Abstract
Objectives
The objective of this study was to determine the distribution and genetic basis of trimethoprim resistance in Actinobacillus pleuropneumoniae isolates from pigs in England.
Methods
Clinical isolates collected between 1998 and 2011 were tested for resistance to trimethoprim and sulphonamide. The genetic basis of trimethoprim resistance was determined by shotgun WGS analysis and the subsequent isolation and sequencing of plasmids.
Results
A total of 16 (out of 106) A. pleuropneumoniae isolates were resistant to both trimethoprim (MIC >32 mg/L) and sulfisoxazole (MIC ≥256 mg/L), and a further 32 were resistant only to sulfisoxazole (MIC ≥256 mg/L). Genome sequence data for the trimethoprim-resistant isolates revealed the presence of the dfrA14 dihydrofolate reductase gene. The distribution of plasmid sequences in multiple contigs suggested the presence of two distinct dfrA14-containing plasmids in different isolates, which was confirmed by plasmid isolation and sequencing. Both plasmids encoded mobilization genes, the sulphonamide resistance gene sul2, as well as dfrA14 inserted into strA, a streptomycin-resistance-associated gene, although the gene order differed between the two plasmids. One of the plasmids further encoded the strB streptomycin-resistance-associated gene.
Conclusions
This is the first description of mobilizable plasmids conferring trimethoprim resistance in A. pleuropneumoniae and, to our knowledge, the first report of dfrA14 in any member of the Pasteurellaceae. The identification of dfrA14 conferring trimethoprim resistance in A. pleuropneumoniae isolates will facilitate PCR screens for resistance to this important antimicrobial.
Keywords: animal infections, antibiotic resistance, respiratory tract
Introduction
Actinobacillus pleuropneumoniae causes porcine pleuropneumonia, an economically important endemic disease that can be difficult to control.1 Good husbandry practices and vaccination can help to reduce the incidence of acute disease, and the early use of effective antimicrobials is essential to limit its spread and severity. A knowledge of the antimicrobial susceptibility patterns of A. pleuropneumoniae is important so that informed treatment decisions can be made.
In the UK, the most commonly used antimicrobials for the treatment of food animals (86% of which are used for pigs and poultry) are tetracyclines, β-lactams and trimethoprim/sulphonamides.2 Sulphonamides have been widely used since the 1930s for the treatment of both human and veterinary diseases.3,4 Trimethoprim, introduced in the 1960s, is often coadministered with sulphonamides.3
Resistance to both trimethoprim and sulphonamides can be mediated either by mutations in the chromosomally encoded target enzymes (dihydropteroate synthase and dihydrofolate reductase, respectively) or by the acquisition of transferable genes encoding alternative drug-insensitive enzymes.3,4 There are three known genes encoding alternative dihydropteroate synthases (sul1, sul2 and sul3)5 and >30 dfr genes encoding trimethoprim-insensitive dihydrofolate reductases.6
Sulphonamide resistance conferred by sul2, carried on small plasmids, has been reported for A. pleuropneumoniae7–9 and other Pasteurellaceae.10 However, little is known regarding the genetic basis of trimethoprim resistance in the Pasteurellaceae. Single bovine and porcine isolates of Pasteurella multocida11 and Pasteurella aerogenes12 have harboured plasmids carrying dfrA20 and dfrA1, respectively, whereas trimethoprim-resistant Haemophilus influenzae has been shown to have mutations in the chromosomally encoded dihydrofolate reductase.13
In this study, we have identified the genetic basis of trimethoprim resistance in A. pleuropneumoniae using WGS followed by plasmid isolation and confirmatory sequencing. Two distinct plasmids carrying dfrA14 were found, the first known description of this gene in the Pasteurellaceae.
Materials and methods
Bacterial strains and antimicrobial resistance testing
A total of 106 clinical isolates of A. pleuropneumoniae, cultured from the pneumonic lungs of pigs submitted for diagnostic investigation to the then Animal Health and Veterinary Laboratory Agency (now Animal and Plant Health Agency) diagnostic laboratories in England between 1998 and 2011, were selected for study. The majority of isolates were from 2005–10 (20, 26, 11, 12, 14 and 8 isolates, respectively), with none from 2000–01 and only 1–4 from each of the other years. Serovars 2 (11%), 6 (6.5%), 7 (8.5%), 8 (72.0%) and 12 (2.0%) were represented, reflecting the serovar distribution in the UK.14 A. pleuropneumoniae MIDG2331 is a plasmid-free serovar 8 clinical isolate that was cultured from pneumonic pig lungs in 1995. MIDG2331 was made NAD-independent by the chromosomal insertion (replacing part of ureC) of the Haemophilus ducreyi nadV gene, yielding MIDG2331ΔureC::nadV.15 All the strains were grown at 37°C with 5% CO2 on brain heart infusion (BHI; Difco) agar supplemented with 0.01% NAD and, when required, with trimethoprim (10 mg/L).
For all isolates, MICs were determined for trimethoprim and sulfisoxazole by agar dilution susceptibility testing, according to the CLSI M31-A3 guidance.16
Genome sequencing and analysis
Genomic DNA was extracted from the 16 trimethoprim-resistant (MIC >32 mg/L) A. pleuropneumoniae isolates (Table 1) using the FastDNA Spin Kit (MP Biomedicals), according to the manufacturer's protocol for bacterial cells, and 0.5 μg was used for library preparation and sequencing as previously described.17
Table 1.
Genes identified by ResFinder in A. pleuropneumoniae isolates from the UK with resistance to trimethoprim and sulfisoxazole
| MIDG number | Year | Location | Serovar | Trimethoprim (mg/L) | Sulfisoxazole (mg/L) |
dfrA14a |
sul2b |
strAc |
strBd |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| contig | length | contig | length | contig | length | contig | length | ||||||
| 2356 | 1998 | Bury St Edmunds | 7 | >32 | >512 | 6 | 3451 | 6 | 3451 | 6 | 3451 | 6 | 3451 |
| 2657 | 2005 | Winchester | 8 | >32 | >512 | 31 | 1757 | 26 | 943 | 31 | 1757 | ||
| 2664 | 2005 | Bury St Edmunds | 8 | >32 | >512 | 65 | 1421 | 55 | 943 | ||||
| 3346 | 2005 | Thirsk | 8 | >32 | >512 | 48 | 1757 | 57 | 943 | 48 | 1757 | ||
| 3201 | 2006 | Bury St Edmunds | 8 | >32 | >512 | 10 | 1765 | 28 | 951 | 10 | 1765 | ||
| 3221 | 2006 | Bristol | 8 | >32 | 256 | 20 | 1761 | 54 | 947 | 20 | 1761 | ||
| 3349 | 2006 | Thirsk | 8 | >32 | >512 | 47 | 1421 | 60 | 943 | ||||
| 3224 | 2007 | Bury St Edmunds | 8 | >32 | >512 | 49 | 3429 | 49 | 3429 | 49 | 3429 | 49 | 3429 |
| 3232 | 2007 | Thirsk | 8 | >32 | >512 | 12 | 1761 | 20 | 947 | 12 | 1761 | ||
| 3370 | 2009 | Thirsk | 8 | >32 | >512 | 57 | 1759 | 106 | 945 | 57 | 1759 | ||
| 3371 | 2009 | Thirsk | 8 | >32 | >512 | 50 | 1759 | 95 | 945 | 50 | 1759 | ||
| 3372 | 2009 | Thirsk | 8 | >32 | >512 | 45 | 1759 | 102 | 945 | 45 | 1759 | ||
| 3378 | 2009 | Bury St Edmunds | 8 | >32 | >512 | 56 | 1753 | 15 | 4128 | 56 | 1753 | ||
| 3388 | 2009 | Thirsk | 8 | >32 | >512 | 6 | 1777 | 22 | 963 | 6 | 1777 | ||
| 3389 | 2009 | Thirsk | 8 | >32 | >512 | 30 | 1610 | 16 | 961 | 30 | 1610 | ||
| 3395 | 2010 | Thirsk | 8 | >32 | >512 | 5 | 636 | 26 | 963 | 74 | 442 | ||
a99.8% identity (483/483 bp) with dfrA14 from Salmonella enterica subsp. enterica serovar Typhimurium (DQ388123).
b100% identity (816/816 bp) with sul2 from Acinetobacter bereziniae (GQ421466).
c100% identity (529/804 bp) with strA from a Shigella flexneri plasmid (AF321551) for MIDG2356 and MIDG3224, and 99.8% identity (529/804 bp) with strA from an Erwinia amylovora plasmid (M96392) for all others with strA (NB: in MIDG3395 only 512/804 bp of the gene were detected).
d99.9% identity (705/837 bp) with strB from an Erwinia amylovora plasmid (M96392).
ResFinder (www.genomicepidemiology.org) was used to identify acquired antimicrobial resistance genes (using a threshold of 98% identity) in the draft genomes. Contigs identified by ResFinder (Table 1) have been submitted to GenBank (accession numbers: contig006_MIDG2356 =KP196974; contig026_MIDG2657 = KP196975; contig031_MIDG2657 =KP196976; contig055_MIDG2664 = KP196977; contig065_MIDG2664 =KP196978; contig010_MIDG3201 = KP196979; contig028_MIDG3201 =KP196980; contig020_MIDG3221 = KP196981; contig054_MIDG3221 =KP196982; contig049_MIDG3224 = KP196983; contig012_MIDG3232 =KP196984; contig020_MIDG3232 = KP196985; contig048_MIDG3346 =KP196986; contig057_MIDG3346 = KP196987; contig047_MIDG3349 =KP196988; contig060_MIDG3349 = KP196989; contig106_MIDG3370 =KP196990; contig050_MIDG3371 = KP196991; contig095_MIDG3371 =KP196992; contig045_MIDG3372 = KP196993; contig102_MIDG3372 =KP196994; contig015_MIDG3378 = KP196995; contig056_MIDG3378 =KP196996; contig006_MIDG3388 = KP196997; contig022_MIDG3388 =KP196998; contig016_MIDG3389 = KP196999; contig030_MIDG3389 =KP197000; contig005_MIDG3395 = KP197001; contig026_MIDG3395 =KP197002; and contig074_MIDG3395 = KP197003).
Isolation and characterization of plasmids
Plasmids were extracted from A. pleuropneumoniae isolates MIDG3224 and MIDG3389, selected as representing two different patterns of resistance genes identified by ResFinder (Table 1), using the QIAprep Spin Miniprep kit (Qiagen). Attempts were made to transform plasmids into Escherichia coli Stellar cells (Clontech) by heat shock, with selection on LB agar containing trimethoprim (10 or 20 mg/L). The conjugal transfer of plasmids from MIDG3224 and MIDG3389 into MIDG2331ΔureC::nadV was carried out as previously described,18 with transconjugants selected on BHI agar (without NAD) supplemented with 10 mg/L trimethoprim.
The MICs of trimethoprim and sulfisoxazole were determined for selected trimethoprim-resistant transconjugants, as described above, and the presence of dfrA14, sul2 and nadV was determined by QiagenFast PCR (Qiagen) using primer pairs dfrA14_for (CATTGATAGCTGCGAAAGCGAAAAACGGC)/dfrA14_rev (ATCGTCGATAAGTGGAGCGTAGAGGC), sul2_for (TCAACATAACCTCGGACAGTTTCTC)/sul2_rev (GGGAATGCCATCTGCCTTGAGC) and nadV_for (CTAGTAACCGAGCCCGCCTAATGAG)/nadV_rev (GGCGGCCGCACTAGTGATTACAAG).
The complete sequences of plasmids pM3389T and pM3224T, isolated from transconjugants, were determined using a primer walking strategy (GenBank accession numbers pM3224T = KP197004 and pM3389T =KP197005). These sequences were subsequently used to search the draft genomes of the remaining trimethoprim-resistant isolates using BLASTn.
Results and discussion
Trimethoprim resistance (MIC >32 mg/L) was detected in 16 out of 106 A. pleuropneumoniae isolates, and all 16 were resistant to sulfisoxazole (MIC ≥256 mg/L) (Table 1). A further 32 isolates were resistant to sulfisoxazole only (data not shown), which is not surprising given that trimethoprim, often coadministered with sulphonamides, was introduced for use 30 years after sulphonamides. Co-resistance to trimethoprim and sulfisoxazole was found in serovar 7 and 8 isolates obtained from four different geographical locations in England as early as 1998 (1 out of 3 isolates), with the largest proportion identified in 2009 (6 out of 14 isolates).
ResFinder analysis (Table 1) of the draft genomes identified the trimethoprim resistance gene dfrA14 on contigs ranging from 636 to 3451 bp in all trimethoprim-resistant isolates. In all but two isolates (MIDG2664 and MIDG3349) a partial strA gene was identified on the same contig as dfrA14, and in two isolates (MIDG2356 and MIDG3224) sul2 and strB were also found on the same contig as dfrA14. The sul2 gene was identified on separate small contigs (768–963 bp) in all other isolates. BLASTx analysis of the dfrA14-containing contigs of MIDG2664 and MIDG3349 revealed partial strA sequences flanking the dfrA14 gene in both cases. Furthermore, alignments of the dfrA14-containing contigs showed that the strA5′-dfrA14-strA3′ sequences were identical in all 16 isolates, although the shorter contigs in MIDG2664 and MIDG3349 were missing the first 205/529 bp of the strA5′ sequence, which was not detected by ResFinder. Alignments of the dfrA14-containing contigs also suggested two different trimethoprim resistance plasmids: contigs from MIDG2356 and MIDG3224 were identical over the 3429 bp common to both, and contigs from the remaining isolates showed 100% identity where alignment was possible, given the different lengths of the contigs.
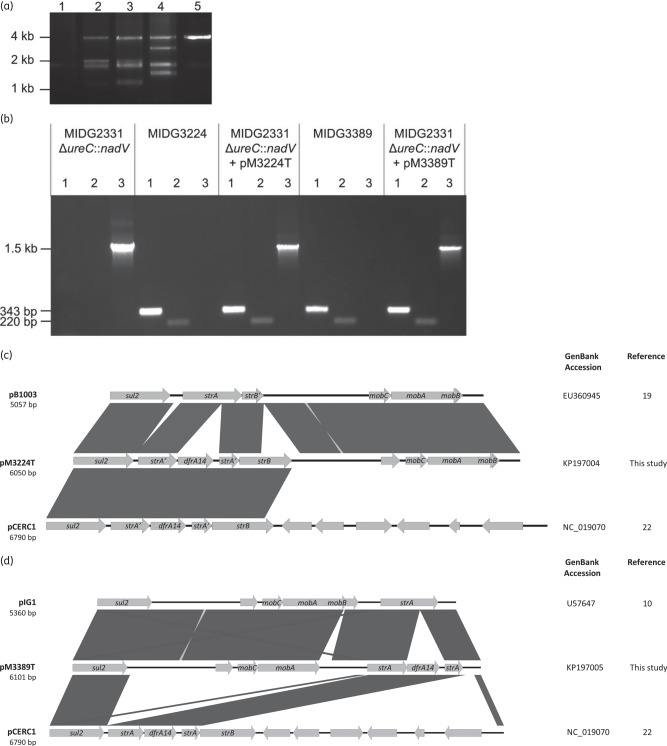
The distribution of sequences among the small contigs suggested the possibility of multiple plasmids sharing common sequences. No known plasmids were detected in the draft genomes using PlasmidFinder (www.genomicepidemiology.org), but an analysis of the endogenous plasmid profiles for MIDG3224 and MIDG3389 suggested multiple plasmids, at least in the latter (Figure 1a). Conjugal transfer from MIDG3224 and MIDG3389 into a plasmid-free recipient strain (MIDG2331ΔureC::nadV) was used to isolate trimethoprim resistance plasmids pM3224T and pM3389T prior to complete nucleotide sequencing (Figure 1a). Trimethoprim-resistant transconjugants were positive for dfrA14 and nadV by PCR, indicating the successful mobilization of plasmids from MIDG3224 and MIDG3389 into MIDG2331ΔureC::nadV (Figure 1b). Furthermore, the amplification of sul2 sequences from transconjugants suggested that both pM3224T and pM3389T also encode sulphonamide resistance (Figure 1b), and the MICs of trimethoprim and sulfisoxazole were the same for the transconjugants as for the donor strains. A primer walking strategy was used to determine the complete nucleotide sequences of pM3224T and pM3389T as representatives of the two different trimethoprim resistance plasmids indicated above.
Figure 1.
Isolation and characterization of newly identified dfrA14-containing A. pleuropneumoniae plasmids. (a) Comparison of plasmid extracts from MIDG2331ΔureC::nadV (Lane 1), conjugal donor strains (Lane 2 = MIDG3224 and Lane 4 = MIDG3389) and respective trimethoprim-resistant transconjugants, showing the transfer of plasmids (Lane 3 = pM3224T and Lane 5 = pM3389T) into MIDG2331ΔureC::nadV. (b) PCR amplification of dfrA14 (343 bp amplicon; Lane 1 in each section), sul2 (220 bp amplicon; Lane 2 in each section) and nadV (1.5 kb amplicon; Lane 3 in each section) from MIDG2331ΔureC::nadV, MIDG3224, MIDG2331ΔureC::nadV+pM3224T, MIDG3389 and MIDG2331ΔureC::nadV+pM3389T, as indicated for each section of the gel. (c) Schematic comparison of pM3224T with the most closely related Pasteurellaceae plasmid, pB1003, and pCERC1, a dfrA14-containing plasmid found in Enterobacteriaceae. (d) Schematic comparison of pM3389T with the most closely related Pasteurellaceae plasmid, pIG1, and pCERC1. Reading frames are indicated by arrows, with arrowheads showing the direction of transcription; only relevant genes have been annotated (sul2: sulphonamide resistance; strA, strB: streptomycin resistance; dfrA14: trimethoprim resistance; mobA, mobB, mobC: plasmid mobilization; strB′: partial strB; strA′: partial strA). Dark grey blocks between sequences indicate ≥99% nucleotide sequence identity.
Plasmid pM3224T (6050 bp) was found to share the greatest similarity (99% identity with 81% coverage) with pB1003 (accession no. EU360945) isolated from P. multocida from pigs in Spain19 (Figure 1c). These two plasmids have identical mobilization genes (306 bp mobC, 972 bp mobA and 261 bp mobB located in the 3′ end of mobA) that belong to the HEN family of relaxases common in mobilizable plasmids in the Pasteurellaceae.19,20 In pB1003, a complete (804 bp) strA and partial (294 bp) strB gene are found downstream of sul2, and a similar gene linkage has been reported in other Pasteurellaceae plasmids.10,21 In pM3224T, however, there is a 711 bp strB gene, and the strA gene is disrupted by the insertion of a 568 bp element carrying dfrA14, a gene arrangement that has previously been reported in plasmids pCERC1 (accession no. NC_019070; Figure 1c) and pSTOJO (accession no. NG_035503) from Enterobacteriaceae isolated from humans,22,23 pYR1521 (accession no. NG_041026) from Yersinia ruckeri isolated from fish24 and pRSB206 (accession no. NC_025062) from an uncultured bacterium from wastewater.25 All of these dfrA14-containing plasmids share an almost identical 3 kb region from sul2 to strB, although the strB gene is truncated (711/837 bp) in pM3224T, suggesting a common origin of this region, with recombination into the different plasmids. The insertion of dfrA14 in a secondary site within strA was first noted in pUK1329 isolated from an E. coli of human origin in Scotland in 1995 (accession no. Z50805) but only a 681 bp fragment was sequenced. Since then, this sequence has been detected in 6.8 kb plasmids in Enterobacteriaceae of human and animal origin from around the world,22 as well as 5 and 53 kb plasmids in Y. ruckeri24 and an uncultured bacterium,25 respectively, but it has not been described in plasmids from any member of the Pasteurellaceae.
The complete nucleotide sequence of pM3389T is 6101 bp and shares greatest similarity (99% identity with 87% coverage) with pIG1 (accession no. U57647) from P. aerogenes10 and an identical plasmid found in P. multocida HN0626 (Figure 1d). These previously identified 5360 bp plasmids encode the strA gene upstream of sul2, as well as the HEN mobilization genes mentioned above, although the mobA gene in these plasmids is 1131 bp in length, with a 273 bp mobB gene encoded within the 3′ end. In pM3389T, there is an insertion of 173 bp that disrupts the end of both mobA and mobB, resulting in a 924 bp mobA gene with an altered 3′ end and no functional mobB gene. In addition, the strA gene is disrupted by the same 568 bp dfrA14-carrying element described above. However, this is the first known description of this gene arrangement upstream of sul2, indicating the separate recombination of just the ΔstrA-dfrA14-ΔstrA cassette instead of the entire sul2-ΔstrA-dfrA14-ΔstrA-strB region.
In both pM3224T and pM3389T, there is an 823 bp sequence upstream of mobC with 99% identity to the putative oriV originally identified in pLS88 (accession no. L23118)27 and common in numerous Pasteurellaceae plasmids.21 Although plasmids with similar oriV regions have been reported to replicate in E. coli, attempts to transform pM3224T and pM3389T into E. coli Stellar cells by heat shock have not been successful. It is possible that these plasmids could be transformed into E. coli by electroporation, but this was not investigated as isolation of the plasmids was achieved by conjugation into MIDG2331ΔureC::nadV. A graphical analysis of the pM3224T and pM3389T sequences revealed that the region containing the oriV and mobilization genes has a GC content of 41%–42%, reflecting the average for Pasteurellaceae, whereas the regions containing the antimicrobial resistance genes have a GC content of 54%–55% and are likely of enterobacterial origin, as previously suggested for antimicrobial resistance genes in other Pasteurellaceae plasmids.28
When the complete sequences of pM3224T and pM3389T were used to search the draft genomes of the remaining trimethoprim-resistant isolates using BLASTn, contigs were identified that could be assembled into plasmids with high identity (99%–100%) to either the 6050 bp plasmid (MIDG2356 and MIDG3224) or the 6101 bp plasmid (all other trimethoprim-resistant isolates). These data indicate that the 6050 and 6101 bp plasmids have been in the UK A. pleuropneumoniae population since at least 1998 and 2005, respectively. The use of trimethoprim/sulphonamide combinations to treat A. pleuropneumoniae infection and other diseases in pigs provides selective pressure for maintenance, and the coexistence of different pathogens may facilitate the transfer of these antimicrobial resistance plasmids between different species.
In conclusion, we report here for the first time, to our knowledge, dfrA14 in the Pasteurellaceae, which will facilitate the development of PCR assays for resistance to trimethoprim, a clinically important antimicrobial.
Funding
This work was supported by a Longer and Larger (LoLa) grant from the Biotechnology and Biological Sciences Research Council (grant numbers BB/G020744/1, BB/G019177/1, BB/G019274/1 and BB/G018553/1), the UK Department for Environment, Food and Rural Affairs, and Zoetis (formerly Pfizer Animal Health) awarded to the Bacterial Respiratory Diseases of Pigs-1 Technology (BRaDP1T) Consortium. M. T. G. H. was supported by the Wellcome Trust (grant number 098051). The MIC work was funded from the former AHVLA's Research and Development Internal Investment Fund (grant number RD0030c). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Transparency declarations
None to declare.
Acknowledgements
We wish to thank Chris Teale from the APHA for his advice and input.
Members of the BRaDP1T Consortium
Duncan J. Maskell, Alexander W. (Dan) Tucker, Sarah E. Peters, Lucy A. Weinert, Jinhong (Tracy) Wang, Shi-Lu Luan and Roy R. Chaudhuri (University of Cambridge; present address for R. Chaudhuri: Department of Molecular Biology and Biotechnology, University of Sheffield, Firth Court, Western Bank, Sheffield S10 2TN, UK); Andrew N. Rycroft, Gareth A. Maglennon and Dominic Matthews (Royal Veterinary College); Brendan W. Wren, Jon Cuccui and Vanessa S. Terra (London School of Hygiene and Tropical Medicine); and Paul R. Langford, Janine T. Bossé and Yanwen Li (Imperial College London).
Contributor Information
Collaborators: on behalf of the BRaDP1T Consortium, Duncan J. Maskell, Alexander W. (Dan) Tucker, Sarah E. Peters, Lucy A. Weinert, Jinhong (Tracy) Wang, Shi-Lu Luan, Roy R. Chaudhuri, Andrew N. Rycroft, Gareth A. Maglennon, Dominic Matthews, Brendan W. Wren, Jon Cuccui, Vanessa S. Terra, Paul R. Langford, Janine T. Bossé, and Yanwen Li
References
- 1.Bossé JT, Janson H, Sheehan BJ et al. . Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect 2002; 4: 225–35. [DOI] [PubMed] [Google Scholar]
- 2.Borriello SP. UK Veterinary Antibiotic Resistance and Sales Surveillance Report 2013. www.vmd.defra.gov.uk/pdf/varss.pdf.
- 3.Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis 2001; 32: 1608–14. [DOI] [PubMed] [Google Scholar]
- 4.Byrne-Bailey KG, Gaze WH, Kay P et al. . Prevalence of sulfonamide resistance genes in bacterial isolates from manured agricultural soils and pig slurry in the United Kingdom. Antimicrob Agents Chemother 2009; 53: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perreten V, Boerlin P. A new sulfonamide resistance gene (sul3) in Escherichia coli is widespread in the pig population of Switzerland. Antimicrob Agents Chemother 2003; 47: 1169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seputiene V, Povilonis J, Ruzauskas M et al. . Prevalence of trimethoprim resistance genes in Escherichia coli isolates of human and animal origin in Lithuania. J Med Microbiol 2010; 59: 315–22. [DOI] [PubMed] [Google Scholar]
- 7.Matter D, Rossano A, Limat S et al. . Antimicrobial resistance profile of Actinobacillus pleuropneumoniae and Actinobacillus porcitonsillarum. Vet Microbiol 2007; 122: 146–56. [DOI] [PubMed] [Google Scholar]
- 8.Ito H, Ishii H, Akiba M. Analysis of the complete nucleotide sequence of an Actinobacillus pleuropneumoniae streptomycin-sulfonamide resistance plasmid, pMS260. Plasmid 2004; 51: 41–7. [DOI] [PubMed] [Google Scholar]
- 9.Kang M, Zhou R, Liu L et al. . Analysis of an Actinobacillus pleuropneumoniae multi-resistance plasmid, pHB0503. Plasmid 2009; 61: 135–9. [DOI] [PubMed] [Google Scholar]
- 10.Kehrenberg C, Schwarz S. Occurrence and linkage of genes coding for resistance to sulfonamides, streptomycin and chloramphenicol in bacteria of the genera Pasteurella and Mannheimia. FEMS Microbiol Lett 2001; 205: 283–90. [DOI] [PubMed] [Google Scholar]
- 11.Kehrenberg C, Schwarz S. dfrA20, a novel trimethoprim resistance gene from Pasteurella multocida. Antimicrob Agents Chemother 2005; 49: 414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehrenberg C, Schwarz S. Trimethoprim resistance in a porcine Pasteurella aerogenes isolate is based on a dfrA1 gene cassette located in a partially truncated class 2 integron. J Antimicrob Chemother 2011; 66: 450–2. [DOI] [PubMed] [Google Scholar]
- 13.de Groot R, Sluijter M, de Bruyn A et al. . Genetic characterization of trimethoprim resistance in Haemophilus influenzae. Antimicrob Agents Chemother 1996; 40: 2131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neill C, Jones SCP, Bossé JT et al. . Prevalence of Actinobacillus pleuropneumoniae serovars in England and Wales. Vet Rec 2010; 167: 661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bossé JT, Soares-Bazzolli DM, Li Y et al. . The generation of successive unmarked mutations and chromosomal insertion of heterologous genes in Actinobacillus pleuropneumoniae using natural transformation. PLoS One 2014; 9: e111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals—Third Edition: Approved Standard M31-A3. CLSI, Wayne, PA, USA, 2008. [Google Scholar]
- 17.Howell KJ, Weinert LA, Luan S-L et al. . Gene content and diversity of the loci encoding biosynthesis of capsular polysaccharides of the 15 serovar reference strains of Haemophilus parasuis. J Bacteriol 2013; 195: 4264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossé JT, Durham AL, Rycroft AN et al. . New plasmid tools for genetic analysis of Actinobacillus pleuropneumoniae and other Pasteurellaceae. Appl Environ Microbiol 2009; 75: 6124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.San Millan A, Escudero JA, Gutierrez B et al. . Multiresistance in Pasteurella multocida is mediated by coexistence of small plasmids. Antimicrob Agents Chemother 2009; 53: 3399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francia MV, Varsaki A, Garcillán-Barcia MP et al. . A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol Rev 2004; 28: 79–100. [DOI] [PubMed] [Google Scholar]
- 21.Matter D, Rossano A, Sieber S et al. . Small multidrug resistance plasmids in Actinobacillus porcitonsillarum. Plasmid 2008; 59: 144–52. [DOI] [PubMed] [Google Scholar]
- 22.Anantham S, Hall RM. pCERC1, a small, globally disseminated plasmid carrying the dfrA14 cassette in the strA gene of the sul2-strA-strB gene cluster. Microb Drug Res 2012; 18: 364–71. [DOI] [PubMed] [Google Scholar]
- 23.Ojo KK, Kehrenberg C, Schwarz S et al. . Identification of a complete dfrA14 gene cassette integrated at a secondary site in a resistance plasmid of uropathogenic Escherichia coli from Nigeria. Antimicrob Agents Chemother 2002; 46: 2054–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Michael GB, Becker R et al. . Pheno- and genotypic analysis of antimicrobial resistance properties of Yersinia ruckeri from fish. Vet Microbiol 2014; 171: 406–12. [DOI] [PubMed] [Google Scholar]
- 25.Eikmeyer F, Hadiati A, Szczepanowski R et al. . The complete genome sequences of four new IncN plasmids from wastewater treatment plant effluent provide new insights into IncN plasmid diversity and evolution. Plasmid 2012; 68: 13–24. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Yang M, Xu Z et al. . Complete genome sequence of Pasteurella multocida HN06, a toxigenic strain of serogroup D. J Bacteriol 2012; 194: 3292–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willson PJ, Albritton WL, Slaney L et al. . Characterization of a multiple antibiotic resistance plasmid from Haemophilus ducreyi. Antimicrob Agents Chemother 1989; 33: 1627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kehrenberg C, Schulze-Tanzil G, Martel JL et al. . Antimicrobial resistance in Pasteurella and Mannheimia: epidemiology and genetic basis. Vet Res 2001; 32: 323–39. [DOI] [PubMed] [Google Scholar]