Abstract
Purpose
To determine long-term outcomes in a clinical trial evaluating the role of taxane type and schedule in operable breast cancer and evaluate the impact of obesity and black race on outcome.
Patients and Methods
A total of 4,954 eligible women with stage II to III breast cancer treated with four cycles of doxorubicin plus cyclophosphamide were randomly assigned to receive paclitaxel or docetaxel every 3 weeks for four doses or weekly for 12 doses using a 2 × 2 factorial design. The primary end point was disease-free survival (DFS). Results are expressed as hazard ratios (HRs) from Cox proportional hazards models. All P values are two sided.
Results
When compared with the standard every-3-week paclitaxel arm, after a median follow-up of 12.1 years, DFS significantly improved and overall survival (OS) marginally improved only for the weekly paclitaxel (HR, 0.84; P = .011 and HR, 0.87; P = .09, respectively) and every-3-week docetaxel arms (HR, 0.79; P = .001 and HR, 0.86; P = .054, respectively). Weekly paclitaxel improved DFS and OS (HR, 0.69; P = .010 and HR, 0.69; P = .019, respectively) in triple-negative breast cancer. For hormone receptor–positive, human epidermal growth factor receptor 2–nonoverexpressing disease, no experimental arm improved OS, and black race and obesity were associated with increased risk of breast cancer recurrence and death.
Conclusion
Improved outcomes initially observed for weekly paclitaxel were qualitatively similar but quantitatively less pronounced with longer follow-up, although exploratory analysis suggested substantial benefit in triple-negative disease. Further research is required to understand why obesity and race influence clinical outcome in hormone receptor–positive disease.
INTRODUCTION
Adjuvant chemotherapy substantially reduces the risk of recurrence and death in operable breast cancer, and sequential anthracycline–taxane regimens are among the most effective.1–3 The Eastern Cooperative Oncology Group (ECOG) E1199 trial was designed to compare taxane type (paclitaxel v docetaxel) and schedule (every 3 weeks v weekly) using a 2 × 2 factorial design as adjuvant therapy in women with stage II to III breast cancer. Our initial report showed that sequential administration of weekly paclitaxel 80 mg/m2 given for 12 consecutive doses after four doses of doxorubicin plus cyclophosphamide every 3 weeks significantly improved disease-free survival (DFS; hazard ratio [HR], 0.79; P = .006) and overall survival (OS; HR, 0.76; P = .01) when compared with paclitaxel 175 mg/m2 given every 3 weeks for four doses in prespecified secondary comparisons, although no differences were found in the primary comparisons of taxane type (paclitaxel v docetaxel) or schedule (every 3 weeks v weekly).4 In addition, exploratory analysis revealed benefit for weekly paclitaxel irrespective of hormone receptor expression in human epidermal growth factor receptor 2 (HER2) –nonoverexpressing disease.
Our initial analysis was reported after a median follow-up of 5.3 years, at which time full information was reached for the primary comparisons of taxane type and schedule. We now report an updated analysis of the secondary comparisons to provide information regarding clinical outcome at 10 years for the four individual treatment arms. The 10-year interval after diagnosis captured information about late recurrence beyond 5 years commonly observed in hormone receptor–positive disease5 and serves as a benchmark in decision aides and multiparameter assays commonly used to counsel patients about potential benefits from adjuvant therapy.6–8
PATIENTS AND METHODS
Patient Selection, Treatment, and End Points
The analysis included patients enrolled onto a National Cancer Institute–sponsored clinical trial (ClinicalTrials.gov identifier NCT00004125) coordinated by the ECOG, the methods and results of which have been previously reported.4 The protocol was reviewed and approved by the institutional review board at each participating institution, and all patients provided written informed consent. Chemotherapy was prescribed by actual body weight. The protocol initially specified 5 years of tamoxifen (20 mg orally daily) in patients with hormone receptor–positive disease and was amended in June 2005 to recommend tamoxifen for 2 to 5 years followed by an aromatase inhibitor for up to 5 years in postmenopausal women to reflect updated American Society of Clinical Oncology (ASCO) guidelines for adjuvant endocrine therapy.9 Of 5,052 patients enrolled, 4,954 were deemed eligible and included in the analysis (4,950 were deemed eligible in original report,4 but four previously ineligible patients were deemed eligible on additional review in this updated report). The allocation of patients by treatment arm and their outcomes are shown in the CONSORT diagram (Fig 1). The primary end point was DFS, and OS was a secondary end point, as previously defined.4
Fig 1.
CONSORT diagram showing No. of patients enrolled (N = 5,052), No. of eligible patients included in efficacy analysis (n = 4,954), and clinical outcomes for patients with predefined breast cancer subtypes of triple-negative breast cancer (TN), hormone receptor–positive, human epidermal growth factor receptor 2 (HER2) –nonoverexpressing breast cancer (HR+), and HER2-overexpressing breast cancer (HER2+). DFS, disease-free survival.
Statistical Analysis Plan
The trial was previously reported when there were a sufficient number of DFS events to perform the primary comparison of taxane type (paclitaxel v docetaxel) and schedule (every 3 weeks v weekly). Per protocol, if one or both of the two primary comparisons were significant, pairwise comparisons of each of the three experimental arms (weekly paclitaxel [P1], docetaxel every 3 weeks [D3], and weekly docetaxel [D1]) with the standard every-3-week paclitaxel arm (P3) were prespecified as secondary comparisons; after approximately 1,400 DFS events, the secondary comparisons would have provided 80% power to detect a 22% reduction in the failure hazard rate for any of the experimental arms (using two-sided nominal 5% level tests, corrected for multiple comparisons). Because there were no differences in the primary comparisons, any subsequent analyses about the secondary comparisons would be regarded as post hoc and exploratory. The updated analysis reported here was planned when at least 85% of surviving patients had been observed for at least 10 years.
The Kaplan-Meier method was used to estimate event-time distributions for DFS and OS. The stratified log-rank test was used to compare DFS and OS distributions between treatment groups. The tests for the comparisons of the individual arms with the P3 arm were stratified on number of positive nodes (zero v ≥ one) and estrogen receptor (ER) status (positive v negative v unknown). Cox proportional hazards models, stratified on number of positive nodes and ER status, were used to estimate the HRs for treatment groups, without adjusting for other variables. Similar to the initial report,4 a post hoc analysis of DFS and OS outcomes according to hormone receptor status and HER2 expression was also conducted as an exploratory analysis. This categorization was based on local institutional assessment of ER, progesterone receptor, and HER2 expression recorded in the patient case report forms. No stratification factors were considered in this exploratory analysis. In addition, multivariable Cox proportional hazards models were fit for hormone receptor–positive disease to identify prognostic factors for DFS and OS. Factors examined in the multivariable Cox models were treatment arm (P1, D3, or D1 v P3), age at registration (continuous), race (black v other), obesity (body mass index [BMI] ≥ 30 kg/m2 v other), tumor size (≥ v < 2 cm), number of positive lymph nodes (zero v one to three v ≥ three), and endocrine therapy (aromatase inhibitor ± tamoxifen v tamoxifen alone v no endocrine therapy or unknown). Endocrine therapy was included as a time-varying covariate, because patients could start it at any time after completing the protocol therapy (discontinuation of endocrine therapy was not considered in model); all other variables were included as time-fixed covariates. The Wald test was used to test for significant covariates in the Cox models. Proportional hazards assumption was tested using Schoenfeld residuals in all Cox models. All P values are two sided. No adjustment was made for multiple comparisons. All analyses were conducted using STATA software (version 13; STATA, College Station, TX).
RESULTS
Patient Demographic and Clinical Characteristics
The four treatment arms were well balanced regarding known prognostic factors, including P3 or P1 administration and D3 or D1 administration (Appendix Table A1, online only). The median age of patients was 51 years (range, 19 to 84 years); 46% were premenopausal, 88% had at least one positive axillary lymph node, 69% had ER-positive disease, 20% had HER2-positive disease, 35% were obese (BMI ≥ 30 kg/m2), and 8% were black.
Primary Comparisons of Taxane Type and Schedule
As of May 1, 2014, there were 1,639 DFS events (33.1% of eligible patients) and 1,283 deaths (25.9%) after a median follow-up of 12.1 years (Appendix Table A2, online only). There were substantially more events than in the original report (1,058 DFS events and 686 deaths after median follow-up of 5.3 years4). As in the original report, there remained no statistical difference in OS or DFS between the paclitaxel-treated groups (P1 and P3) and the docetaxel-treated groups (D1 and D3; stratified log-rank P = .977 for OS and .322 for DFS) and no difference between the two schedules (P1 and D1 v P3 and D3; stratified log-rank P = .795 for OS and .876 for DFS). The results were similar from the stratified Cox models, without adjusting for any other variables (data not shown). As in the original report, in a stratified Cox model that included the taxane administered (docetaxel v paclitaxel), the schedule of administration (weekly v every 3 weeks), and their interaction, the taxane-by-schedule interaction test was significant for DFS (HR, 1.46 for interaction term; P < .001) and OS (HR, 1.36 for interaction term; P = .007), which indicates that P1 and D3 were superior to P3.
Secondary Comparisons of Experimental Arms (P1, D3, D1) With Standard Arm (P3)
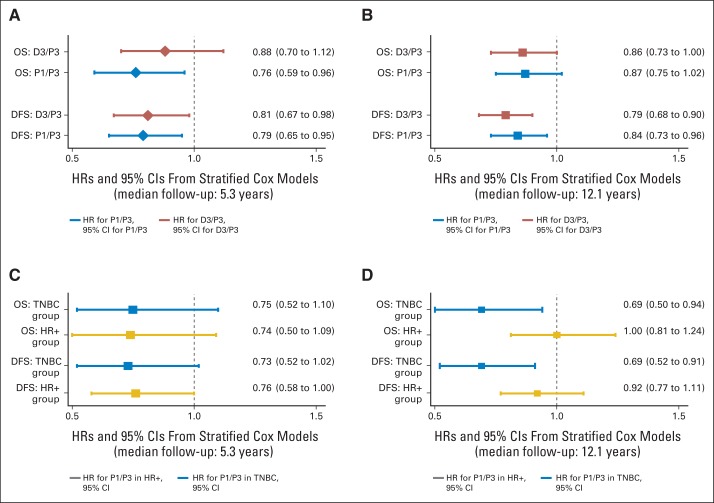
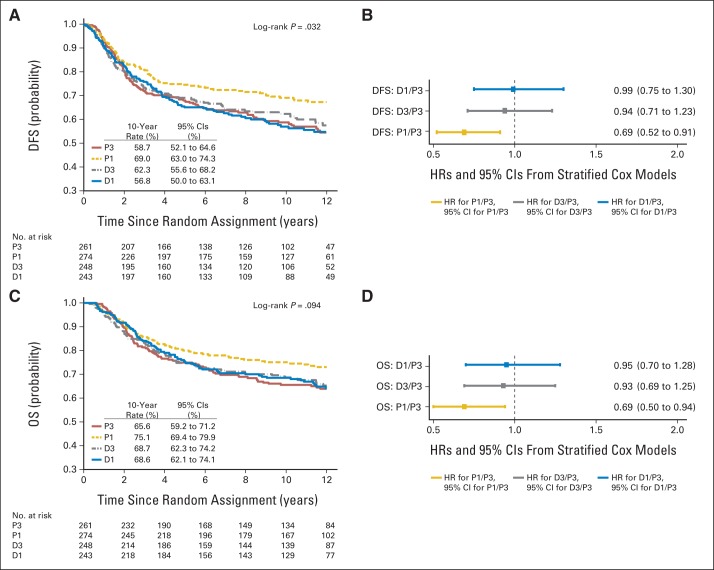
The Kaplan-Meier curves for the four treatment groups are shown for DFS in Figure 2A. The DFS curves were statistically different between the four treatment groups (stratified log-rank P = .0014). Stratified Cox model analysis also showed that patients in the P1 (HR, 0.84; P = .011) and D3 arms (HR, 0.79; P = .001), but not D1 arm, had significantly lower hazard of DFS failure compared with patients in the P3 arm (Fig 2B). The OS curves shown in Figure 2C were not statistically different between the four treatment groups (stratified log-rank P = .0578). Stratified Cox model analysis shows that patients in the P1 (HR, 0.87; P = .09) and D3 arms (HR, 0.86; P = .054) had marginally lower hazard of death compared with patients in the P3 arm, but neither reached statistical significance (Fig 2D). When compared with the original report after a median follow-up of 5.3 years, the results were similar for the D3 arm after a median follow-up of 12.1 years. However, for the P1 arm, the results were qualitatively similar but quantitatively less pronounced in both the secondary comparisons in the entire population (Figs 3A and 3B) and were no longer apparent in the exploratory analysis in hormone receptor–positive, HER2-nonoverexpressing disease (Figs 3C and 3D). The 10-year rates were on average 11% to 12% lower for DFS and 8% to 11% lower for OS compared with the 5-year rates, reflecting the fact that approximately 35% of the DFS events and 47% of the deaths occurred after year 5 (Appendix Table A3, online only).
Fig 2.
(A, B) Disease-free survival (DFS) and (C, D) overall survival (OS) in entire population of 4,954 eligible patients, based on (A, C) Kaplan-Meier estimates by treatment arm and (B, D) estimated hazard ratios (HRs) and 95% CIs from stratified univariable Cox models in experimental arms (weekly paclitaxel [P1], docetaxel every 3 weeks [D3], and weekly docetaxel [D1]) compared with standard arm (paclitaxel every 3 weeks [P3]).
Fig 3.
Disease-free survival (DFS) and overall survival (OS) hazard ratios (HRs) and 95% CIs for weekly paclitaxel (P1) and docetaxel every 3 weeks (D3) compared with paclitaxel every 3 weeks (P3) in overall population (A, B) and in patients with triple-negative breast cancer (TNBC) and hormone receptor–positive/human epidermal growth factor receptor 2–nonoverexpressing disease (HR+; C, D), including the prior report and current report.
Exploratory Analysis by Breast Cancer Subtype
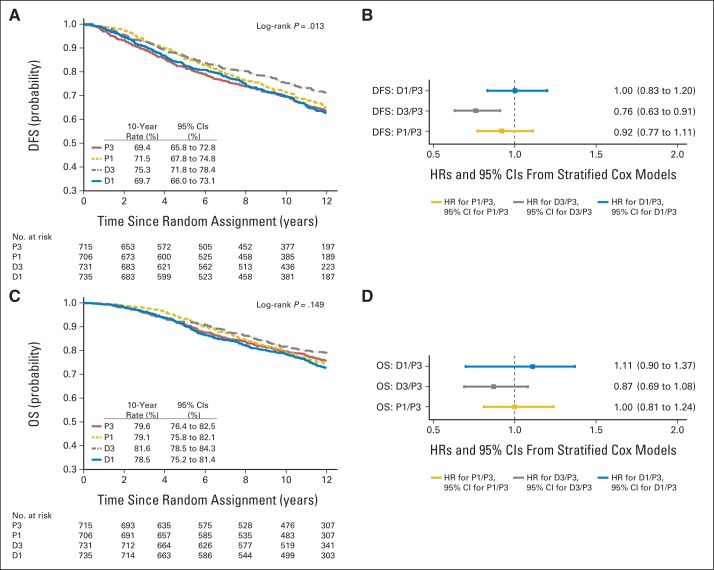
For the 1,025 patients with triple-negative breast cancer (TNBC), P1 was associated with significantly improved DFS (HR, 0.69; P = .001) and OS (HR, 0.69; P = .019); similar benefits were not observed for docetaxel administered via either schedule (Figs 4A to 4D). For the 2,879 patients with hormone receptor–positive and HER2-negative/unknown disease, D3 was associated with improved DFS (HR, 0.76; P = .004) but not OS; for the P1 or D1 arms, no significant results were observed for either DFS or OS (Figs 5A to 5D). For the 971 patients with HER2-overexpressing disease, there was no significant difference by treatment arm (data not shown).
Fig 4.
(A, B) Disease-free survival (DFS) and (C, D) overall survival (OS) in 1,025 patients with triple-negative breast cancer, based on (A, C) Kaplan-Meier estimates by treatment arm and (B, D) estimated hazard ratios (HRs) and 95% CIs from univariable Cox models in experimental arms (weekly paclitaxel [P1], docetaxel every 3 weeks [D3], and weekly docetaxel [D1]) compared with standard arm (paclitaxel every 3 weeks [P3]).
Fig 5.
(A, B) Disease-free survival (DFS) and (C, D) overall survival (OS) in 2,879 patients with hormone receptor–positive, human epidermal growth factor receptor 2–nonoverexpressing breast cancer, based on (A, C) Kaplan-Meier estimates by treatment arm and (B, D) estimated hazard ratios (HRs) and 95% CIs from univariable Cox models in experimental arms (weekly paclitaxel [P1], docetaxel every 3 weeks [D3], and weekly docetaxel [D1]) compared with standard arm (paclitaxel every 3 weeks [P3]).
Relation Between Obesity, Race, and Clinical Outcome
In previous analysis of this trial population after a median follow-up of 7.9 years, we found a relationship between higher BMI10 and black race,11 with worse clinical outcomes in patients with hormone receptor–positive, HER2-nonoverexpressing disease. Here we evaluated multivariable Cox models after a median follow-up of 12.1 years. Obesity was a significant prognostic factor for a higher risk of a DFS event (HR, 1.24; P = .003) and death (HR, 1.29; P = .002) in nonblack patients. Black race was significantly associated with poor DFS and OS compared with nonblacks in nonobese patients (HR, 2.29; P < .001 for DFS; HR, 2.24; P < .001 for OS), but not in obese patients (HR, 1.27; P = .169 for DFS; HR, 1.28; P = .212 for OS), indicating black women have poorer survival, particularly for nonobese women, compared with nonblack women after adjustment for the factors included in the multivariable model.
To more fully characterize the association between obesity and clinical outcome, we evaluated the smoothed average hazard rate for breast cancer recurrence estimated using Kernel estimator by cancer subtype in the presence or absence of obesity (Fig 6). Similar to prior reports, TNBC was associated with high recurrence rates within 5 years of diagnosis, whereas hormone receptor–positive disease was associated with lower risk of recurrence within the first 5 years, but a continuous risk of recurrence beyond 5 years. Here we also show that obesity was associated with higher risk of recurrence in hormone receptor–positive disease from approximately 3 to 8 years after diagnosis.
Fig 6.
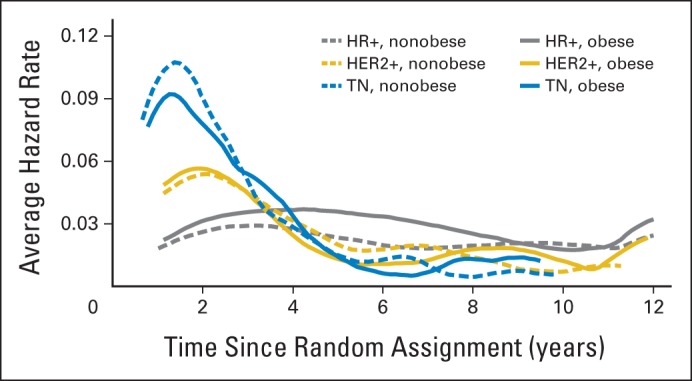
Smoothed average hazard rate for breast cancer recurrence estimated by Kernel estimator according to breast cancer subtype in presence or absence of obesity. HER2, human epidermal growth factor receptor 2; HER2+, HER2-overexpressing breast cancer; HR+, hormone receptor–positive, HER2–nonoverexpressing breast cancer; TN, triple-negative breast cancer.
DISCUSSION
Here we report long-term outcomes after a median follow-up of 12.1 years of a trial that was designed to determine the optimal taxane and schedule in stage II to III breast cancer. We previously reported no significant differences in outcome between taxane (paclitaxel v docetaxel) and schedule (every 3 weeks v weekly) in the primary comparisons after a median follow-up of 5.3 years and improved DFS and OS for the P1 arm compared with the P3 arm in the secondary comparisons.4 As a consequence of this study and others,12,13 the P1 regimen emerged as a component of a preferred adjuvant chemotherapy regimen recommended by the National Comprehensive Cancer Center Network clinical practice guidelines.14 Although D3 was also associated with improved DFS, OS was not improved, and there was more overall toxicity than with P1. With substantially longer follow-up for at least 10 years, the results are qualitatively similar overall but quantitatively less pronounced for the P1 arm. P1 and D3 were both associated with significantly improved DFS (HR, 0.84; P = .011 and HR, 0.79; P = .001, respectively), but OS was only marginally improved (HR, 0.87; P = .09 and HR, 0.86; P = .054, respectively).
Breast cancer is a heterogeneous disease, and clinical outcome and response to therapy vary by subtype. This study was performed between 1999 and 2001, before adjuvant trastuzumab became standard therapy for HER2-overexpressing disease,15 and thus, no patients received such therapy. We therefore performed exploratory analyses evaluating clinical outcome in HER2-nonoverexpressing disease, where the comparisons would be most clinically relevant. This analysis revealed that P1, but not the other experimental arms, was associated with approximately a 30% reduction in the risk of recurrence and death in TNBC, which translated into approximately a 10% absolute improvement in DFS and OS at 10 years. This clearly establishes sequential doxorubicin plus cyclophosphamide followed by P1 as an effective adjuvant chemotherapy option for stage II to III TNBC.
Although P1 was associated with similar benefits irrespective of hormone receptor status in HER2-nonoverexpressing disease after a median of 5.3 years, the benefits initially seen in hormone receptor–positive disease waned with longer follow-up. This seems inconsistent with findings from the Early Breast Cancer Trialists Collaborative Group, which found that the proportional risk reductions associated with chemotherapy were little affected by ER expression.3 Adjuvant chemotherapy primarily prevents early recurrences within 5 years of diagnosis,3 whereas endocrine therapy prevents early and late recurrences.16–19 As exemplified by our study and described in prior reports, patients with hormone receptor–positive disease have a continuous annual risk of recurrence that persists beyond 5 years.20,21 All patients included in this trial had a large tumor (> 2 cm) and/or positive axillary lymph nodes, factors known to be prognostic for recurrence overall and especially for late recurrence in hormone receptor–positive disease.21 Although the type of adjuvant endocrine therapy administered within 5 years of diagnosis was comparable in the four treatment arms,4 and the protocol was updated approximately 4 years after completion of accrual to reflect updated ASCO practice guidelines recommending consideration of extended adjuvant therapy,9 we do not have accurate information regarding the proportion of patients who received > 5 years of endocrine therapy. It is conceivable that early benefits associated with adjuvant chemotherapy in hormone receptor–positive disease may be mitigated by a suboptimal duration of endocrine therapy in patients at risk for late relapse; if true, however, it would be expected to affect relapse patterns irrespective of taxane or taxane schedule and not specifically in the P1 arm. Taken together, however, our findings suggest that breast cancer heterogeneity influences benefit from adjuvant taxane therapy. Although P1 was the most effective option tested for TNBC and was also associated with early gains in hormone receptor–positive disease, the taxane regimen employed may be less important in patients at risk for late relapse, where attention to the use of extended adjuvant endocrine therapy may be more impactful.
Although our study focused on evaluating the effects of differing taxanes and schedules adjusted for disease-specific prognostic factors, we also evaluated host-specific factors unrelated to the underlying disease that are not integrated into current staging but nevertheless influence prognosis. We found a statistically significant association between obesity at diagnosis and inferior DFS and OS in patients with hormone receptor–positive, HER2-nonoverexpressing disease, but not other breast cancer subtypes, a finding that was not consistently observed in prior analyses.22 This subtype-specific effect was confirmed in a meta-analysis of approximately 80,000 patients, indicating that obesity at diagnosis was associated with a 35% increase in the risk of recurrence and death in ER-positive breast cancer, primarily in women who were premenopausal at diagnosis, but not in ER-negative disease.23 Moreover, when the timing of recurrence was evaluated, we observed that obesity was associated with later recurrences between 3 and 8 years after diagnosis. Potentially contributing factors may include hyperestrogenemia,24 hyperinsulinemia,25,26 elevation of certain lipids,27,28 inflammation,29,30 or a combination of factors.31 This raises the possibility that there may be sufficient lead time between diagnosis and recurrence to permit currently recommended dietary and lifestyle interventions to have a significant impact in reducing the risk of recurrence,32 which is being evaluated in a variety of ongoing and planned trials.33
Although breast cancer incidence and mortality have declined by approximately 35% in the United States since 1990, mortality rates have declined less in black women, resulting in a substantially higher mortality rate for black compared with nonblack women.34 A substantial body of evidence indicates a racial divide in breast cancer35 and other hormone-dependent cancers, such as uterine and prostate cancers, but generally not other cancer types.36,37 Contributing factors in breast cancer include higher rates of advanced-stage disease38 and/or more aggressive TNBC,39 less adherence to chemotherapy40 and endocrine therapy,41 more comorbidities,42 and disparities in care.43–45 Here we find that despite selection of relatively healthy women lacking significant comorbidities who had access to state-of-the-art care, black race was associated with inferior outcome, specifically in hormone receptor–positive breast cancer, a finding that was not explained by a higher obesity rate, more advanced-stage disease, or differing adherence to chemotherapy or endocrine therapy in black study participants.11 Similar findings have been described in other reports.46 Further research is required to determine why black women with hormone receptor–positive breast cancer have a higher risk of recurrence.
In conclusion, sequential doxorubicin plus cyclophosphamide followed by weekly paclitaxel was the most effective adjuvant chemotherapy option tested in this trial for TNBC and represents a reasonable treatment option for this population. Similar benefits were not observed for patients with hormone receptor–positive, HER2-nonoverexpressing disease, where obesity and black race were found to be independently associated with worse clinical outcome.
Appendix
Table A1.
Demographic and Clinical Characteristics (N = 4954)
| Characteristic | Paclitaxel Every 3 Weeks (n = 1,253) | Weekly Paclitaxel (n = 1,232) | Docetaxel Every 3 Weeks (n = 1,236) | Weekly Docetaxel (n = 1,233) |
|---|---|---|---|---|
| Age, years | ||||
| Median | 51 | 51 | 51 | 51 |
| Range | 26-79 | 22-84 | 25-79 | 19-81 |
| Race, % | ||||
| White | 84.2 | 83.2 | 85.0 | 85.4 |
| Black | 8.1 | 8.7 | 9.7 | 7.1 |
| Other | 7.7 | 8.1 | 5.3 | 7.5 |
| BMI, % | ||||
| Normal (< 25 kg/m2) | 29.5 | 30.1 | 31.5 | 28.9 |
| Overweight (25 to 30 kg/m2) | 31.6 | 32.0 | 29.4 | 31.4 |
| Obese (≥ 30 kg/m2) | 35.8 | 33.9 | 35.4 | 35.8 |
| Unknown | 3.1 | 4.1 | 3.7 | 4.0 |
| Tumor size, % | ||||
| < 2 cm | 39.0 | 36.1 | 36.6 | 36.5 |
| ≥ 2 cm | 60.7 | 63.8 | 63.1 | 63.3 |
| Unknown | 0.2 | 0.1 | 0.3 | 0.2 |
| Positive lymph nodes, % | ||||
| 0 | 12.1 | 12.2 | 11.9 | 11.8 |
| 1-3 | 54.8 | 55.8 | 55.5 | 55.8 |
| 4-9 | 22.9 | 22.5 | 23.1 | 22.2 |
| ≥ 10 | 10.1 | 9.5 | 9.1 | 10.0 |
| Unknown | 0.2 | 0.1 | 0.3 | 0.2 |
| Expression of ER and/or PR, % | ||||
| Yes | 71.0 | 70.2 | 71.7 | 72.8 |
| No | 27.9 | 28.7 | 27.0 | 26.3 |
| Unknown | 1.2 | 1.1 | 1.3 | 1.0 |
| Expression of HER2 protein, % | ||||
| Yes | 20.6 | 19.2 | 19.3 | 19.6 |
| No | 70.2 | 69.6 | 70.6 | 68.4 |
| Unknown | 9.2 | 11.2 | 10.1 | 12.1 |
| Premenopausal or age < 50 years, % | 46.2 | 45.8 | 47.0 | 45.6 |
| Breast-sparing surgery, % | 39.4 | 39.0 | 39.2 | 38.9 |
Abbreviations: BMI, body mass index; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Table A2.
DFS and OS Events by Treatment Arm Among Eligible Patients (N = 4,954)
| Events | Paclitaxel Every 3 Weeks (n = 1,253) | Weekly Paclitaxel (n = 1,232) | Docetaxel Every 3 Weeks (n = 1,236) | Weekly Docetaxel (n = 1,233) | Total (N = 4,954) |
|---|---|---|---|---|---|
| Ipsilateral recurrence | 38 | 33 | 33 | 40 | 144 |
| Locoregional recurrence | 68 | 54 | 66 | 75 | 263 |
| Distant recurrence | 290 | 247 | 226 | 257 | 1,020 |
| Any recurrence* | 332 | 284 | 253 | 303 | 1,172 |
| Second invasive breast cancer | 48 | 28 | 28 | 36 | 140 |
| Second invasive breast cancer without recurrence* | 39 | 22 | 22 | 26 | 109 |
| Death without recurrence/second invasive breast cancer* | 78 | 84 | 95 | 101 | 358 |
| Death without recurrence | 84 | 86 | 98 | 104 | 372 |
| Death with recurrence | 261 | 220 | 203 | 241 | 925 |
| DFS events* | 449 | 390 | 370 | 430 | 1,639 |
| OS events | 339 | 304 | 298 | 342 | 1,283 |
Abbreviations: DFS, disease-free survival; OS, overall survival.
Total of 31 patients had both recurrence and second invasive breast cancer during follow-up.
Table A3.
5- and 10-Year DFS and OS Rates by Treatment Arm Among Eligible Patients (N = 4,954)
| Arm | DFS |
OS |
||
|---|---|---|---|---|
| Rate | 95% CI | Rate | 95% CI | |
| 5 years | ||||
| P3 | 0.771 | 0.746 to 0.794 | 0.865 | 0.844 to 0.883 |
| P1 | 0.819 | 0.795 to 0.839 | 0.894 | 0.875 to 0.910 |
| D3 | 0.814 | 0.791 to 0.835 | 0.873 | 0.853 to 0.891 |
| D1 | 0.779 | 0.754 to 0.801 | 0.865 | 0.845 to 0.884 |
| 10 years | ||||
| P3 | 0.655 | 0.627 to 0.682 | 0.753 | 0.727 to 0.777 |
| P1 | 0.707 | 0.679 to 0.733 | 0.777 | 0.752 to 0.801 |
| D3 | 0.719 | 0.692 to 0.744 | 0.785 | 0.760 to 0.808 |
| D1 | 0.671 | 0.643 to 0.698 | 0.759 | 0.733 to 0.782 |
Abbreviations: D1, weekly docetaxel; D3, docetaxel every 3 weeks; DFS, disease-free survival; OS, overall survival; P1, weekly paclitaxel; P3, paclitaxel every 3 weeks.
See accompanying editorial on page 2334
Support information appears at the end of this article.
Presented at the 2014 San Antonio Breast Cancer Symposium, San Antonio, TX, December 11, 2014.
This study was coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group (group co-chairs Robert L. Comis, MD, Mitchell D. Schnall, MD, PhD). Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00004125.
Support
Supported in part by Public Health Service Grants No. CA180794, CA180820, CA189859, CA180888, CA180821, CA180867, CA180867, CA180790, CA190140, CA180802, CA180795, CA180816, CA180864, and CA180844 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Joseph A. Sparano, Antonio C. Wolff, George W. Sledge Jr, William C. Wood, Nancy E. Davidson
Administrative support: Joseph A. Sparano, Silvana Martino, Jennifer A. Ligibel, Edith A. Perez, Thomas Saphner, Antonio C. Wolff, George W. Sledge Jr, William C. Wood, Nancy E. Davidson
Provision of study materials or patients: Joseph A. Sparano, Silvana Martino, Jennifer A. Ligibel, Edith A. Perez, Thomas Saphner, Antonio C. Wolff, George W. Sledge Jr, Nancy E. Davidson
Collection and assembly of data: Joseph A. Sparano, Fengmin Zhao, Antonio C. Wolff
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Joseph A. Sparano
No relationship to disclose
Fengmin Zhao
No relationship to disclose
Silvana Martino
Consulting or Advisory Role: Amgen, Novartis, Rockwell, Celgene, AstraZeneca, Boehringer Ingelheim
Travel, Accommodations, Expenses: Amgen, Novartis, Rockwell, Celgene, AstraZeneca
Jennifer A. Ligibel
No relationship to disclose
Edith A. Perez
No relationship to disclose
Thomas Saphner
No relationship to disclose
Antonio C. Wolff
Consulting or Advisory Role: Mersana
Research Funding: Myriad Genetics
George W. Sledge Jr
Leadership: Syndax
Honoraria: Genentech, Symphogen
Consulting or Advisory Role: Symphogen
Research Funding: Genentech/Roche (Inst)
William C. Wood
No relationship to disclose
Nancy E. Davidson
No relationship to disclose
REFERENCES
- 1.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 2.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 3.Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 6.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 7.Albain KS, Barlow WE, Ravdin PM, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: A phase 3, open-label, randomised controlled trial. Lancet. 2009;374:2055–2063. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor–positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 9.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor–positive breast cancer: Status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 10.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118:5937–5946. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104:406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martín M, Rodríguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 13.Martin M, Ruiz A, Ruiz Borrego M, et al. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high-risk, node-negative breast cancer: Results from the GEICAM/2003-02 study. J Clin Oncol. 2013;31:2593–2599. doi: 10.1200/JCO.2012.46.9841. [DOI] [PubMed] [Google Scholar]
- 14.Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 15.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 16.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–1955. doi: 10.1200/JCO.2007.11.6798. [DOI] [PubMed] [Google Scholar]
- 19.Jin H, Tu D, Zhao N, et al. Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: Analyses adjusting for treatment crossover. J Clin Oncol. 2012;30:718–721. doi: 10.1200/JCO.2010.34.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dignam JJ, Dukic V, Anderson SJ, et al. Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Res Treat. 2009;116:595–602. doi: 10.1007/s10549-008-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sestak I, Dowsett M, Zabaglo L, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–1511. doi: 10.1093/jnci/djt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niraula S, Ocana A, Ennis M, et al. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: A meta-analysis. Breast Cancer Res Treat. 2012;134:769–781. doi: 10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- 23.Pan H, Gray RG. Effect of obesity in premenopausal ER+ early breast cancer: EBCTCG data on 80,000 patients in 70 trials. J Clin Oncol. 2014;32(suppl 15s):5s. abstr 503. [Google Scholar]
- 24.Lunardi G, Del Mastro L, Bighin C, et al. Effects of body mass index (BMI) on plasma levels of estrone sulfate (ES) in postmenopausal women with breast cancer (BC) during letrozole (L) treatment. J Clin Oncol. 2011;29(suppl):48s. abstr 515. [Google Scholar]
- 25.Goodwin PJ, Ennis M, Bahl M, et al. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. 2009;114:517–525. doi: 10.1007/s10549-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 26.Irwin ML, Duggan C, Wang CY, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: The health, eating, activity, and lifestyle study. J Clin Oncol. 2011;29:47–53. doi: 10.1200/JCO.2010.28.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Ishikawa T, Sirianni R, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: New insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book. 2013:46–51. doi: 10.1200/EdBook_AM.2013.33.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson ER, Brown KA. Minireview: Obesity and breast cancer: A tale of inflammation and dysregulated metabolism. Mol Endocrinol. 2013;27:715–725. doi: 10.1210/me.2013-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain R, Strickler HD, Fine E, et al. Clinical studies examining the impact of obesity on breast cancer risk and prognosis. J Mammary Gland Biol Neoplasia. 2013;18:257–266. doi: 10.1007/s10911-013-9307-3. [DOI] [PubMed] [Google Scholar]
- 32.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ligibel JA, Strickler HD. Obesity and its impact on breast cancer: Tumor incidence, recurrence, survival, and possible interventions. Am Soc Clin Oncol Educ Book. 2013:52–59. doi: 10.14694/EdBook_AM.2013.33.52. [DOI] [PubMed] [Google Scholar]
- 34.Menashe I, Anderson WF, Jatoi I, et al. Underlying causes of the black-white racial disparity in breast cancer mortality: A population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: A meta-analysis of 14 studies involving over 10,000 African-American and 40,000 white American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 36.Albain KS, Unger JM, Crowley JJ, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach PB, Schrag D, Brawley OW, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 38.Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88:114–123. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 40.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 41.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 42.Tammemagi CM, Nerenz D, Neslund-Dudas C, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 43.Bhargava A, Du XL. Racial and socioeconomic disparities in adjuvant chemotherapy for older women with lymph node-positive, operable breast cancer. Cancer. 2009;115:2999–3008. doi: 10.1002/cncr.24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider EC, Zaslavsky AM, Epstein AM. Racial disparities in the quality of care for enrollees in medicare managed care. JAMA. 2002;287:1288–1294. doi: 10.1001/jama.287.10.1288. [DOI] [PubMed] [Google Scholar]
- 45.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y, Ma H, Malone KE, et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29:3358–3365. doi: 10.1200/JCO.2010.34.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]