The authors documented a low compliance with guidelines to screen for skin malignancy in a community-dwelling cohort of patients with newly diagnosed chronic lymphocytic leukemia.
Abstract
Purpose:
Assess compliance with skin cancer screening guidelines in a community-dwelling cohort of patients with newly diagnosed chronic lymphocytic leukemia (CLL) and evaluate the clinical utility of such screening.
Methods:
We identified patients diagnosed at Mayo Clinic with CLL between January 1, 2004, and June 1, 2012, who resided within 30 miles of Mayo Clinic. We evaluated adherence to skin cancer screening and identified the prevalence of skin malignancies during follow-up. Medical records were reviewed to document skin cancer screening and diagnosis of a skin malignancy.
Results:
Collectively, 113 individuals who met criteria were diagnosed with CLL during the study interval. Forty-one patients (36%) had a whole body skin examination by either a dermatologist or primary care provider documented within 6 months of diagnosis; of these; nine (8% of overall cohort; 22% of examined patients) had a skin malignancy identified. Fifteen additional skin malignancies were diagnosed during follow-up. There were a total of 24 skin malignancies (21% of cohort) diagnosed, including basal cell carcinoma (n = 10), squamous cell carcinoma (n = 11), sebaceous carcinomas (n = 2), and melanoma (n = 1).
Conclusion:
We documented a low compliance with guidelines to screen for skin malignancy in a community-dwelling cohort of patients with newly diagnosed CLL. Standardized and systems-based approaches are likely to increase compliance with skin cancer screening guidelines in patients with CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in the United States, with more than 16,000 estimated cases in 2012.1 CLL is commonly diagnosed incidentally after the finding of lymphocytosis seen on a complete blood count obtained for another purpose. The clinical course of patients with CLL can be quite variable: whereas some patients have a benign course, others succumb to an aggressive disease that relapses or is refractory to treatment. Some patients experience transformation of CLL to a more aggressive lymphoid malignancy.
Patients with CLL are at high risk for second malignancies, including cancers of the skin (eg, malignant melanoma, Merkel cell carcinoma, basal cell carcinoma, and squamous cell carcinoma).2 In addition to a high incidence of skin malignancies, patients with CLL also appear to experience more aggressive behavior of these cancers once they develop.
Patients with CLL who develop basal cell carcinoma and squamous cell carcinoma are more likely to experience locally aggressive disease (resulting in potentially disfiguring surgery) and/or metastatic disease than those without CLL. Similarly, the survival of patients with malignant melanoma and Merkel cell carcinoma is worse, stage for stage, in patients with CLL than in patients without CLL.2,3
As a result of these facts, it is thought that early detection of skin cancer is critical to improving skin cancer outcomes in patients with CLL. To promote early diagnosis and education on skin cancer prevention, it has been recommended that patients with CLL be referred for full-body skin evaluation within 6 months of diagnosis.4–6 Nonetheless, evidence to demonstrate the clinical utility of such screening is lacking. In this study, we sought to determine adherence to skin cancer screening guidelines in a community-dwelling cohort of patients newly diagnosed with CLL and to identify the prevalence of skin malignancies in patients with newly diagnosed CLL.
Methods
In the present study, we identified a community-dwelling cohort of patients newly diagnosed with CLL who lived within 30 miles of the Mayo Clinic and were diagnosed with CLL between January 1, 2004, and June 1, 2012; patient cases were identified through the Mayo Clinic CLL database. We chose this period for investigation because our group recommended full-body skin examinations in 2004.4 It is standard recommended care at Mayo Clinic for patients to undergo a full-skin examination within 6 months of diagnosis of CLL.4,5,6 The provider may perform this examination, refer patients to primary care or dermatology, or notify the referring physician of this recommendation. The Mayo Clinic in Rochester is located in a largely rural area in southeastern Minnesota and is both a tertiary referral center and the primary care center for the surrounding region of southeastern Minnesota, northern Iowa, and western Wisconsin. The Mayo Clinic Institutional Review Board approved the chart review of primary care patients via the electronic medical record system. Patients who listed their primary address as a hotel but lived more than 30 miles from the Mayo Clinic or who died within 6 months of diagnosis of CLL were excluded from the study. Records were reviewed for details of diagnosis regarding CLL and any skin malignancy. Standard clinicopathologic features were also abstracted for review.
Charts were reviewed to document referral to dermatology or primary care for full-body skin examination within 6 months of diagnosis of CLL. Diagnosis of a skin malignancy at initial screening and during follow-up was identified from clinical notes and pathology records. In order to ensure completeness of records, a second chart review was performed using the Mayo Clinical Notes Search Tool. In addition, our findings were cross-referenced with the Mayo Clinic tumor registry, the Mayo CLL patient database, and a Mayo Clinic dermatology database of skin malignancies. Patients who received their primary care elsewhere in Olmsted County, MN, were identified through the Rochester Epidemiology Project and had their records reviewed at the site of their primary care as approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. The Rochester Epidemiology Project was established in 1966 in order to maintain a comprehensive medical records linkage system for residents of Olmsted County, Minnesota. The project and its applications have been described elsewhere.7–10
Basic summary statistics were generated to characterize our study population. Time to skin cancer diagnosis was defined as the time between the date of CLL diagnosis and the date of skin cancer for those with a diagnosis, or date of last follow-up or death for those without skin cancer. An estimate of time to skin cancer was calculated using the Kaplan-Meier method. Multivariable Cox regression analyses were conducted to determine whether there were baseline factors associated with development of skin cancer. Because data were incomplete, we were not able to include all variables in the Cox model; hence, we ran multiple Cox models. Each model was adjusted for age, sex, and Rai stage, and one final variable (CD38, ZAP-70, IGVH mutation status, CD49 days, fluorescence in situ hybridization [FISH], or treatment) was included. P values ≤ .05 were considered significant, and all statistical analyses were performed using the SAS 9.2 software package (SAS Institute; Cary, NC).
Results
There were 113 patients newly diagnosed with CLL between January 1, 2004, and June 1, 2012, who resided within 30 miles of the Mayo Clinic in Rochester, MN. The median age at diagnosis was 68 years, the majority of patients were male (59%), and most patients had a low risk Rai score (56%; Table 1). Consistent with being a cohort of patients newly diagnosed with CLL, most patients had a more favorable prognostic profile with respect to CD38, ZAP70, IGHV, and cytogenetic analysis by FISH.
Table 1.
Characteristics of Patients With CLL Who Resided Within 30 Miles of Mayo Clinic and Were Diagnosed Between January 1, 2004, and June 1, 2012
| Characteristic | No. | % |
|---|---|---|
| Age at CLL diagnosis, years | ||
| Median | 68 | |
| Interquartile range | 59-76 | |
| Sex | ||
| Male | 67 | 59 |
| Female | 46 | 41 |
| Rai stage | ||
| Low risk (0) | 63 | 56 |
| Intermediate risk (1-2) | 45 | 40 |
| High risk (3-4) | 5 | 4 |
| CD38 | ||
| Negative | 76 | 74 |
| Positive | 27 | 26 |
| Missing | 10 | |
| ZAP-70 | ||
| Negative | 66 | 67 |
| Positive | 32 | 33 |
| Missing | 15 | |
| IGVH mutation status | ||
| Mutated | 41 | 56 |
| Unmutated | 32 | 44 |
| Missing | 40 | |
| CD49d | ||
| Negative | 55 | 68 |
| Positive | 26 | 32 |
| Missing | 32 | |
| FISH | ||
| Normal | 23 | 23 |
| 13q- | 41 | 41 |
| Trisomy 12 | 21 | 21 |
| 11q- | 8 | 8 |
| 17p- | 6 | 6 |
| Missing | 14 | |
Abbreviations: CLL, chronic lymphocytic leukemia; FISH, fluorescence in situ hybridization.
Collectively, 41 (36%) patients had a documented full-body skin exam by either a dermatologist or primary care provider within 6 months of diagnosis. Nine of these 41 patients (22% of the examined patients; 8% of the overall cohort) were found to have a malignancy of the skin within 6 months of diagnosis of CLL: two patients had basal cell carcinoma, six had squamous cell carcinoma, and one had malignant melanoma. Education regarding skin cancer prevention was documented in the clinical notes for only 28 (25%) of patients.
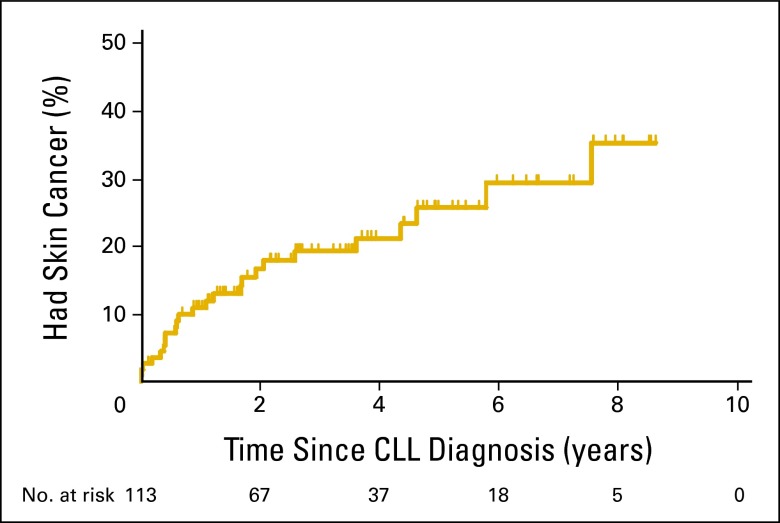
An additional 15 skin cancers were diagnosed in this cohort during extended follow-up: there were eight additional cases of basal cell carcinoma, five more cases of squamous cell carcinoma, and two sebaceous carcinomas. After a median follow-up of 3.6 years, 24 (21%) of patients with CLL developed a skin malignancy, including 11 cases of squamous cell carcinomas, 11 cases of basal cell carcinoma, one melanoma, and two sebaceous carcinomas. Median time to skin cancer development was not reached (Figure 1). After adjustment for age, sex, and Rai stage, we did not find any association between FISH risk category, CD38, ZAP70, IGVH, or CD49 days status and skin cancer risk (Table 2), suggesting that the increased risk of skin malignancy in patients with CLL is independent of prognostic factors.
Figure 1.
Time from diagnosis of chronic lymphocytic leukemia (CLL) to skin cancer diagnosis; median time was not reached. The number of patients at risk in each year post CLL diagnosis is displayed below the plot.
Table 2.
Multivariable Cox Regression Analysis Results for Time to Any Skin Cancer Diagnosis
| Factor | HR | 95% CI | P |
|---|---|---|---|
| CD38 (positive) | 1.2 | 0.5 to 2.9 | .71 |
| ZAP-70 (positive) | 1.4 | 0.6 to 3.3 | .42 |
| IGVH mutation status (unmutated) | 1.3 | 0.5 to 3.9 | .60 |
| CD49d (positive) | 1.1 | 0.4 to 3.0 | .85 |
| FISH (17p-/11q-versus normal/13q-/trisomy 12) | 0.6 | 0.1 to 2.6 | .48 |
NOTE. Each row represents a different model. Age, sex, and Rai stage were included in each model.
Abbreviations: FISH, fluorescence in situ hybridization; HR, hazard ratio.
Discussion
Patients with CLL are at high risk for second malignancies, including skin malignancies. Screening for skin malignancies within 6 months of diagnosis of CLL has been recommended, but there are few data on efficacy.4,5,6 We sought to determine compliance with these recommendations in a community-dwelling cohort of patients newly diagnosed with CLL and to determine the incidence of skin malignancies in this cohort during follow-up. Fewer than 40% of patients had a documented full-body examination within 6 months of their CLL diagnosis. Notably, 8% of the overall cohort had a skin malignancy identified within 6 months of their CLL diagnosis (number needed to screen to detect one skin cancer = 13). Of the patients who underwent screening within 6 months of diagnosis, 22% were found to have a skin malignancy (number needed to screen = 4.3), including one case of melanoma. During follow-up, 21% of patients with CLL had developed at least one skin cancer. Given that the age-adjusted rate of skin cancer, adjusted to the 2000 US standard population, is 24.3 of 100,000 (< 1%) in Minnesota,11 the prevalence of skin cancer in our cohort of patients with CLL is dramatically elevated.
When considered with the evidence that skin cancers behave more aggressively in patients with CLL,2,3 we believe that these data support routine screening for skin cancer at the time of diagnosis in patients with CLL. In fact, the immediate clinical implications of skin cancer screening at the time of diagnosis are likely of greater clinical significance to an individual patient than many other routine studies performed in patients with newly diagnosed CLL (eg, prognostic evaluations for CD38, ZAP-70, and IGVH). Early detection of skin cancers has the potential to reduce the need for disfiguring surgery in patients with basal cell and squamous cell carcinoma and to positively affect overall survival for those with melanoma or Merkel cell carcinoma.3
Unfortunately, our study also suggests that skin cancer screening is performed in a minority of patients with newly diagnosed with CLL. In some cases, the hematologists did not perform the examinations or make a referral to primary care or dermatology. In other cases, the hematologists' recommendations to perform a full-body examination were ignored by the referring provider. The relevance of skin examinations in this cohort may not be fully recognized. Although our group was the first, to our knowledge, to recommend routine screening and to advocate its use, our own adherence to these recommendations was poor. This illustrates the need to develop systematic approaches to improve screening. Such approaches could include automatic referrals to dermatology or primary care provider for full-body skin examinations in patients with newly diagnosed CLL, systematic distribution of dermatology referral materials at the time of initial hematology consultation, or dermatology consultation hot buttons on a CLL order page for those with an electronic ordering system. Such processes are in development at the Mayo Clinic to increase adherence to skin cancer screening in all newly diagnosed CLL patients.
The exact mechanism for the increased incidence of skin malignancies in patients with CLL is uncertain. Many associations between immunosuppression and skin cancers have been made previously.2,12 CLL is thought to represent a state of immunosuppression. Treatment for CLL is thought to further worsen immunosuppression in these patients, but we were not able to determine whether treatment was predictive for the development of a skin cancer with our follow-up.
This study is the first, to our knowledge, to report the incidence of skin malignancies at the time of diagnosis and during follow-up in a community-dwelling cohort of patients with CLL. Our study has a number of limitations. First, patients were classified by residence at the time of CLL diagnosis. Not all patients resided within 30 miles the of Mayo Clinic for the duration of this study. Although many of these patients continued their care at Mayo Clinic or Olmsted Medical Center after moving, not all did, and some cases of skin cancer could have been missed (ie, our estimate of skin cancers in patients with CLL is conservative). Second, the analysis represents a single center experience with adherence to skin cancer screening in patients with CLL. Nonetheless, we were among the first to advocate universal skin cancer screening at diagnosis,4,13,5,6 and it is stated to be standard practice for members of our group. This finding illustrates the challenge of implementing this guideline without a standardized approach. Third, we have a very small sample size and limited power to detect an association between time to skin cancer and prognostic biomarkers, so results from the multivariable analysis should be interpreted with caution. In addition, the medical record may not always reflect whether a skin examination was performed or the extent of the examination. As there are a limited number of providers in Olmsted County, and we had access to all primary care and dermatology records for our cohort, we likely captured any diagnosis of skin malignancy in our cohort with the exception of the few patients who moved or were diagnosed outside of the county. Accordingly, although it reflects a comprehensive ascertainment, our data may slightly underestimate the true incidence of skin malignancies in patients with newly diagnosed CLL.
In conclusion, we documented a low compliance with guidelines to screen for skin malignancy in a community-dwelling cohort of patients with newly diagnosed CLL. Of the patients who underwent full-body skin examinations within 6 months of their diagnosis of CLL, 22% were found to have an active malignancy of the skin, and 21% of patients with CLL had developed a malignancy of the skin by the end of the study. Referrals for screening skin examinations at diagnosis in patients with CLL could have an immediate impact for this at-risk population. Standardized and systems-based approaches are likely to increase compliance with skin cancer screening guidelines in patients with CLL.
Acknowledgment
Study data were obtained from the Rochester Epidemiology Project, which is supported by the National Institute on Aging under Award No. R01 AG034676. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Portions of this study were presented at the ASCO Quality Care Symposium, San Diego, CA, November 30-December 1, 2012.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Tait D. Shanafelt, Genentech, GlaxoSmithKline, Celgene, Cephalon, Hospira, Polyphenon E International Expert Testimony: None Patents, Licenses or Royalties: None Other Remuneration: None
Author Contributions
Conception and design: Aaron S. Mansfield, Kari G. Rabe, Timothy G. Call, Jerry D. Brewer, Tait D. Shanafelt
Financial support: Tait D. Shanafelt
Administrative support: Tait D. Shanafelt
Provision of study materials or patients: Aaron S. Mansfield, Kari G. Rabe, Susan M. Schwager, Tait D. Shanafelt
Collection and assembly of data: Aaron S. Mansfield, Kari G. Rabe, Susan L. Slager, Susan M. Schwager, Tait D. Shanafelt
Data analysis and interpretation: Aaron S. Mansfield, Kari G. Rabe, Susan L. Slager, Timothy G. Call, Jerry D. Brewer, Tait D. Shanafelt
Manuscript writing: Aaron S. Mansfield, Kari G. Rabe, Susan L. Slager, Jerry D. Brewer, Tait D. Shanafelt
Final approval of manuscript: All authors
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Onajin O, Brewer JD. Skin cancer in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. Clin Adv Hematol Oncol. 2012;10:571–576. [PubMed] [Google Scholar]
- 3.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30:843–849. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Call TG. Current approach to diagnosis and management of chronic lymphocytic leukemia. Mayo Clin Proc. 2004;79:388–398. doi: 10.4065/79.3.388. [DOI] [PubMed] [Google Scholar]
- 5.Shanafelt TD, Byrd JC, Call TG, et al. Narrative review: Initial management of newly diagnosed, early-stage chronic lymphocytic leukemia. Ann Intern Med. 2006;145:435–447. doi: 10.7326/0003-4819-145-6-200609190-00007. [DOI] [PubMed] [Google Scholar]
- 6.Shanafelt TD, Kay NE. Comprehensive management of the CLL patient: A holistic approach. Hematology Am Soc Hematol Educ Program. 2007:324–331. doi: 10.1182/asheducation-2007.1.324. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: Half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: An illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: The Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention, National Program of Cancer Registries. United States cancer statistics: 1999-2009 cancer incidence and mortality data. http://www.cdc.gov/uscs.
- 12.Gerlini G, Romagnoli P, Pimpinelli N. Skin cancer and immunosuppression. Crit Rev Oncol Hematol. 2005;56:127–1236. doi: 10.1016/j.critrevonc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Shanafelt TD, Call TG. Current approach to diagnosis and management of chronic lymphocytic leukemia. Mayo Clin Proc. 2004;79:388–398. doi: 10.4065/79.3.388. [DOI] [PubMed] [Google Scholar]