body size. For many years, the poultry industry has administered antibiotics given at subtherapeutic levels to increase growth and overall health of the birds when exposed to conventional housing systems over the growth period (Dibner et al., 2005). However, concerns expressed by the public over the use of antibiotics has resulted in the search for alternatives. Phytogenic additives are one such area that are effective as a replacement for these antibiotics.
In the first study, a phytogenic feed additive and a common industry antibiotic were compared to observe differences between performance data and oxidative stress. In the second part, ESR was employed to find a possible mechanism to explain the results found in the LOA obtained.
In the first study, growth and performance data were not significantly different between the PFA and BMD groups. Although, Group I birds had significantly higher live weight gain during the starter period. There is a wide range of data concerning performance of birds given phytogenic or phytogenic-type additives. After investigation of oregano-based phytogenic feed additives, Cross et al. (2007) determined that weight gain is dependent on the components of the phytogenic feed additive. Buchanan et al. (2008) found that while one brand of PFA had a positive effect on live weight gain, a similar PFA had no effect on live weight gain in Cobb 500 broilers further illustrating the variability in composition between additives. Essential oils are believed to be important in the biological effects of the PFA additives. However, it has not been determined whether essential oils have a positive effect on performance due to stabilization of feed components, improvement of the gut environment via the natural microflora, or by stimulation of digestive and pancreatic enzymes (Windisch et al., 2007). It is also unknown whether the effectiveness of the essential oil is dose dependent or whether a certain combination of essential oils have a positive effect on broiler performance. Lee et al. (2003) investigated the inclusion of dietary essential oils in the diet of female broilers and found that feed intake, weight gain and feed:grain ratio were not different among any of the groups fed the additive. Essential oils were thought to be responsible for the improvement of performance parameters in birds. However, Jamroz et al. (2005) fed birds a plant origin based diet with either a maize or wheat and barley base mixed with the additive and while growth was not significantly affected, feed conversion ratio was improved in both diet types. It has also been suggested that essential oils can reduce free radical production in the gut. Youdim and Dean (2000) found that there were higher levels of polyunsaturated fatty acids in the phospholipid portions of various tissues in birds fed the supplement than those fed control diets. It was suggested that components of the supplements acted as free radical scavengers and influenced the in vivo antioxidant defense mechanisms. Scavenging free radicals in the gut would reduce oxidative stress and thereby reduce gut inflammation associated with feeding. A reduction in oxidative stress could decrease feed intake, improve feed conversion and support the hypothesis that PFA’s can, in fact, increase growth and performance in birds.
A reduction in oxidative stress was measured in chicks fed the PFA as compared to birds in the BMD or DLC groups. While the exact components of the PFA used for this study are not known, it was advertised that pungent substances were used. Pungent substances can include black pepper, cumin, ginger and capsaicin among others, which have been reported to exhibit antioxidant capabilities. The antioxidant mechanism for black pepper shows a strong hydrogen donating ability in addition to metal chelation (Gulcin, 2005). This suggests that black pepper would be a good scavenger of hydrogen peroxide and superoxide among other free radicals. However, this effect was not observed with the PFA in this study. Along with pungent substances, certain herbs have also shown potential as antioxidants. Thyme, in particular shows the ability to lower oxidative stress. A study with Japanese quail that were administered thyme oil and thyme water extracts revealed that these two treatments significantly reduced DNA damage induced by oxidative stress and had a significantly higher total antioxidant response (Sengul et al., 2008). The PFA is advertised to contain these component types, which may thus contribute to the observed results.
Once it was determined that feeding PFA and BMD had an effect on oxidative stress, electron spin resonance was used to measure their ability to quench specific radicals. For this experiment, both feed additives were investigated to determine how well each reduced peak height on ESR measurement of hydroxyl and superoxide radicals. Reduction in peak height indicates that the additive effectively reduced free radical production.
The PFA demonstrated peak height reduction in the Fenton chemistry production of hydroxyl radicals. At 40 mg/mL of day old preparation, the PFA was shown to be a more effective scavenger of these radicals as compared to BMD. PFA was also effective at quenching the hydroxyl radicals at 20 mg/mL. Thus, it is likely that the feed components responsible for the reduction of oxidative stress in vivo are also functioning in vitro to quench hydroxyl radicals. On the other hand, PFA did not quench superoxide radical production. When analyzing the ESR spectra, the PFA actually increased superoxide peak height, although the mechanism for this observation in not clear. PFA was also ineffective in quenching radicals when exposed to RAW 264.7 mouse peritoneal cells.
Hydroxyl and superoxide radicals were quenched with the addition of BMD in their specific reaction sequences. Interestingly, both an age and dose dependency were observed with the BMD additive. However, day old preparations did not scavenge radicals as efficiently as fresh preparations. Also, BMD at 5 mg/mL did not significantly reduce hydroxyl radicals and was not as effective as higher doses in quenching superoxide radical reduction. In the RAW 264.7 cells, BMD showed a significant peak height reduction at 20 mg/mL. Again, the efficiency of usage of BMD is dose dependent and a function of storage of the antibiotic when added to the feed.
Conclusion
In conclusion, there was no effect of the additives on live weight gain, feed intake, or growth of the birds. However, there was a significant decrease in oxidative stress of the birds fed the PFA comparable to the observed effects of the antibiotic. Results from this study suggest that the PFA reduces hydroxyl radical production, revealing a partial in vitro mechanism. However, more research is needed to determine the mechanism explaining the reduction of oxidative stress in the birds. BMD worked effectively as a scavenger of both hydroxyl and superoxide radicals and reduced oxidative stress. Although, the analysis of phytogenic feed additives and how they affect the animals’ physiology as well as performance is still relatively new and the results from these studies demonstrate PFA’s show promise as an alternative to antibiotics.
REFERENCES
- Aruoma O. Free radicals, oxidative stress and antioxidants in human health and disease. JAOCS. 1998;75:199–211. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan NP, Hott JM, Cutlip SE, Rack AL, Asamer A, Moritz JS. The Effects of a Natural Antibiotic Alternative and a Natural Growth Promoter Feed Additive on Broiler Performance and Carcass Quality. J. Appl. Poult. Res. 2008;17:202–210. [Google Scholar]
- Cross DE, McDevitt RM, Hillman K, Acamovic T. The effects of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Gulcin I. The antioxidant and radical scavenging activities of black pepper (piper nigrum) seeds. Int. J. Food Sci. and Nutr. 2005;56:491–499. doi: 10.1080/09637480500450248. [DOI] [PubMed] [Google Scholar]
- Holmes DJ, Austad SN. The evolution of avian senescence patters; implications for understanding primary aging processes. Am. Zool. 1995;35:307–317. [Google Scholar]
- Jamroz D, Wiliczkiewicz A, Wertelecki T, Orda J, Skorupiska J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005;46:485–493. doi: 10.1080/00071660500191056. [DOI] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Hayete B, Lawerence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Lee KW, Everts H, Kappert HJ, Frehner M, Losa R, Beynen AC. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta. Physiol. Scanda. 1986;548:87–99. [PubMed] [Google Scholar]
- SAS Institute Inc. SAS user's guide: Statistics. Cary, NC: SAS Institute Inc.; 2002. [Google Scholar]
- Sengul T, Yurtseven S, Cetin M, Kocyigit A, Sogut B. Effect of thyme (T. vulgaris) extracts on fattening, performance, some blood parameters, oxidative stress and DNA damage in Japanese quails. J. Anim. and Feed Sci. 2008;17:608–620. [Google Scholar]
- Shobana S, Naidu A. Antioxidant activity of selected Indian spices. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2000;62:107–110. doi: 10.1054/plef.1999.0128. [DOI] [PubMed] [Google Scholar]
- Simoyi M, VanDyke K, Klandorf H. Manipulation of plasma uric acid in broiler chicks and its effect on leukocyte oxidative activity. Am. J. Physiol. Regulatory Integrative Comp. Phsyiol. 2002;282:791–796. doi: 10.1152/ajpregu.00437.2001. [DOI] [PubMed] [Google Scholar]
- Singh G, Marimuthu P, Catalan C, deLampasona MP. Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. J. Sci. Food and Agri. 2004;84:1878–1884. [Google Scholar]
- VanDyke K, VanDyke C, Woodfork K. Luminescence Biotechnology Instruments and Applications. CRC Press LLC; 2002. [Google Scholar]
- Windisch WM, Schedle K, Plitznerand C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2007;86:140–148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Deans SG. Effect of thyme oil and thymol dietary supplementation of the antioxidant status and fatty acid composition of the ageing rat brain. Br. J. Nutr. 2000;83:87–93. [PubMed] [Google Scholar]