Abstract
Background
Pregabalin may reduce postoperative pain and opioid use. Higher doses may be more effective, but may cause sedation and confusion. This prospective, randomized, blinded, placebo-controlled study tested the hypothesis that pregabalin reduces pain at 2 weeks after total knee arthroplasty, but increases drowsiness and confusion.
Methods
Patients (30 per group) received capsules containing pregabalin (0, 50, 100, or 150 mg); two capsules before surgery, one capsule twice a day until postoperative day (POD) 14, one on POD15, and one on POD16. Multimodal analgesia included femoral nerve block, epidural analgesia, oxycodone–paracetamol, and meloxicam. The primary outcome was pain with flexion (POD14).
Results
Pregabalin did not reduce pain at rest, with ambulation, or with flexion at 2 weeks (P=0.69, 0.23, and 0.90, respectively). Pregabalin increased POD1 drowsiness (34.5, 37.9, 55.2, and 58.6% in the 0, 50, 100, and 150 mg arms, respectively; P=0.030), but did not increase confusion (0, 3.5, 0, and 3.5%, respectively; P=0.75). Pregabalin had no effect on acute or chronic pain, opioid consumption, or analgesic side-effects. Pregabalin reduced POD14 patient satisfaction [1–10 scale, median (first quartile, third quartile): 9 (8, 10), 8 (7, 10), 8 (5, 9), and 8 (6, 9.3), respectively; P=0.023). Protocol compliance was 63% by POD14 (50.0, 70.0, 76.7, and 56.7% compliance, respectively), with no effect of dose on compliance. Per-protocol analysis of compliant patients showed no effect of pregabalin on pain scores.
Conclusions
Pregabalin had no beneficial effects, but increased sedation and decreased patient satisfaction. This study does not support routine perioperative pregabalin for total knee arthroplasty patients.
Clinical trial registration.
ClinicalTrials.gov: http://www.clinicaltrials.gov/ct2/show/study/NCT01333956.
Keywords: analgesia, anticonvulsants, arthroplasty, knee, pain management, perioperative care, replacement
Editor's key points.
Pain in the recovery phase after knee arthroplasty can be problematic and affect function.
Evidence for pregabalin reducing pain and opioid use after surgery is inconsistent.
This study explored the effect of different pregabalin doses on pain 14 days after surgery.
Pregabalin did not improve analgesia, increased immediate side-effects, and decreased patient satisfaction.
Further work is needed to elucidate the role of pregabalin in multimodal perioperative analgesia.
Perioperative pregabalin may reduce postoperative pain and opioid use,1 although this is controversial. Among total knee arthroplasty (TKA) patients, pregabalin 300 mg daily reduced acute pain, chronic pain, and opioid intake.2 Pregabalin did not reduce analgesic use after cosmetic3 or ankle surgery.4 A meta-analysis5 found that pregabalin reduced postoperative pain and reduced analgesic drug intake, but only at doses ≥300 mg daily. Limitations of this meta-analysis include the limited dose–response information that is available for pregabalin in the acute pain setting and the possibility that publication bias may have exaggerated the benefit.6
Epidural analgesia combined with femoral nerve block and oral analgesia initially provides good analgesia for TKA.7 However, pain after TKA can be severe and persistent. One report found that ∼45% of patients had significant postoperative pain (visual analog scale >40) at 1 month, with a gradual decline to ∼12% of patients at 1 year.8 Another study reported control pain scores 1 week after discharge [approximately postoperative day (POD) 10] of 3.7 (interquartile range 2.9–4.7).9 Our own observational pilot study (conducted by J.T.Y.) found a mean (sd) Numeric Rating Scale (NRS) pain score with activity of 4.8 (2.3) and at rest 2.4 (1.9) at 2 weeks after TKA in 40 patients (J.T.Y., unpublished data). This corresponds to moderate to severe pain. This is clinically important because of the correlation between postoperative pain and development of chronic pain.10 Buvanendran and colleagues2 administered pregabalin for 2 weeks (followed by a rapid taper). In order to investigate the effects of pregabalin on subacute pain after TKA, it seemed logical to assess pain at the end of a 2 week period of pregabalin administration.
Side-effects of pain management may impair participation in physical therapy and diminish patient satisfaction. At least 25% of patients on POD1 after TKA have an Opioid-Related Symptom Distress Scale (ORSDS) score >1 for nausea, drowsiness, itchiness, and fatigue.11 Pregabalin could possibly reduce side-effects by reducing opioid use, or alternatively, could increase side-effects by causing sedation or confusion. Doses of 300 mg day−1 increased sedation and confusion on POD1.2
This prospective, randomized study compared placebo with three doses of pregabalin [50, 100, and 150 mg twice daily (BID)], in the hope that a lower pregabalin dose would improve analgesia without increasing side-effects. The study tested the hypothesis that pregabalin reduces pain after TKA and determined dose-related side-effects. Subacute pain (at 14 days) was studied because of the perception that pain at 14 days was more of a major clinical problem than chronic pain.
Methods
After providing informed written consent, 120 patients with osteoarthritis who were to undergo primary TKA with a participating surgeon were enrolled (from May 2011 to March 2013). This study was approved by the Hospital for Special Surgery Institutional Review Board and registered with clinicaltrials.gov (NCT01333956). Eligible patients were 18- to 80-yr-old English speakers judged able to follow the protocol, planned to have regional anaesthesia and to be discharged home or to a participating rehabilitation centre. Exclusion criteria included planned general anaesthesia, allergy, or intolerance to one of the study medications, ASA physical status of IV, hepatic failure, renal failure (estimated creatinine clearance <30 ml min−1), difficult-to-manage diabetes mellitus (including insulin dependence), chronic gabapentin or pregabalin use (regular use for longer than 3 months), chronic opioid use (regular use for longer than 3 months), and major prior ipsilateral open knee surgery.
Patients received preoperative oral meloxicam (Lupin Pharmaceuticals- Goa, India) (7.5 or 15 mg; lower dose used for age ≥75 yr) and dexamethasone (6 mg). An ultrasound-guided femoral nerve block was performed [0.25% bupivacaine (Hospira- Lake Forest, IL, USA), 30 ml, with adrenaline (Amphastar- So El Monte, CA, USA), 5 μg ml−1], followed by a combined spinal and epidural [10, 12.5, or 15 mg 0.5% plain bupivacaine, Lake Forest, IL, USA]. Sedation consisted of midazolam (Hospira- Lake Forest, IL, USA) and propofol (Hospira- Lake Forest, IL, USA) only. Postoperative patient-controlled epidural analgesia [bupivacaine (Lake Forest, IL, USA) 0.06% plus hydromorphone (Akorn Pharmaceuticals- Lake Forest, IL, USA) 10 μg ml−1] was begun at 4 ml h−1; 4 ml bolus dose; 10 min lock-out; 20 ml h−1 maximum. The basal rate became 2 ml h−1 at 07.00 h on POD1, and 0 ml h−1 at 17.00 h on POD1. The epidural was discontinued at noon on POD2. Patients received postoperative daily meloxicam and oxycodone–paracetamol (Mallinckrodt- Hazelwood, MO, USA) (5 mg oxycodone – 325 mg paracetamol every 4 hr as needed).
Patients were randomized to receive capsules containing 0, 50, 100, or 150 mg pregabalin (four groups of 30 patients per group). Two capsules were given ∼30 min before transfer to the operating room. Patients received one capsule twice a day until POD14 (total daily dose of 0, 100, 200, or 300 mg pregabalin), then one capsule at bedtime on POD15 and POD16. The computer-generated randomization table was prepared by a research assistant not otherwise involved in the study. The hospital pharmacy prepared indistinguishable capsules. No other study personnel were aware of group assignment. Patients were discharged with prescriptions for meloxicam and oxycodone–paracetamol (Hazelwood, MO, USA) (5 mg–325 mg), unless intolerance had occurred. Open-label use of pregabalin or gabapentin was not allowed.
Preoperative data collection included the following patient characteristics: age, sex, race, BMI, ASA status, and pain scores at rest and with movement. Subsequent data collected included the following: POD1 pain rating (NRS) at time of assessment and with activity, confusion assessment method (CAM),12 opioid usage, ORSDS, and other side-effects; POD3 NRS pain scores, opioid usage, and ORSDS; and POD14 NRS pain scores, opioid usage, ORSDS, compliance with administration of study drug, blinding assessment, and satisfaction with pain management. Drowsiness was assessed as a component of the ORSDS, which was administered on POD1, 3, and 14. Satisfaction was assessed by asking, ‘On a scale of 1 to 10, with 1 being ‘very dissatisfied’ and 10 being ‘very satisfied,’ how satisfied or dissatisfied are you with the overall performance of the pain medication that you received?’
At 3 months after surgery, opioid usage and neuropathic pain were assessed. Neuropathic pain was evaluated as a binary outcome [using a cut-off of 12 on the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS)] and by comparison of LANSS scores as a continuum. The LANSS was administered at 3 months only.
Patients can meet criteria for delirium by CAM by having acute onset of inattention and either disorganized thinking or altered level of consciousness. Patients without acute onset can also meet criteria for delirium if inattention, disorganized thinking, and altered level of consciousness are all present, with at least one factor judged to be fluctuating.13 The CAM has been widely applied12 and has been specifically used to evaluate elderly TKA patients receiving epidural analgesia and femoral nerve block.14
The ORSDS is a four-point scale that evaluates 12 symptoms (nausea, vomiting, constipation, difficulty passing urine, difficulty concentrating, drowsiness or difficulty staying awake, feeling lightheaded or dizzy, feeling confused, feelings of general fatigue or weakness, itchiness, dry mouth, and headache) via three symptom distress dimensions (frequency, severity, and bothersomeness).15 It is validated for use after orthopaedic surgery, specifically including TKA patients receiving epidural analgesia and femoral nerve block.11
The LANSS has seven yes/no questions about five symptoms and two signs associated with neuropathic pain; a self-administered LANSS was validated to identify neuropathic pain.16 The LANSS was used as the primary outcome (at 3 and 6 months) to evaluate pregabalin administration among TKA patients who received epidural analgesia.2
Study data were collected and managed using REDCap electronic data capture tools hosted at the Hospital for Special Surgery.17
Statistical analysis
An observational pilot study conducted by J.T.Y. at the Hospital for Special Surgery from May 19, 2010 to May 18, 2011 in anticipation of powering a randomized interventional trial found a mean (sd) NRS pain score with activity of 4.8 (2.3) and at rest 2.4 (1.9) at 2 weeks after TKA in 40 patients (J.T.Y., unpublished data). We expected mean NRS pain scores with flexion on POD14 (the primary outcome) of 4.8, 4.0, 3.0, and 2.0 in the 0, 50, 100, and 150 mg pregabalin groups, respectively, with a within-group sd of 2.5 points. We calculated that 30 patients per arm (120 patients in total) would provide 99% power with two-sided α of 0.05 to detect a linear dose–response relationship between pregabalin dose and NRS pain with flexion using anova. We chose a sample size large enough to achieve high power for the primary outcome to allow for precise estimation of postoperative sedation and confusion rates. There were no stopping guidelines or interim analyses.
The primary efficacy analysis used all available data, with each patient analysed in the group to which they were originally assigned. Continuous outcome variables were compared between treatment arms via omnibus and linear-trend anovas. Effect sizes corresponding with anovas were quantified as η2 values along with 95% confidence intervals (CIs). Ordinal variables were compared between groups with Kruskal–Wallis and Jonckheere–Terpstra tests, and binary variables were analysed using χ2 or Fisher's exact tests and Cochran–Armitage trend tests. Continuous data measured at multiple time points were analysed with the generalized estimating equations method (GEE)18,19 using an autoregressive [AR(1)] correlation structure and adjusting for patient characteristics and baseline value, where applicable. Bang blinding indices were calculated along with 95% CIs to assess the success of blinding.20
As a result of the unexpectedly low compliance rate by POD14, we performed two per-protocol analyses of the primary outcome (NRS pain with flexion scores on POD14) as follows: (i) including only patients who took at least 70% of capsules;21 and (ii) including only patients who took 100% of capsules. We performed post hoc power calculations to re-examine our power, given the reduced sample sizes. Compliance rates by POD14 were tested for linear and quadratic trends using logistic regression.
All statistical analyses were performed with SAS Version 9.3 (SAS Institute, Cary, NC, USA). All statistical tests were two sided, with a value of P<0.05 considered statistically significant.
Results
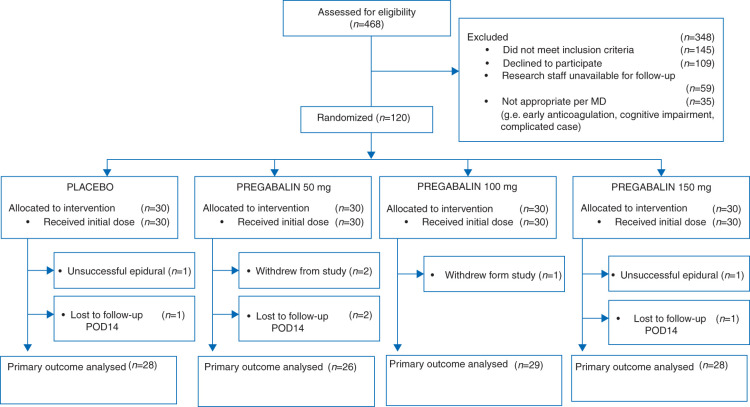
One hundred and twenty patients were enrolled (Fig. 1 and Table 1). Two patients had failed epidurals. The primary outcome was analysed in 111 patients.
Fig 1.
Consolidated Standards of Reporting Trials diagram of patient flow through the study. POD, postoperative day.
Table 1.
Patient characteristics. BID, twice daily
| Characteristic | Pregabalin (0 mg; n=30) |
Pregabalin (50 mg BID; n=30) |
Pregabalin (100 mg BID; n=30) | Pregabalin (150 mg BID; n=30) |
|---|---|---|---|---|
| Age [yr; mean (range)] | 66 (34–79) | 67 (54–77) | 65 (53–79) | 68 (44–80) |
| Sex (male/female; %) | 53%/47% | 40%/60% | 57%/43% | 23%/77% |
| BMI [kg m−2; mean (sd)] | 32 (5) | 32 (6) | 32 (6) | 30 (7) |
| Race | ||||
| Caucasian | 27 | 23 | 28 | 27 |
| Other | 3 | 7 | 2 | 3 |
| ASA (I/II/III) | 0/26/4 | 1/20/9 | 2/23/5 | 1/22/7 |
Pain outcomes and opioid use
Pregabalin did not produce an overall difference among groups for POD14 NRS pain score with flexion (the primary outcome). Moreover, pregabalin did not produce a dose-dependent decrease in POD14 NRS pain score with flexion (Table 2). Effect sizes (η2 values) indicated that 2% of the variability in POD14 flexion NRS score can be explained by pregabalin dose.
Table 2.
Pain scores. BID, twice daily; CI, confidence interval; GEE, generalized estimating equations; POD, postoperative day
| Time of assessment | Analysis type | Pregabalin (0 mg) |
Pregabalin (50 mg BID) | Pregabalin (100 mg BID) | Pregabalin (150 mg BID) | Omnibus |
Linear trend |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=28) | (n=26) | (n=29) | (n=28) | η2 (95% CI) | P-value | η2 (95% CI) | P-value | |||
| Pain with flexion | POD14 | Intent to treat | 4.0 (2.3) | 4.8 (2.1) | 4.5 (2.4) | 4.0 (2.1) | 0.02 (0, 0.07) | 0.55 | 0 (0–0.03) | 0.90 |
| Pain with ambulation | POD14 | Intent to treat | 3.6 (2.0) | 3.8 (1.6) | 3.5 (1.8) | 3.1 (1.9) | 0.02 (0, 0.07) | 0.57 | 0.01 (0–0.08) | 0.23 |
| Pain at rest | POD14 | Intent to treat | 2.9 (2.3) | 2.9 (2.0) | 2.8 (1.8) | 2.7 (2.1) | 0 (0, 0) | 0.98 | 0 (0–0.05) | 0.69 |
| GEE: pain with flexion | POD1, 3, and 14, and 3 months | Intent to treat | – | – | – | – | – | 0.72 | – | 0.47 |
| GEE: pain with ambulation | POD1, 3, and 14, and 3 months | Intent to treat | – | – | – | – | – | 0.59 | – | 0.17 |
| GEE: pain at rest | POD1, 3, and 14, and 3 months | Intent to treat | – | – | – | – | – | 0.91 | – | 0.99 |
| (n=15) | (n=23) | (n=23) | (n=17) | |||||||
| Pain with flexion | POD14 | Per protocol (≥70% compliant) | 3.9 (2.2) | 4.7 (2.1) | 4.9 (2.3) | 3.2 (1.7) | 0.10 (0, 0.21) | 0.05 | 0.01 (0, 0.09) | 0.40 |
| Pain with ambulation | POD14 | Per protocol (≥70% compliant) | 3.3 (2.2) | 3.6 (1.7) | 3.7 (1.9) | 2.4 (1.6) | 0.07 (0, 0.18) | 0.13 | 0.02 (0, 0.12) | 0.18 |
| Pain at rest | POD14 | Per protocol (≥70% compliant) | 2.8 (2.4) | 3.0 (2.0) | 2.8 (1.6) | 2.4 (2.0) | 0.01 (0, 0.06) | 0.81 | 0.01 (0, 0.08) | 0.49 |
| GEE: pain with flexion | POD1, 3, and 14, and 3 months | Per protocol (≥70% compliant) | – | – | – | – | – | 0.59 | – | 0.30 |
| GEE: pain with ambulation | POD1, 3, and 14, and 3 months | Per protocol (≥70% compliant) | – | – | – | – | – | 0.35 | – | 0.07 |
| GEE: pain at rest | POD1, 3, and 14, and 3 months | Per protocol (≥70% compliant) | – | – | – | – | – | 0.97 | – | 0.82 |
| (n=15) | (n=21) | (n=21) | (n=17) | |||||||
| Pain with flexion | POD14 | Per protocol (100% compliant) | 3.9 (2.2) | 4.8 (2.2) | 4.9 (2.5) | 3.2 (1.7) | 0.10 (0, 0.21) | 0.07 | 0.01 (0, 0.10) | 0.38 |
| Pain with ambulation | POD14 | Per protocol (100% compliant) | 3.3 (2.2) | 3.6 (1.7) | 3.6 (1.9) | 2.4 (1.6) | 0.07 (0, 0.17) | 0.19 | 0.03 (0, 0.13) | 0.18 |
| Pain at rest | POD14 | Per protocol (100% compliant) | 2.8 (2.4) | 3.0 (1.9) | 2.7 (1.7) | 2.4 (2.0) | 0.01 (0, 0.07) | 0.79 | 0.01 (0, 0.09) | 0.46 |
| GEE: pain with flexion | POD1, 3, and 14, and 3 months | Per protocol (100% compliant) | – | – | – | – | – | 0.57 | – | 0.32 |
| GEE: pain with ambulation | POD1, 3, and 14, and 3 months | Per protocol (100% compliant) | – | – | – | – | – | 0.28 | – | 0.05 |
| GEE: pain at rest | POD1, 3, and 14, and 3 months | Per protocol (100% compliant) | – | – | – | – | – | 0.99 | – | 0.78 |
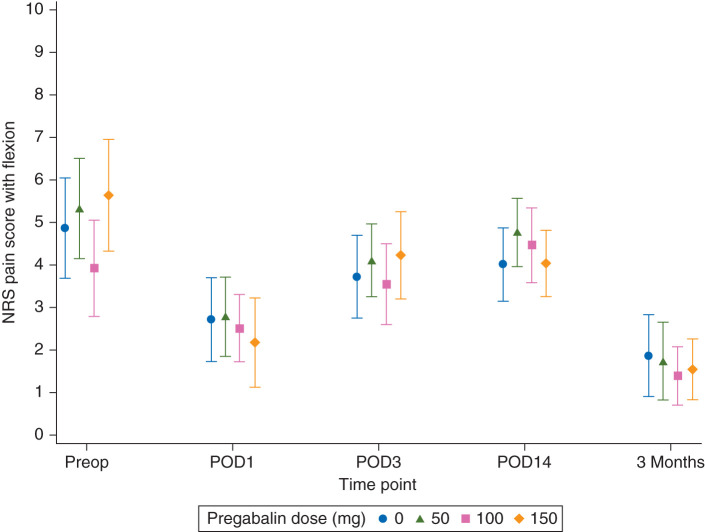
The GEE analysis of NRS pain score with flexion at all postoperative time points (POD1, 3, and 14, and 3 months) did not suggest an interaction between time point and pregabalin dose (i.e. the relationship between pregabalin dose and NRS remained constant over time). The GEE analysis found no evidence of a difference in mean NRS pain scores with flexion between treatment groups, after taking into account the postoperative longitudinal measurements and adjusting for age, sex, BMI, and baseline score (Table 2 and Fig. 2).
Fig 2.
Pain scores with flexion over time. Data are plotted as means with 95% confidence intervals. POD, postoperative day; NRS, Numeric Rating Scale pain score.
Pregabalin did not produce an overall difference among groups or a dose-dependent decrease in NRS pain scores with ambulation or at rest on POD14 (Table 2). The GEE analysis also showed no differences for pain scores with ambulation and at rest over time (Table 2).
The overall incidence of neuropathic pain at 3 months was low [3/93; 3.2% (95% CI 1.1–9.0)]. There was no evidence of a difference in LANSS between groups (Table 3).
Table 3.
Confusion, drowsiness, nausea, length of stay, satisfaction, LANSS, and compliance. BID, twice daily; CAM, confusion assessment method; LANSS, Leeds Assessment of Neuropathic Symptoms and Signs; ORSDS, Opioid-Related Symptom Distress Scale; POD, postoperative day; Q, quartile
| Parameter | Pregabalin (0 mg) | Pregabalin (50 mg BID) | Pregabalin (100 mg BID) | Pregabalin (150 mg BID) | Omnibus |
P-value linear trend |
Quadratic trend |
|---|---|---|---|---|---|---|---|
| Neuropathic pain [LANSS; median (Q1, Q3)] | 0 (0, 3) | 0 (0, 6) | 0 (0, 0) | 0 (0, 3) | 0.30 | 0.94 | – |
| Side-effect composite score, POD1 [ORSDS; median (Q1, Q3)] | 0.4 (0.2, 0.7) | 0.4 (0.2, 0.6) | 0.5 (0.2, 0.7) | 0.4 (0.2, 0.8) | 0.84 | 0.97 | – |
| Side-effect composite score, POD14 [ORSDS; median (Q1, Q3)] | 0.3 (0.1, 0.5) | 0.3 (0.1, 0.5) | 0.3 (0.1, 0.5) | 0.3 (0, 0.4) | |||
| Drowsiness, POD1 [ORSDS; n/total (%)] | 10/29 (34.5%) | 11/29 (37.9%) | 16/29 (55.2%) | 17/29 (58.6%) | 0.16 | 0.03 | – |
| Drowsiness, POD14 [ORSDS; n/total (%)] | 8/28 (28.6%) | 6/26 (23.1%) | 12/29 (41.4%) | 10/28 (35.7%) | 0.49 | 0.32 | – |
| Nausea, POD1 [ORSDS; n/total (%)] | 10/29 (34.5%) | 12/29 (41.4%) | 13/29 (44.8%) | 9/29 (31.0%) | 0.69 | 0.86 | – |
| Confusion, POD1 [CAM; n/total (%)] | 0/29 (0%) | 1/28 (3.5%) | 0/29 (0%) | 1/28 (3.5%) | 0.99 | 0.75 | – |
| Satisfaction, POD14 [median (Q1, Q3)] | 9 (8, 10) | 8 (7, 10) | 8 (5, 9) | 8 (6, 9.3) | 0.07 | 0.02 | – |
| Length of stay [days; median (Q1, Q3)] | 3 (3, 4) | 4 (3, 4) | 3 (3, 4) | 4 (3, 4) | 0.17 | 0.84 | – |
| Compliance | |||||||
| POD14≥70% compliant [n/total (%)] | 15/29 (51.7%) | 23/30 (76.7%) | 23/30 (76.7%) | 17/29 (58.6%) | 0.09 | 0.62 | 0.01 |
| POD14 100% compliant [n/total (%)] | 15/29 (51.7%) | 21/30 (70.0%) | 21/30 (70.0%) | 17/29 (58.6%) | 0.38 | 0.62 | 0.10 |
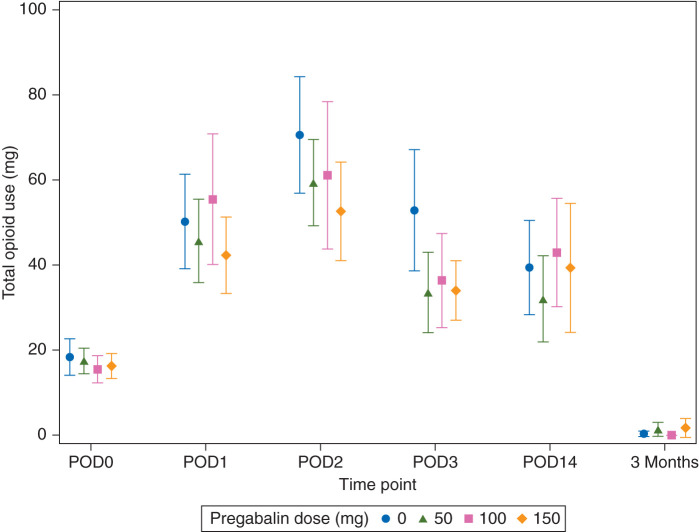
There was no evidence of an association between pregabalin dose and total opioid use after taking into account longitudinal measurements and adjusting for patient characteristics (omnibus P=0.59, linear trend P=0.33, Fig. 3; total opioid use, POD0, 1, 2, 3, and 14, and 3 months). Total opioid use represents daily opioid use on POD0, 1, 2, 3, and 14, and 3 months.
Fig 3.
Opioid use over time. Data are plotted as means with 95% confidence intervals. POD, postoperative day.
Side-effects and other secondary outcomes
The ORSDS evaluated side-effects on POD1, 3, and 14 (Table 3). The only dose-dependent effect was an increase in drowsiness on POD1.
Confusion, as assessed by CAM on POD1, was rare, and there was no evidence of a difference between groups (Table 3).
There was a dose-dependent decrease in satisfaction with higher doses of pregabalin. There was no evidence of a difference in hospital length of stay among groups (Table 3).
Although most patients (73%) were unwilling to guess treatment assignment, there was a statistically significant excess of correct guesses of treatment assignment by patients taking pregabalin. The Bang blinding index20 was 0.10 (pregabalin; 95% CI 0.01–0.19, P=0.041) and −0.07 (placebo; 95% CI −0.23 to 0.09, P=0.76). The blinding index can range from 1 (complete unblinding) to −1 (opposite guessing); blinding index values near 0 indicate random guessing and support successful blinding.
Protocol compliance and per-protocol analysis
Protocol compliance can be categorized as ‘perfect compliance’, ‘partial compliance’, and ‘non-compliance.’22 Partial compliance can be defined as taking at least 70% of intended drug doses.21 Compliance rates were reasonable by POD3 (94% of patients were at least 70% compliant, and 91% of patients were 100% compliant).
Eighteen patients discontinued pregabalin in the hospital for the following reasons: placebo group, one discontinuation (patient unwilling to continue); 50 mg group, five discontinuations [one for confusion, one for difficulty concentrating and unusual dreams (patient requested discontinuation), and three unwilling to continue]; 100 mg group, four discontinuations (one for cardiac arrest, one for sedation and hallucinations, and two unwilling to continue); and 150 mg group, eight discontinuations [six for sedation or confusion, one for atrial fibrillation (patient requested discontinuation), and one unwilling to continue].
By POD14, 66% of patients took at least 70% of study pills and 63% of patients took 100% of study pills (Table 3). Exploratory per-protocol analyses of NRS pain with flexion scores using either (i) patients who took at least 70% of capsules or (ii) patients who took 100% of capsules found no difference in NRS pain scores with flexion between treatment groups (Table 2). These results are similar to those of the primary efficacy analysis. The sample sizes available for the per-protocol analyses provide at least 80% power to find a linear trend or an overall difference between treatment groups (at the a priori estimates of mean and sd of NRS pain score with flexion and α=0.05).
Discussion
This dose–response study was conducted with the hope of finding a pregabalin dose that would reduce pain without causing major side-effects. The first study hypothesis, that pregabalin reduces pain 2 weeks after TKA, was not supported by the trial. The second study hypothesis, that pregabalin increases rates of drowsiness and confusion on POD1, was confirmed for drowsiness, but not for confusion. No beneficial effects of pregabalin were noted; no reduction occurred in acute pain, chronic pain, opioid consumption, or analgesic side-effects.
Pregabalin was associated with two adverse effects: increased sedation and dose-dependent decreased patient satisfaction. The pregabalin-associated reduction in satisfaction is probably clinically relevant. Satisfaction scores [median (quartile 1, quartile 3)] decreased from 9 (8, 10) for placebo to 8 (6, 9.3) for 300 mg daily pregabalin. The minimal clinically significant difference on a 0–100 visual analog satisfaction scale was 7–11 mm,23 among emergency department patients.
This study reaches similar conclusions to the conclusions found in a gabapentin study. Gabapentin (like pregabalin, a gabapentinoid) did not reduce morphine consumption or pain scores and did not improve patient satisfaction after TKA.24 However, Buvanendran and colleagues2 showed that administration of 300 mg pregabalin daily after TKA reduced pain scores but increased sedation and confusion on POD1.
The discordant result may be attributable to the many differences between this study and the study by Buvanendran and colleagues.2 Buvanendran and colleagues2 reported that NRS pain scores at discharge with passive range of motion were 6.0 (2.3) for pregabalin and 7.0 (2.2) for control patients. The POD3 pain scores with flexion among study patients were lower, at 4.2 (2.9) for pregabalin 150 mg BID and 3.7 (2.6) for placebo. The difference in pain control may be related to different analgesic protocols. Buvanendran and colleagues2 used epidural analgesia (bupivacaine plus fentanyl, Lake Forest, IL, USA), oral opioids, and celecoxib; in the present study, we used a single-injection bupivacaine (Lake Forest, IL, USA) femoral nerve block, epidural analgesia (bupivacaine plus hydromorphone, Lake Forest, IL, USA), oral opioids, dexamethasone, and meloxicam. The primary outcome of the study by Buvanendran and colleagues2 was neuropathic pain at 6 months, whereas in the present study we used the primary outcome of pain (NRS) at 14 days. Buvanendran and colleagues2 found that pregabalin reduced the incidence of neuropathic pain at 3 months from 8.7 to 0%. In the present study, the overall rate of neuropathic pain at 3 months was lower (3.2%) and was not influenced by pregabalin.
Pain after TKA can be severe if not treated aggressively.25 High levels of acute pain after TKA are associated with increased rates of chronic postsurgical pain,10 suggesting that improved treatment of acute pain may reduce the incidence of chronic pain. However, pressure to mobilize patients rapidly may preclude the use of analgesic regimens that interfere with participation in postoperative physical therapy. The results of the present study suggest that addition of pregabalin to the described analgesic regimen will not improve patient outcomes, decreases satisfaction, and increases sedation. The increased sedation could interfere with physical therapy.
Strengths of the present study include the design; this was a prospective, blinded, randomized dose–response trial, with pregabalin prescribed for more than 14 days. Numerous outcomes were assessed (pain scores at multiple times in multiple conditions, opioid intake, analgesic side-effects, and patient satisfaction).
Weaknesses of the study include insufficient power for some secondary outcomes, incomplete patient compliance, and the fact that this is a negative study for the primary outcome. Trials that fail to support their primary outcomes have a longer time to publication and are less likely to be published.26 A recent pregabalin meta-analysis was not definitive because publication bias may have exaggerated the benefits of pregabalin,6 supporting publication of negative pregabalin trials to reduce bias. Power calculations are prominent in evaluation of negative studies. The data used for the power calculation [NRS pain score of 4.8 (2.3)] were comparable to the observed primary result [4.0 (2.3), 4.8 (2.1), 4.5 (2.4), and 4.0 (2.1)]. This suggests that the preliminary data used for the power analysis were valid and appropriate. Besides referring to the power analysis, it can be useful to compare study size among similar studies. This study had 120 patients; the three studies that demonstrated reduction of chronic pain by pregabalin6 had 40,27 70,28 or 2402 subjects. Thus, this study does not have an unusually small size compared with its peers.
Compliance in clinical trials is often not reported.29 The patient adherence rate in the present study was 63% for 14 days. To place this in context, withdrawal rates in studies of antiepileptic drugs for chronic pain are typically 30% or more for 12 weeks.30 In the present study, rates of discontinuation were not apparently influenced by group; rates were similar in patients receiving placebo (50% compliant) and the highest dose of pregabalin (56.7% compliant). This suggests that non-compliance was not attributable to side-effects of pregabalin. Protocol adherence diminished after discharge. Many outpatients do not take prescribed medication. For example, after an acute myocardial infarction the adherence rates for cardiovascular drugs are 66–76%.31 This study suggests that, in the real world outside of hospital-based clinical trials, if pregabalin is prescribed for pain after TKA, many patients will not take it for the full 14 days. Per-protocol analysis indicated that among compliant patients no analgesic benefit was noted, which suggests that even if we could compel compliance, the pregabalin would have no benefit.
Future research on pregabalin for TKA patients could focus on different analgesic regimens, on selection of patient groups likely to benefit, on use of pregabalin for patients reporting difficulties with postoperative pain management, or on alternative scheduling of pregabalin.
Pregabalin might improve analgesia after TKA if given to patients receiving a different, less comprehensive analgesic regimen. This study used an optimized analgesic regimen consisting of neuraxial anaesthesia, epidural analgesia combined with peripheral nerve block,7 and multimodal oral analgesia. Total knee arthroplasty patients managed with general anaesthesia and opioids have high pain scores25 that might be improved by addition of pregabalin. Alternative anaesthetic and analgesic regimens could include combinations of general anaesthesia, i.v. opioids, continuous nerve block (femoral, adductor canal, or sciatic), or local infiltration analgesia.
In summary, this study failed to find benefit from pregabalin for analgesia after TKA. Pregabalin was associated with increased drowsiness and reduced satisfaction with analgesia.
Authors' contributions
Study design: J.T.Y., Y.L., D.J.M., E.A.G., M.M.A., D.E.P., R.L.K., K.M.J.-E., A.S.R., D.D.B., G.H.W.
Participant recruitment: J.T.Y., Y.L., D.J.M., E.A.G., M.M.A., D.E.P., R.L.K., K.M.J.-E., A.S.R., D.D.B., G.H.W., A.K.G., J.C.
Data collection: A.K.G., J.C.
Data analysis: J.T.Y., K.G.F., A.K.G.
Drafting the manuscript: J.T.Y., K.G.F., A.K.G.
Declaration of interest
J.T.Y. is an editorial review consultant: Acta Anaesthesiologica Scandinavica 2013; Anesthesia and Analgesia 1994–1998, 2004 to the present, guest editor 2011 to the present; Anesthesiology 1998, 2014; British Journal of Anaesthesia 2008, 2013; Clinical Orthopaedics and Related Research 2013, 2014; HSS Journal (editor) 2009 to the present; Regional Anesthesia and Pain Medicine 2009 to the present, associate editor 2012 to the present. M.M.A. is a consultant for Biomet Inc. D.E.P. has received royalties and is a consultant for MAKO Surgical; a Board Member of The Hip Society; and a consultant for Medical Compression Systems, Israel. A.S.R. has conducted research for Stryker; holds stocks in Nova; and is a consultant for Depuy, Conformis, Mako, Ceramtec, Medtronic, Pipeline, and Convatec. G.H.W. is a paid consultant and a paid speaker for, and receives research funding from, Exactech, Stryker, and DJO Global, Inc.; and is a board member of Eastern Orthopaedic Association and the Knee Society. Other authors: none declared.
Funding
Hospital for Special Surgery Anesthesiology Department, New York, NY, USA (Research and Education Fund); the REDCap electronic data capture tools are funded by the CTSC grant (grant number UL1 TR000457-06) from the National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD, USA.
Acknowledgements
The authors would like to thank Heather Reel for assistance with data collection, Dr Heejung Bang for initial statistical consultation, and Matthew Rade for assistance with the early phases of the study.
References
- 1.Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology 2013; 119: 1215–21 [DOI] [PubMed] [Google Scholar]
- 2.Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg 2010; 110: 199–207 [DOI] [PubMed] [Google Scholar]
- 3.Chaparro LE, Clarke H, Valdes PA, Mira M, Duque L, Mitsakakis N. Adding pregabalin to a multimodal analgesic regimen does not reduce pain scores following cosmetic surgery: a randomized trial. J Anesth 2012; 26: 829–35 [DOI] [PubMed] [Google Scholar]
- 4.YaDeau JT, Paroli L, Kahn RL, et al. Addition of pregabalin to multimodal analgesic therapy following ankle surgery: a randomized double-blind, placebo-controlled trial. Reg Anesth Pain Med 2012; 37: 302–7 [DOI] [PubMed] [Google Scholar]
- 5.Engelman E, Cateloy F. Efficacy and safety of perioperative pregabalin for post-operative pain: a meta-analysis of randomized-controlled trials. Acta Anaesthesiol Scand 2011; 55: 927–43 [DOI] [PubMed] [Google Scholar]
- 6.Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012; 115: 428–42 [DOI] [PubMed] [Google Scholar]
- 7.YaDeau JT, Cahill JB, Zawadsky MW, et al. The effects of femoral nerve blockade in conjunction with epidural analgesia after total knee arthroplasty. Anesth Analg 2005; 101: 891–5 [DOI] [PubMed] [Google Scholar]
- 8.Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res 2003; 416: 27–36 [DOI] [PubMed] [Google Scholar]
- 9.Buvanendran A, Kroin JS, Tuman KJ, et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA 2003; 290: 2411–8 [DOI] [PubMed] [Google Scholar]
- 10.Liu SS, Buvanendran A, Rathmell JP, et al. A cross-sectional survey on prevalence and risk factors for persistent postsurgical pain 1 year after total hip and knee replacement. Reg Anesth Pain Med 2012; 37: 415–22 [DOI] [PubMed] [Google Scholar]
- 11.YaDeau JT, Liu SS, Rade MC, Marcello D, Liguori GA. Performance characteristics and validation of the Opioid-Related Symptom Distress Scale for evaluation of analgesic side effects after orthopedic surgery. Anesth Analg 2011; 113: 369–77 [DOI] [PubMed] [Google Scholar]
- 12.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–8 [DOI] [PubMed] [Google Scholar]
- 13.Inouye SK. The Confusion Assessment Method (CAM): Training Manual and Coding Guide. New Haven: Yale University School of Medicine, 2003 [Google Scholar]
- 14.Rade MC, YaDeau JT, Ford C, Reid MC. Postoperative delirium in elderly patients after elective hip or knee arthroplasty performed under regional anesthesia. HSS J 2011; 7: 151–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apfelbaum JL, Gan TJ, Zhao S, Hanna DB, Chen C. Reliability and validity of the perioperative opioid-related symptom distress scale. Anesth Analg 2004; 99: 699–709 [DOI] [PubMed] [Google Scholar]
- 16.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS scores for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 2005; 6: 149–58 [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Mazumdar M, Memtsoudis SG. Beyond repeated-measures analysis of variance: advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med 2012; 37: 99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988; 44: 1049–60 [PubMed] [Google Scholar]
- 20.Bang H, Flaherty SP, Kolahi J, Park J. Blinding assessment in clinical trials: a review of statistical methods and a proposal of blinding assessment protocol. Clin Res Regul Aff 2010; 27: 42–51 [Google Scholar]
- 21.Cnaan A, Zhao H, Silber JH. Measuring compliance and its effect on analyses in longitudinal clinical trials. Proceedings of the ENAR Spring Meeting—Biometric Section to Include ENAR & WNAR. Washington, DC, USA: 2002; 591–95 [Google Scholar]
- 22.Vander Stichele R. Measurement of patient compliance and the interpretation of randomized clinical trials. Eur J Clin Pharmacol 1991; 41: 27–35 [DOI] [PubMed] [Google Scholar]
- 23.Singer AJ, Thode HC., Jr Determination of the minimal clinically significant difference on a patient visual analog satisfaction scale. Acad Emerg Med 1998; 5: 1007–11 [DOI] [PubMed] [Google Scholar]
- 24.Paul JE, Nantha-Aree M, Buckley N, et al. Gabapentin does not improve multimodal analgesia outcomes for total knee arthroplasty: a randomized controlled trial. Can J Anaesth 2013; 60: 423–31 [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Boctor B, Verner J. The effect of single-injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Reg Anesth Pain Med 2002; 27: 139–44 [DOI] [PubMed] [Google Scholar]
- 26.Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. Br Med J 1997; 315: 640–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg 2010; 110: 1180–5 [DOI] [PubMed] [Google Scholar]
- 28.Pesonen A, Suojaranta-Ylinen R, Hammarén E, et al. Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: a randomized placebo-controlled trial. Br J Anaesth 2011; 106: 873–81 [DOI] [PubMed] [Google Scholar]
- 29.Gossec L, Tubach F, Dougados M, Ravaud P. Reporting of adherence to medication in recent randomized controlled trials of 6 chronic diseases: a systematic literature review. Am J Med Sci 2007; 334: 248–54 [DOI] [PubMed] [Google Scholar]
- 30.Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA 2014; 312: 182–331 [DOI] [PubMed] [Google Scholar]
- 31.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009; 119: 3028–35 [DOI] [PubMed] [Google Scholar]