Abstract
The neurological outcome for infants with Grade I/II intraventricular hemorrhage (IVH) is debated. The aim of this study was to determine whether very low birth weight infants (VLBW, < 1500 g) with Grade I /II (IVH) have altered visuocortical activity compared with infants with no IVH. We assessed the quantitative swept parameter Visual Evoked Potential (sVEP) responses evoked by three different visual stimuli. Data from 52 VLBW infants were compared with data from 13 infants with Grade I or II IVH, enrolled at 5 – 7 months corrected age. Acuity thresholds and suprathreshold response amplitudes were compared. Grating Acuity (GA), Contrast Sensitivity (CS) and Vernier Acuity (VA) were each worse in the Grade I/ II IVH compared with the no IVH groups (8.24 cpd in IVH group vs 13.07 cpd in no IVH group for GA; 1.44% vs 1.18% for CS and 1.55 arcmin vs 0.58 arcmin for VA). The slopes of the response amplitude for CS and VA were significantly lower in IVH infants. The spatial frequency tuning function was shifted downward on the spatial frequency axis, without a change in slope. These results indicate that Grade I/II IVH are associated with deleterious effects on cortical vision development and function.
Keywords: Prematurity; Intraventricular Hemorrhage, Swept parameter Visual Evoked Potentials; Contrast Sensitivity; Spatial Frequency; Vernier Acuity
1. Introduction
Advances in neonatal and perinatal medicine have resulted in improved survival of Very Low Birtheweight (VLBW) infants (Stevenson, Wright, Lemons, Oh, Korones, Papile, Bauer, Stoll, Tyson, Shankaran, Fanaroff, Donovan, Ehrenkranz & Verter, 1998). However, these infants remain at high risk for neurodevelopmental handicap in the long term (Hack, Klein & Taylor, 1995, McCormick, Brooks-Gunn, Workman-Daniels, Turner & Peckham, 1992). Cognitive impairments in these children can range from severe mental retardation to subtle neurological dysfunction such as attention deficits, behavior problems, visuo-motor, visuo-spatial, and fine and gross motor dysfunction (Hack & Taylor, 2000, Woodward, Edgin, Thompson & Inder, 2005). Several of the more subtle problems are not detectable until school age, and school performance is compromised in a large percentage of VLBW infants (Hille, den Ouden, Bauer, van den Oudenrijn, Brand & Verloove-Vanhorick, 1994, Hunt, Cooper & Tooley, 1988).
Several studies have shown that intraventricular hemorrhage (IVH), particularly Grade III/IV IVH is one of the major causes of adverse neurological outcome (Vohr, Allan, Westerveld, Schneider, Katz, Makuch & Ment, 2003). Each of these studies has utilized cranial ultrasound to diagnose IVH. An IVH occurs when blood vessels near the lateral ventricles of the brain bleed. Grade I is blood in the periventricular germinal matrix; Grade II, blood within the lateral ventricles without ventricular dilatation; Grade III, blood within and distending the lateral ventricles; and Grade IV, blood within the ventricular system and parenchymal (i.e., direct brain) involvement. Hack et al reported the following incidence of IVH in a cohort of VLBW infants; Grade I –17%, Grade II – 10%, Grade III – 11% and Grade IV – 7% (Hack, Horbar, Malloy, Tyson, Wright & Wright, 1991). Although Grade I and II hemorrhage is the most common cranial ultrasound abnormality in preterm infants, the effect of these milder hemorrhages on neurological outcome is less apparent. Some studies have shown milder IVH to have no effect on measures of cognitive outcome when compared to infants with normal ultrasounds (Whitaker, Johnson, Sebris, Pinto, Wasserman, Kairam, Shaffer & Paneth, 1990, Whitaker, Feldman, Van Rossem, Schonfeld, Pinto-Martin, Torre, Blumenthal & Paneth, 1996). In contrast other studies, including the most recent by Patra et al. (2006) (Patra, Wilson-Costello, Taylor, Mercuri-Minich & Hack, 2006), have shown a subtle downward (worse) trend in the scores on the Bayley Scales of Infant Development and other neuropsychological test measures (Patra et al., 2006, Stevenson et al., 1998).
Given the role of periventricular subplate neurons in cortical formation, the possibility exists that injury to this area, even of a mild nature, could result in impaired neurological function (Evrard, Gressens & Volpe, 1992). Subplate neurons, which play a pivotal role in cortical development, are present in the region that is damaged by IVH, adjacent to the lateral ventricles, and vulnerable to injury at the time when IVH occurs developmentally. The anatomical and temporal overlap for subplate neurons at a time of greatest risk to periventricular regions places the developing visual cortex squarely at risk for developmental injury from an IVH (McQuillen & Ferriero, 2005).
The swept parameter Visual Evoked Potential (sVEP) is a sensitive tool that can provide a quantitative electrophysiological measure of different visual functions by determining a sensory threshold (i.e. the minimal stimulus that produces a cortical visual response), as well as response amplitudes (i.e. the strength of the response) to suprathreshold stimuli (Norcia, 1993). We measured sVEP responses for three different visual tasks, each of which has a different rate of development; contrast sensitivity (CS), a measure of the ability to detect slight changes in luminance across space (Norcia, Tyler & Hamer, 1990), grating acuity (GA), a test of spatial resolution that measures the finest grating producing a visual response (Skoczenski & Norcia, 2002) and vernier acuity (VA) which measures the minimum spatial offset that can be detected between repetitive bar patterns (Skoczenski & Norcia, 1999).
The goal of this study was to determine if infants with Grade I or II IVH, as detected by head ultrasound, had changes in sVEP when compared to VLBW infants with normal head ultrasounds. Such changes may ultimately be indicative or predictive of future visuo-motor or visuo-spatial problems.
2. Methods
2.1 Participants
A total of 65 VLBW infants were enrolled in the study, 52 infants had normal head ultrasounds and 13 infants had Grade I or Grade II IVH. Table 1 shows the characteristics of the infants. There were no significant differences between the groups, except in birth weight. Infants with an IVH tended to be smaller at birth. Inclusion criteria for VLBW infants included Retinopathy of Prematurity (ROP) ≤ Stage II (i.e., a demarcation ridge dividing vascular from avascular retina, with no neovascularization; this is considered mild ROP), and no Plus disease and no congenital eye or other anomalies; i.e., no cataract, retinal, or optic nerve anomaly. No infant in this study had strabismus or known amblyopia (we excluded one infant with esotropia and another one with left eye ptosis due to hemangioma from the IVH group). Plus disease is defined as vascular dilatation and tortuosity of posterior retinal vessels, a sign of severe ROP. Information regarding birth weight, gestational age at birth, highest stage of ROP as recorded by an ophthalmologist and results of the head ultrasound done between 7 – 10 days of age for all enrolled infants was obtained from the medical record. Gestational age was assessed by the best obstetrical estimate using the last menstrual period and ultrasound examination. Post conceptional age was determined by adding chronologic age in weeks to gestational age in weeks at birth. Infants were examined between 5 – 7 months of age corrected for prematurity. We chose this time period for two reasons: many infants need extra weeks or months in the nursery, so at 5 – 7 months we were sure that all would have been discharged; recordings for contrast, grating, and vernier acuity can be made at this age. Head ultrasounds were graded according to the system described previously (Papile, Burstein, Burstein & Koffler, 1978); Grade I, blood in the periventricular germinal matrix; Grade II, blood within the lateral ventricles without ventricular dilatation; Grade III, blood within and distending the lateral ventricles; and Grade IV, blood within the ventricular system and parenchymal involvement.
Table 1. Characteristics of enrolled infants.
| Demographics | No IVH (n=52) |
Grade I/II IVH (n=13) |
P- value |
|---|---|---|---|
| Gender | M = 21, F = 31 | M = 4, F = 9 | |
| Race | |||
| White | 35 | 7 | |
| Hispanic | 9 | 6 | |
| Asian | 7 | ||
| Other | 1 | ||
| Any ROP, but < Stage II and no plus | 9 | 5 | |
| Birth weight (g) | 1203 + 28 | 1063 + 71 | <0.05 |
| Gestational age (wks) | 29 + 2 | 29 + 2 | 0.219 |
| Post-conceptional age at exam (wks) | 26 + 7 | 27 + 8 | 0.846 |
| Mean chronologic age at exam (wks) | 37 + 1 | 38 + 2 | 0.574 |
The Institutional Review Board for human subjects research at Stanford University and Smith-Kettlewell Eye Research Institute approved the study. The research adhered to the tenets of the Declaration of Helsinki. Signed informed consent was obtained from the parents of the enrolled infants after explanation of the study procedures.
2.2 Electroencephalogram Recording
The electroencephalogram (EEG) signal was amplified using a Grass© Model 12 amplifier (filter settings: 1–100Hz at –6 dB) at a gain of 20,000. Active electrodes were placed over the infant’s scalp at the location O1, Oz, O2 of the International 10–20 system. A reference electrode was placed at Cz and a ground electrode was placed at Pz. Electrode impedance was equal to or less than 10 kΩ. During an experimental session, the participants were seated in their parent’s lap in front of the monitor. The sVEP responses were measured under binocular viewing conditions in all observers. The experimenter attracted the participant’s attention to the stimulus with small toys (about 1 – 2 cm in size) dangled over the center of the display. Recordings were interrupted when the participant was judged not to be attending to the stimulus and were resumed when the participant looked back at the screen. When interruptions occurred, the program interrupted the sweep but not the stimulus appearance or modulation. When the trial resumed after an interruption, data collection recommenced with the stimulus set to its value at 0.5 seconds before the interruption. In this study, the stimuli were repeated 4 – 8 times to increase the signal to noise ratio through averaging out the uncorrelated background EEG activity. We attempted to acquire at least 4 sweep trials in a given condition so that the response function would be representative of the individual’s peak amplitude of response. We allowed collection of up to 8 trials in a given condition if the first 4 trials did not result in a scorable threshold. By this strategy, all voltage vs amplitude functions could be entered into the group average curves.
2.3 Stimulus Presentation
Stimuli were presented on a high-bandwidth monochrome monitor (MR2000HB-MED, Richardson Electronics) at a screen resolution of 1600×1200 pixels and a 60 Hz vertical refresh rate. The stimulus field was 18° × 25°. Viewing was binocular and the infants viewed the screen from 100 cm while seated in their parent’s lap. Mean luminance of the display was 102 cd/m2. The stimulus details have been described previously (Mirabella, Kjaer, Norcia, Good & Madan, 2006). In brief, there were three swept parameter conditions: spatial frequency, an 80% contrast and 3.76 Hz phase-reversing grating was swept from 2 to 16 cpd in 10-linear steps; contrast, a 3.76 Hz phase-reversing and 2 cpd sinusoidal grating was swept from 0.5 to 20% contrast in equal 10-logarithmic steps and vernier offset, vernier displacements were periodically introduced and removed at 3.76 Hz from a 2 cpd, 80% contrast square-wave grating. The size of the vernier displacements was swept from 8 to 0.5 arcmin in 10 equal logarithmic steps. The sweep duration for all three measures was 10 seconds.
2.4 Statistical Analysis
The sVEP technique has been described in detail previously (Mirabella et al., 2006, Norcia, Tyler, Hamer & Wesemann, 1989, Skoczenski & Norcia, 2002). In order to quantify statistical differences in swept parameter response functions between IVH and no IVH infants, we constructed mean swept parameter responses for each stimulus for each group of children. Each epoch of these group response functions represented the complex average of the Fourier coefficients from the corresponding epoch in the individual swept parameter response functions. To estimate the standard errors of thresholds and slopes of the group average response functions, we used a jackknife procedure (Sprent, 1989). Significant response differences between the groups were identified by two-tailed, heteroscedastic t-tests (Tables 2 and 3).
Table 2. Group response function thresholds in no IVH and Grade I/II IVH groups.
Thresholds differed for the vernier offset (VA), grating acuity/spatial frequency (GA) and contrast (CS) sweeps. IVH, intraventricular hemorrhage. The number of infants (n) in the calculation of each mean is shown below each entry in the table.
| Group Response | GA(c/deg) | CS (%) | VA(arcmin) | |
|---|---|---|---|---|
| IVH | Threshold ± SEM |
8.235 ±0.673 (n = 9) |
1.436 ±0.097 (n = 13) |
1.554 ±0.169 (n = 10) |
| No IVH | Threshold ±SEM |
13.066 ±.454 (n = 43) |
1.178 ±0.041 (n = 52) |
0.579 ±0.045 (n = 39) |
| Difference | t-score p-value |
12.635 <0.001 |
8.749 <0.001 |
22.539 <0.001 |
Table 3. Slopes of the regression lines used to determine group sVEP thresholds.
The slopes differ for contrast (CS) and vernier offset (VA) sweeps but not for grating / spatial frequency (GA) sweeps. The number of infants (n) in the calculation of each mean is shown below each entry in the table. IVH, intraventricular hemorrhage.
| Group Response | GA (uV/(c/deg)) |
CS (uV/%) |
VA (uV/arcmin) |
|
|---|---|---|---|---|
| IVH | Slope ±SEM |
−0.737 ±0.419 (n = 9) |
0.384 ±0.159 (n = 13) |
1.343 ±0.805 (n = 10) |
| No IVH | Slope ± SEM |
−0.696 ±0.136 (n = 43) |
0.714 ±0.089 (n = 52) |
0.956 ±0.106 (n = 39) |
| Difference | t-score p-value |
0.318 0.752 |
5.454 <0.001 |
2.439 0.018 |
3. Results
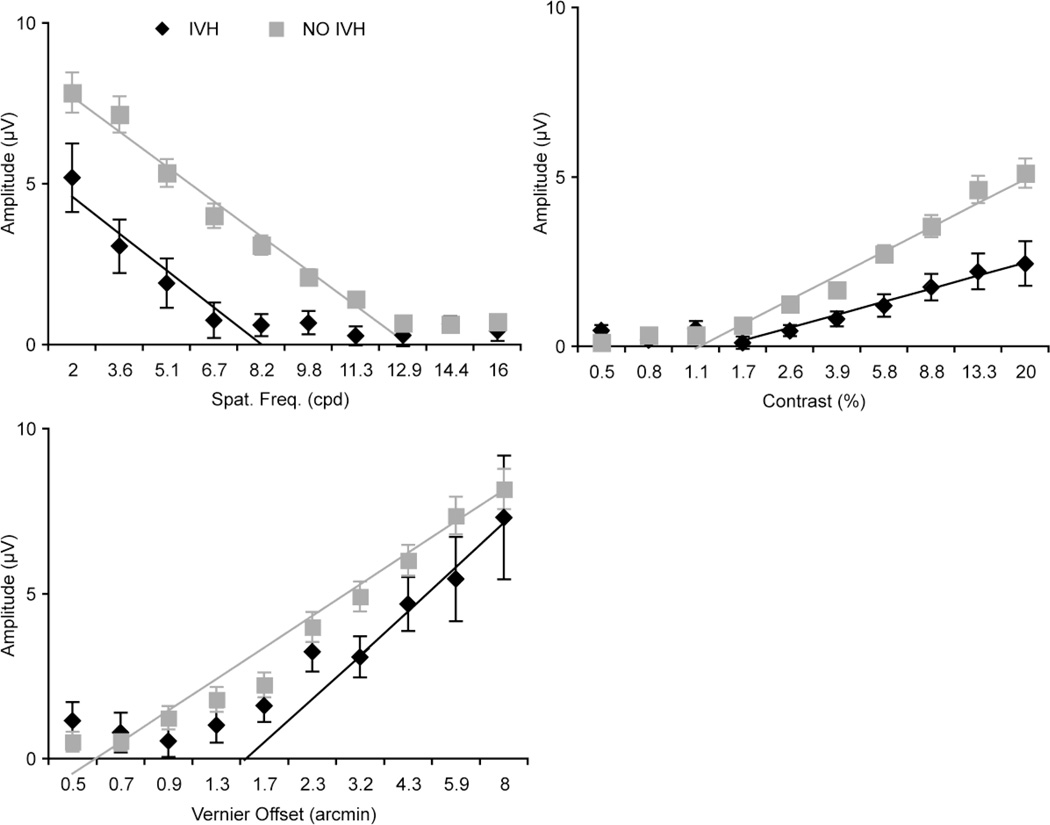
Table 1 shows baseline characteristics of infants with a Grade I or II IVH, compared to no IVH. There were no significant differences between the groups, except in birth weight (two-tailed, heteroscedastic t-tests). Infants with an IVH tended to be smaller at birth. However, gestational ages at birth were similar between the groups. The sVEP group response functions at each recording electrode (O1, Oz and O2) for each of the stimuli were analyzed, and the response amplitude at Oz showed the highest amplitude among the 3 active electrodes. Figure 1 plots sVEP group response functions for each of the stimuli at Oz. Thresholds estimated from the group response functions (intercept of the regression lines shown in Figure 1) differed for all 3 conditions (Table 2). A significant worsening of threshold was noted for grating acuity (GA: 8.23 cpd in the IVH group vs. 13.06 cpd in the no IVH group, p<.001); for contrast sensitivity (CS: 1.44% vs. 1.178%, p<.001), and for vernier acuity (VA: 1.55 arcmin vs. 0.579 arcmin, p<.001).
Figure 1. Mean response functions for each of the three visual measures.
Vector averaged sVEP response functions at Oz derivation in infants with IVH (black diamonds) and without IVH (gray squares) for (clockwise from bottom left) vernier offset, grating acuity, and contrast sensitivity. Error bars are +/− 1 standard error of the means. The solid lines are the regression lines used to estimate threshold and slope of the function (see Table 2 and 3). Amplitudes were lower for the all visual measures in the Grade I/II IVH group compared to no IVH group. Statistically significant differences are present across the entire sweep range for all 3 functions, except for vernier acuity, as shown.
Group response functions were monotonically increasing functions of contrast and vernier offset size and were decreasing functions of spatial frequency for both the IVH and no IVH groups. The slope of the spatial frequency response function was the same in the two groups: the spatial frequency response function was shifted as a whole towards lower spatial frequencies. Response amplitudes in the no IVH group were about 50% larger than those in the IVH group at the lowest spatial frequency. The slope of contrast response function was lower in the IVH group, leading to peak amplitudes that were a factor of approximately 2.5 lower than those measured in the no IVH group. The slope of the vernier response function was steeper in the IVH group, leading to lower (worse thresholds). The vernier response function of the IVH group converges towards that of the no IVH group for the largest offsets. The values of the slopes and the statistical testing results are shown in Table 3.
4. Discussion
Previous studies of the effects of IVH on neurodevelopment have relied on neurological deficits in the newborn period, or have utilized long-term follow-up examinations to assess cognitive and other behavioral measures of development. Often these studies require years of observation before the participants are able to perform the required tasks. In this study, we have shown that infants with low grade IVH (Grades I and II) have measurable and significant neurophysiological changes in cortical responsiveness at 5 – 7 months corrected age.
The main finding in this study of increased thresholds for all three stimulus conditions, indicating decreased sensitivity to visual stimuli, is potentially significant for functional vision in patients with a low grade IVH. The finding is also significant since these three response functions are likely subserved by different cortical mechanisms. However this effect is small in magnitude and may ameliorate with age. It will be of interest to determine if VEP abnormalities persist in this population and whether they are predictive of other developmental outcomes, either in infancy or in later childhood.
We also found reductions in the amplitude of the evoked response in each of the measures of visual function: grating acuity, contrast sensitivity and vernier acuity. These amplitude reductions took different forms for the different stimuli. For the contrast sweep functions, suprathreshold amplitudes became increasingly abnormal as the stimulus became more visible (slope change). The contrast response function thus starts at a higher contrast (the threshold deficit) and becomes progressively abnormal as stimulus contrast increases. For the vernier offset function the amplitude difference decreased towards suprathreshold values. The abnormality in the vernier function is thus primarily in the near threshold region. The spatial frequency response function was shifted towards the lower spatial frequency range, with a non-significant change in response slope. This leftward shift of the response function led to a decrease in the estimated grating acuity.
The significance of these suprathreshold response findings is more difficult to interpret than threshold changes, yet potentially offers insights into visual cortical functioning in infants who had an IVH. Pending future investigations that could link neurophysiology findings to anatomical and neurodevelopmental changes, we can offer the following possible interpretations. A reduction in amplitude of the evoked response could be caused by a number of factors. A decrease in neuronal mass, as occurs in profound brain damage has been shown to adversely affect the response amplitude (Good, 2001). It is plausible, given what is known about the effects of more severe grades of IVH, to consider that this occurs even in low grade IVH. Another theoretical explanation includes the possibility that the normal balance of neuronal excitation and inhibition is altered in preterm infants with neurological injury. A shift of this balance towards inhibition, either from reduced excitation or increased inhibition, would have the effect of reducing the signal amplitude. It is also possible that decreases in the temporal precision (synchronization) of synaptic activity could occur and this could result in reduced response amplitudes via a loss of temporal summation of the afferent volley.
This study has several limitations. One of these is the small sample size of IVH infants. Also, we did not specifically examine the presence of white matter injury or periventricular leukomalacia, conditions that are known to affect cortical visual function. Infants in the IVH group had a lower mean birth weight, which can independently affect neurodevelopmental outcome and therefore could contribute to the decrease in visual function seen in this study. A larger sample would be needed to control for this effect statistically. Also, infants in the IVH group were more likely to have had mild ROP. ROP that progresses to Stage III, or to plus disease, is associated with changes in refraction and, in some cases, reduced visual acuity (Quinn, Dobson, Davitt, Hardy, Tung, Pedroza & Good, 2008). In this study, there were infants with mild ROP (≤ Stage II) in both groups (9 in no IVH group and 5 in IVH group), but none of the infants had Stage III or plus disease. Mild ROP does not influence visual acuity outcome or development of refractive errors (O'Connor, Stephenson, Johnson, Tobin, Moseley, Ratib, Ng & Fielder, 2002). Finally, two studies have found that infants with IVH often have non-cortical ocular complications such as strabismus or high refractive errors (Harvey, Dobson, Luna & Scher, 1997, O'Keefe, Kafil-Hussain, Flitcroft & Lanigan, 2001). In our study, we excluded one infant with esotropia and another one with left eye ptosis due to hemangioma from IVH group.
Refractive errors were not measured in this study. There are several reasons that we do not believe that the presence of myopia is causing the effects we observed. First the patterns of supra-threshold amplitude losses in the spatial frequency and contrast sweeps are inconsistent with differences in refractive error between the two groups. Refractive error should preferentially affect high spatial frequencies, but the entire spatial tuning function is shifted rightward, rather than showing the steeper decline with increasing spatial frequency that would be expected from excess blur. Secondly, the contrast response function measured at low spatial frequencies shows a change in slope with increasing contrast. Refractive error reduces the input contrast on the retina and would shift the contrast response function to the right without a change in slope. Moreover, there would need to be a very significant refractive error difference between groups to create an effect at 2 cpd stimuli. The prevalence of high refractive errors in premature infants, as a group is quite low (Sahni, Subhedar & Clark, 2005), and low grade IVH is not known to increase the risk for high myopia. Nonetheless, there may have ocular defects in our cohort that are below our detection threshold. Therefore the possibility exists that we are measuring a mixture of ocular and cortical changes in our cohort.
This study demonstrates the presence of significant threshold and suprathreshold neurophysiological changes in infants with Grades I and II IVH. These changes are detectable many months after the injury and are present in all three visual measures, suggesting a more generalized and at least partially persistent effect of Grades I and II IVH on vision development. The three response measures (grating acuity, contrast sensitivity and vernier acuity) are likely limited by critical immaturities (Brown & Lindsey, 2009) at different stages of visual pathway anatomy and development and may selectively drive different downstream cortical mechanisms subserving different aspects of functional vision (Skoczenski & Good, 2004). Whether these changes actually portend subclinical, or clinically-important alterations in mature behavioral functioning is an open question requiring longer followup and additional investigations.
Acknowledgements
This work was supported in part by grant 1 RO1 EY015228-01A2 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD (WVG, AM, AMN); grant 5 M01 RR000070 from the National Center for Research Resources, National Institutes of Health, Department of Health and Human Services, Bethesda, MD (Stanford University); funds from the Children’s Eye Foundation of the American Association for Pediatric Ophthalmology and Strabismus (WVG). The authors thank Patricia Hartsell, Sharon Cassinelli and Judith Y. Hall for their assistance in recruiting and co-coordinating participants’ visits.
List of Abbreviations
- VLBW
Very Low Birth Weight
- sVEP
Swept parameter Visual Evoked Potentials
- ROP
Retinopathy of Prematurity
- IVH
Intraventricular Hemorrhage
- PVL
Periventricular Leukomalacia
- CS
Contrast Sensitivity
- GA
Grating Acuity
- VA
Vernier Acuity
Footnotes
Presented in part at the 2007 meetings of Society for Pediatric Research and Association for Research in Vision and Ophthalmology.
References
- Brown AM, Lindsey DT. Contrast insensitivity: the critical immaturity in infant visual performance. Optom Vis Sci. 2009;86(6):572–576. doi: 10.1097/OPX.0b013e3181a72980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard P, Gressens P, Volpe JJ. New concepts to understand the neurological consequences of subcortical lesions in the premature brain. Biol Neonate. 1992;61(1):1–3. doi: 10.1159/000243525. [DOI] [PubMed] [Google Scholar]
- Good WV. Development of a quantitative method to measure vision in children with chronic cortical visual impairment. Trans Am Ophthalmol Soc. 2001;99:253–269. [PMC free article] [PubMed] [Google Scholar]
- Hack M, Horbar JD, Malloy MH, Tyson JE, Wright E, Wright L. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics. 1991;87(5):587–597. [PubMed] [Google Scholar]
- Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. Future Child. 1995;5(1):176–196. [PubMed] [Google Scholar]
- Hack M, Taylor HG. Perinatal brain injury in preterm infants and later neurobehavioral function. Jama. 2000;284(15):1973–1974. doi: 10.1001/jama.284.15.1973. [DOI] [PubMed] [Google Scholar]
- Harvey EM, Dobson V, Luna B, Scher MS. Grating acuity and visual-field development in children with intraventricular hemorrhage. Dev Med Child Neurol. 1997;39(5):305–312. doi: 10.1111/j.1469-8749.1997.tb07436.x. [DOI] [PubMed] [Google Scholar]
- Hille ET, den Ouden AL, Bauer L, van den Oudenrijn C, Brand R, Verloove-Vanhorick SP. School performance at nine years of age in very premature and very low birth weight infants: perinatal risk factors and predictors at five years of age. Collaborative Project on Preterm and Small for Gestational Age (POPS) Infants in The Netherlands. J Pediatr. 1994;125(3):426–434. doi: 10.1016/s0022-3476(05)83290-1. [DOI] [PubMed] [Google Scholar]
- Hunt JV, Cooper BA, Tooley WH. Very low birth weight infants at 8 and 11 years of age: role of neonatal illness and family status. Pediatrics. 1988;82(4):596–603. [PubMed] [Google Scholar]
- McCormick MC, Brooks-Gunn J, Workman-Daniels K, Turner J, Peckham GJ. The health and developmental status of very low-birth-weight children at school age. Jama. 1992;267(16):2204–2208. [PubMed] [Google Scholar]
- McQuillen PS, Ferriero DM. Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol. 2005;15(3):250–260. doi: 10.1111/j.1750-3639.2005.tb00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Kjaer PK, Norcia AM, Good WV, Madan A. Visual development in very low birth weight infants. Pediatr Res. 2006;60(4):435–439. doi: 10.1203/01.pdr.0000238249.44088.2c. [DOI] [PubMed] [Google Scholar]
- Norcia AM. Improving infant evoked response measurement. In: Simons K, editor. Early visual development, normal and abnormal. New York: Oxford University Press; 1993. pp. 536–552. [Google Scholar]
- Norcia AM, Tyler CW, Hamer RD. Development of contrast sensitivity in the human infant. Vision Res. 1990;30(10):1475–1486. doi: 10.1016/0042-6989(90)90028-j. [DOI] [PubMed] [Google Scholar]
- Norcia AM, Tyler CW, Hamer RD, Wesemann W. Measurement of spatial contrast sensitivity with the swept contrast VEP. Vision Res. 1989;29(5):627–637. doi: 10.1016/0042-6989(89)90048-5. [DOI] [PubMed] [Google Scholar]
- O'Connor AR, Stephenson T, Johnson A, Tobin MJ, Moseley MJ, Ratib S, Ng Y, Fielder AR. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109(1):12–18. doi: 10.1542/peds.109.1.12. [DOI] [PubMed] [Google Scholar]
- O'Keefe M, Kafil-Hussain N, Flitcroft I, Lanigan B. Ocular significance of intraventricular haemorrhage in premature infants. Br J Ophthalmol. 2001;85(3):357–359. doi: 10.1136/bjo.85.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I–II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149(2):169–173. doi: 10.1016/j.jpeds.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Quinn GE, Dobson V, Davitt BV, Hardy RJ, Tung B, Pedroza C, Good WV. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study: findings to 3 years of age. Ophthalmology. 2008;115(6):1058–1064. e1051. doi: 10.1016/j.ophtha.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Sahni J, Subhedar NV, Clark D. Treated threshold stage 3 versus spontaneously regressed subthreshold stage 3 retinopathy of prematurity: a study of motility, refractive, and anatomical outcomes at 6 months and 36 months. Br J Ophthalmol. 2005;89(2):154–159. doi: 10.1136/bjo.2004.045815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoczenski AM, Good WV. Vernier acuity is selectively affected in infants and children with cortical visual impairment. Dev Med Child Neurol. 2004;46(8):526–532. doi: 10.1017/s001216220400088x. [DOI] [PubMed] [Google Scholar]
- Skoczenski AM, Norcia AM. Development of VEP Vernier acuity and grating acuity in human infants. Invest Ophthalmol Vis Sci. 1999;40(10):2411–2417. [PubMed] [Google Scholar]
- Skoczenski AM, Norcia AM. Late maturation of visual hyperacuity. Psychol Sci. 2002;13(6):537–541. doi: 10.1111/1467-9280.00494. [DOI] [PubMed] [Google Scholar]
- Sprent P. Applied non parametric statistical methods. London: Chapman & Hall; 1989. [Google Scholar]
- Stevenson DK, Wright LL, Lemons JA, Oh W, Korones SB, Papile LA, Bauer CR, Stoll BJ, Tyson JE, Shankaran S, Fanaroff AA, Donovan EF, Ehrenkranz RA, Verter J. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179(6 Pt 1):1632–1639. doi: 10.1016/s0002-9378(98)70037-7. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, Ment LR. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111(4 Pt 1):e340–e346. doi: 10.1542/peds.111.4.e340. [DOI] [PubMed] [Google Scholar]
- Whitaker A, Johnson J, Sebris S, Pinto J, Wasserman G, Kairam R, Shaffer D, Paneth N. Neonatal cranial ultrasound abnormalities: association with developmental delay at age one in low birth weight infants. J Dev Behav Pediatr. 1990;11(5):253–260. [PubMed] [Google Scholar]
- Whitaker AH, Feldman JF, Van Rossem R, Schonfeld IS, Pinto-Martin JA, Torre C, Blumenthal SR, Paneth NS. Neonatal cranial ultrasound abnormalities in low birth weight infants: relation to cognitive outcomes at six years of age. Pediatrics. 1996;98(4 Pt 1):719–729. [PubMed] [Google Scholar]
- Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128(Pt 11):2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]