Abstract
Purpose
On the basis of evidence that resistance to vascular endothelial growth factor (VEGF) receptor inhibition is caused by hypoxia-driven residual VEGF and other proangiogenic factors, combinations of agents from these classes were hypothesized to improve treatment outcomes relative to single-agent VEGF pathway blockade.
Patients and Methods
A total of 361 patients with metastatic clear cell renal cell carcinoma were randomly assigned equally to arm A (bevacizumab monotherapy 10 mg/kg intravenously [IV] every 2 weeks), B (bevacizumab 10 mg/kg IV every 2 weeks and temsirolimus 25 mg IV every week), C (bevacizumab 5 mg/kg IV every 2 weeks and sorafenib 200 mg orally twice daily on days 1 to 5, 8 to 12, 15 to 19, and 22 to 26), or D (sorafenib 200 mg twice daily and temsirolimus 25 mg IV weekly). Progression-free survival was the primary end point.
Results
Among 331 eligible treated patients, median PFS was 7.5 months for bevacizumab alone (90% CI, 5.8 to 10.8 months), 7.6 months for bevacizumab plus temsirolimus (90% CI, 6.7 to 9.2 months), 9.2 months for bevacizumab plus sorafenib (90% CI, 7.5 to 11.4 months), and 7.4 months for sorafenib plus temsirolimus (90% CI, 5.6 to 7.9 months). Hazard ratios from stratified Cox proportional hazards models were 1.01, 0.89, and 1.07 (with respective P values of .95, .49, and .68) for the three combinations, respectively, compared with bevacizumab alone. Adverse events did not differ significantly among treatment arms.
Conclusion
The activity of sorafenib, temsirolimus, and bevacizumab administered in doublet combinations did not significantly improve median progression-free survival in comparison with bevacizumab monotherapy.
INTRODUCTION
The treatment of patients with metastatic clear cell renal cell carcinoma (ccRCC) has been revolutionized by the recognition that the frequent loss of the von Hippel-Lindau gene product in ccRCC results in dysregulated angiogenesis1 and upregulation of proangiogenic cytokines, such as vascular endothelial growth factor (VEGF).2 VEGF targeted antibody bevacizumab was the first agent to demonstrate improved disease control,3 confirmed in subsequent phase III trials of the addition of bevacizumab to interferon (IFN) versus IFN alone.4,5 Several small-molecule inhibitors of the VEGF receptor (VEGFR) and receptors of other proangiogenic cytokines, including platelet-derived growth factor (PDGF), have demonstrated single-agent efficacy in randomized trials.6–9 Rapamycin analog mammalian target of rapamycin (mTOR) inhibitors have been suggested to downregulate the von Hippel-Lindau–regulated hypoxia inducible factor (HIF) –1α,10 and two such agents have demonstrated improved outcomes for patients with metastatic ccRCC in randomized trials.11,12
With objective response rates ranging from 10% to 40% in treatment-naive patients and median progression-free survival of < 1 year with sorafenib, sunitinib, pazopanib, temsirolimus, and bevacizumab with or without IFN, there remains a need for improved therapy in patients with metastatic ccRCC (particularly for those with adverse prognostic features).3,7,11,13
Interrogation of circulating angiogenic cytokines in patients receiving VEGFR-targeted therapies demonstrated a marked upregulation in VEGF and soluble VEGFRs early in the course of treatment.14,15 This motivated the investigation of bevacizumab and sorafenib specifically. Early clinical investigations of combinations of VEGF, VEGFR, and mTOR inhibitors were motivated by the hypothesis that VEGF and mTOR signaling in endothelial cells is incompletely blocked when each agent is administered at its maximum-tolerated dose.
Phase I clinical trials were conducted investigating two-drug combinations of bevacizumab, sorafenib, and temsirolimus,16–18 and recommended phase II doses were established. Doses for sorafenib combined with either bevacizumab or temsirolimus were lower than those established in single-agent trials because of overlapping toxicities. Despite the need for dose attenuation, promising combination activity was observed with these doublet regimens in patients with ccRCC enrolled onto the phase Ib trials. Therefore, we proposed a randomized phase II trial evaluating each two-drug combination regimen of sorafenib, temsirolimus, and bevacizumab relative to a benchmark of single-agent bevacizumab. Single-agent bevacizumab has been established to be well tolerated and active (response rate, 10%; median progression-free survival [PFS] comparable to other single agents investigated in study), justifying its choice as the reference standard for this randomized phase II trial.3
We aimed to demonstrate an improvement in PFS with two-drug therapy versus monotherapy with a statistical design attuned to seeking a signal of efficacy. Further investigation would then be warranted in a definitive phase III trial.
PATIENTS AND METHODS
Eligible patients were required to be age ≥ 18 years and have unresectable histologically confirmed ccRCC, with < 25% of any other histology. Patients had to have undergone prior nephrectomy, unless the primary tumor was < 5 cm or the patient had extensive liver metastases (> 30% of liver parenchyma) or multiple (> five) bone metastases. Normal organ function and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 were required. Patients could not have had > one prior regimen containing a vaccine or cytokine-based immunotherapy for advanced disease. Exclusion criteria included prior antiangiogenic therapy, including but not limited to sunitinib, sorafenib, pazopanib, vandetanib, or bevacizumab, or prior treatment with mTOR inhibitors (temsirolimus, everolimus, or investigational). Prior thalidomide or IFN-α-2b were allowed for either adjuvant or advanced-disease therapy. History or clinical evidence of CNS disease was prohibited, as were other medical conditions judged by the treating physician to raise the risk of toxicities related to VEGF/VEGFR-targeted therapy. All patients provided written voluntary informed consent using a document approved at the central and local institutional review board levels.
Prior radiation therapy was permitted, but toxicities from radiation had to have resolved, and a minimum of 2 weeks had to have elapsed before random assignment. Pregnant (requiring testing within 7 days before random assignment and treatment) and breastfeeding women were excluded. HIV-positive patients receiving combination antiretroviral therapy were excluded because of possible pharmacokinetic interactions with study drugs.
The primary objective was to determine median PFS for each study arm. PFS was defined as time from random assignment to radiographic or clinical evidence of disease progression (as defined by RECIST [version 1.0]19) or death resulting from any cause without progression. Patients alive without progression were censored at the date of last disease assessment. On the basis of evidence from Bukowski et al,20 median PFS of approximately 9 months was to be expected from single-agent bevacizumab. The study was designed to detect a 67% improvement to 15 months (target PFS hazard ratio, 0.60) in any combination cohort, assuming bevacizumab-treated patients confirmed the prestudy assumption. The design called for enrollment of 80 eligible patients onto each arm over 12 months, with 12 additional months of follow-up. This design provided 90% power using a one-sided log-rank test stratified on prior treatment and risk category with 10% type I error. Full information would exist when 104 of 160 patients in a pair of cohorts had experienced progression or died. To assure that 80 patients per arm were eligible, targeted accrual was 90 patients per arm (360 total patients).
A secondary objective was to estimate the proportion of patients with stable disease at 6 months in each arm. Patients whose date of progression was after 6 months or who were disease free at last follow-up beyond 6 months were considered to be stable at 6 months, and all other patients were not.
The protocol stated that the final interpretation of study results, including the suitability of a doublet for further testing, would be based on the entirety of the outcome data and not on a single formal rule. For example, a positive outcome for the primary end point of PFS would need to be taken in context with regard to toxicity and other supportive efficacy evidence to conclude whether further investigation would be warranted. Secondary end points included response as assessed using RECIST and changes in tumor burden and overall survival (OS). OS was defined as time from random assignment to death. Patients alive at last contact were censored on the date of last contact.
Patients were randomly assigned equally to the four arms. Stratification factors were as follows: prior therapy (cytokine or vaccine v neither) and modified Memorial Sloan-Kettering Cancer Center risk category (favorable v intermediate v poor), based on performance status, hemoglobin, corrected calcium, and prior nephrectomy.21
Institutions obtained treatment assignments from a Web registration program. The primary efficacy analysis was performed including eligible treated patients. A sensitivity analysis of PFS was performed including all randomly assigned patients. The safety population included all treated patients. The evaluation of changes in tumor burden included all patients with measurable disease per RECIST.
Descriptive statistics were used to characterize patients, their disease, and prior treatment at baseline. Differences in categorical variables were evaluated using χ2 or Fisher's exact test.22 The Wilcoxon rank sum test23 or Kruskal-Wallis test was used to test for differences in continuous variables by group.24
The Kaplan-Meier method was used to graphically portray PFS and OS.25 Stratified proportional hazards models and stratified log-rank tests were used to estimate the PFS and OS distributions for each arm and for comparisons with bevacizumab alone (arm A).26 The method described by Grambsch and Therneau27 was used to test the proportional hazards assumption of the PFS model. Unless otherwise indicated, P values are two sided, and no adjustments were made for multiple comparisons.
RESULTS
The study was activated on September 14, 2007, and closed to accrual on December 10, 2010, after accrual of 361 patients. The data cutoff for this report was February 7, 2014. Patient disposition is detailed in Fig 1. The primary efficacy population included the 331 eligible patients who received treatment. The toxicity analysis included all 355 treated patients. Table 1 lists patient characteristics by treatment arm. Median age was 61 years. The arms were well balanced with respect to baseline characteristics.
Fig 1.

CONSORT diagram.
Table 1.
Patient Demographic and Disease Characteristics
| Characteristic | Arm A (bevacizumab alone) No. (%) | Arm B (bevacizumab plus temsirolimus) No. (%) | Arm C (bevacizumab plus sorafenib) No. (%) | Arm D (sorafenib plus temsirolimus) No. (%) | Total No. (%) |
|---|---|---|---|---|---|
| No. of patients | 84 | 80 | 83 | 84 | 331 |
| Sex | |||||
| Male | 62 (74) | 55 (69) | 57 (69) | 67 (80) | 241 (73) |
| Female | 22 (26) | 25 (31) | 26 (31) | 17 (20) | 90 (27) |
| Race | |||||
| White | 79 (95) | 69 (90) | 75 (94) | 80 (96) | 303 (94) |
| African American | 2 (2) | 5 (7) | 4 (5) | 2 (2) | 13 (4) |
| Asian | 2 (2) | 1 (1) | 0 (0) | 1 (1) | 4 (1) |
| Native American | 0 (0) | 2 (3) | 1 (1) | 0 (0) | 3 (1) |
| Unknown/missing | 1 | 3 | 3 | 1 | 8 |
| Ethnicity | |||||
| Hispanic | 1 (1) | 3 (4) | 2 (3) | 0 (0) | 6 (2) |
| Non-Hispanic | 78 (99) | 74 (96) | 78 (98) | 75 (100) | 305 (98) |
| Unknown/missing | 5 | 3 | 3 | 9 | 20 |
| Age category, years | |||||
| ≤ 50 | 8 (10) | 12 (15) | 18 (22) | 14 (17) | 52 (16) |
| 50-59 | 27 (32) | 24 (30) | 20 (24) | 32 (38) | 103 (31) |
| 60-69 | 31 (37) | 30 (38) | 34 (41) | 27 (32) | 122 (37) |
| ≥ 70 | 18 (21) | 14 (18) | 11 (13) | 11 (13) | 54 (16) |
| Histology | |||||
| Clear cell | 80 (95) | 78 (98) | 77 (83) | 77 (92) | 312 (94) |
| Mixed | 4 (5) | 2 (3) | 6 (7) | 7 (8) | 19 (6) |
| Risk category as stratified | |||||
| Favorable | 29 (35) | 30 (38) | 28 (34) | 27 (32) | 114 (34) |
| Intermediate | 31 (37) | 27 (34) | 35 (42) | 32 (38) | 125 (38) |
| Poor | 24 (29) | 23 (29) | 20 (24) | 25 (30) | 92 (28) |
| Risk category | |||||
| Favorable | 26 (31) | 27 (34) | 25 (30) | 31 (37) | 109 (33) |
| Intermediate | 34 (41) | 31 (39) | 37 (45) | 31 (37) | 133 (40) |
| Poor | 24 (29) | 22 (28) | 21 (25) | 22 (26) | 89 (27) |
| Primary tumor ≤ 5 cm* | |||||
| No | 6 (55) | 7 (78) | 3 (38) | 7 (64) | 23 (59) |
| Yes | 5 (46) | 2 (22) | 5 (63) | 4 (36) | 16 (41) |
| Extensive liver involvement* | |||||
| No | 6 (55) | 8 (89) | 5 (63) | 8 (73) | 27 (69) |
| Yes | 5 (46) | 1 (11) | 3 (38) | 3 (27) | 12 (31) |
| Multiple (> five) bone metastases* | |||||
| No | 9 (82) | 7 (78) | 7 (88) | 7 (64) | 30 (77) |
| Yes | 2 (18) | 2 (22) | 1 (13) | 4 (36) | 9 (23) |
| T stage | |||||
| 0 | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 2 (1) |
| 1A | 4 (5) | 5 (6) | 5 (6) | 1 (1) | 15 (5) |
| 1B | 7 (9) | 8 (10) | 11 (13) | 10 (12) | 36 (11) |
| 2 | 16 (20) | 16 (21) | 16 (20) | 19 (24) | 67 (21) |
| 3 | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 2 (1) |
| 3A | 17 (21) | 24 (31) | 18 (22) | 19 (24) | 78 (24) |
| 3B | 20 (25) | 17 (22) | 21 (26) | 25 (31) | 83 (26) |
| 3C | 1 (1) | 1 (1) | 3 (4) | 1 (1) | 6 (2) |
| 4 | 11 (14) | 4 (5) | 3 (4) | 2 (3) | 20 (6) |
| X | 1 (1) | 2 (3) | 5 (6) | 4 (5) | 12 (4) |
| Unknown/missing | 4 | 2 | 1 | 3 | 10 |
| N stage | |||||
| 0 | 35 (46) | 34 (44) | 28 (36) | 30 (38) | 127 (41) |
| 1 | 5 (7) | 5 (6) | 4 (5) | 9 (11) | 23 (7) |
| 2 | 8 (10) | 9 (12) | 12 (16) | 9 (11) | 38 (12) |
| X | 29 (38) | 30 (39) | 33 (43) | 31 (39) | 123 (40) |
| Unknown/missing | 7 | 2 | 6 | 5 | 20 |
| M stage | |||||
| 0 | 27 (34) | 20 (25) | 24 (30) | 25 (31) | 96 (30) |
| 1 | 35 (44) | 37 (47) | 35 (43) | 40 (49) | 147 (46) |
| X | 17 (22) | 22 (28) | 22 (28) | 17 (21) | 78 (24) |
| Unknown/missing | 5 | 1 | 2 | 2 | 10 |
Among patients with no prior nephrectomy.
Efficacy
By hazard ratio, there were no significant differences in PFS between the three combination arms and the bevacizumab arm (Fig 2); the two-sided CIs are wider than the 80% CIs that would correspond to the 10% one-sided type I error of the study design. Patients treated with the combination of bevacizumab and sorafenib had a median PFS (9.2 months) that was approximately 1 to 2 months longer than that of the other arms (7.4 to 7.6 months) that was not significant. Inclusion of the ineligible patients in the analysis did not change the conclusion that no combination improved PFS compared with bevacizumab alone.
Fig 2.
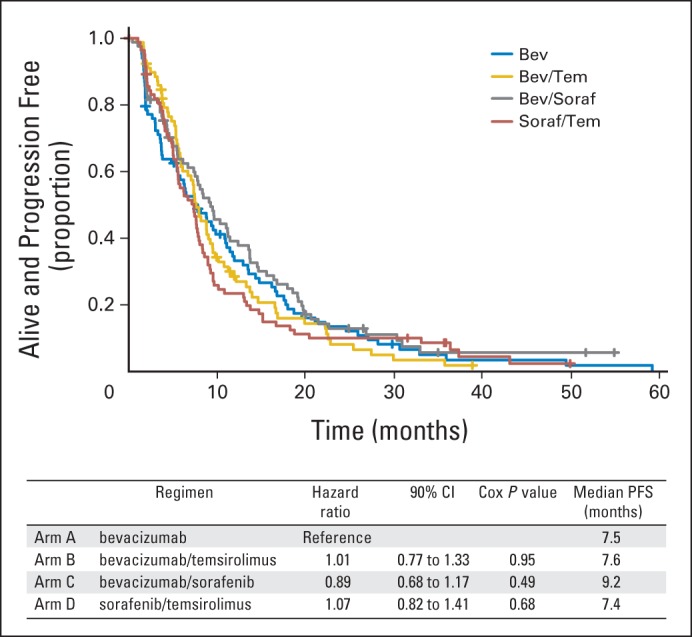
Progression-free survival (PFS). Bev, bevacizumab; Soraf, sorafenib; Tem, temsirolimus.
A secondary objective was to estimate the proportion of patients with stable disease at 6 months in each arm. The rates of 6-month PFS were essentially the same: bevacizumab alone, 55%; bevacizumab plus temsirolimus, 56%; bevacizumab plus sorafenib, 59%; and sorafenib plus temsirolimus, 54%.
There were no differences in OS by arm (Fig 3). Median OS was 28.6 months in the bevacizumab-alone arm relative to bevacizumab plus temsirolimus (24.7 months), bevacizumab plus sorafenib (27.5 months), and sorafenib plus temsirolimus (24.3 months).
Fig 3.
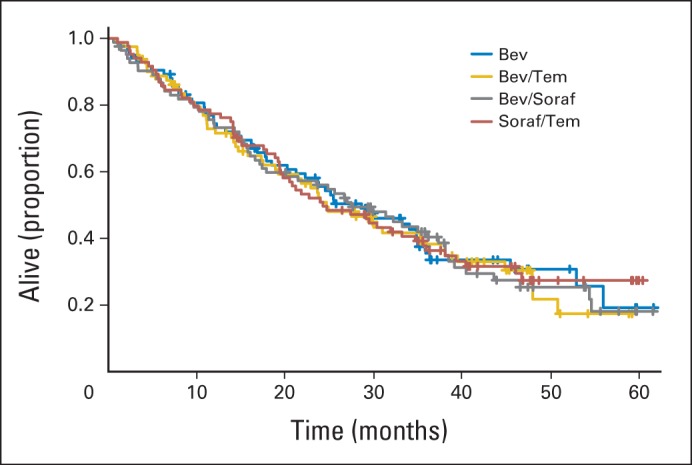
Overall survival. Bev, bevacizumab; Soraf, sorafenib; Tem, temsirolimus.
Response was assessed by RECIST (version 1.0). Overall response rates (including complete and partial responses) are listed in Table 2, along with 95% exact binomial CIs. Response rates were higher among patients treated with combinations including bevacizumab compared with bevacizumab alone, but the response rate in the sorafenib plus temsirolimus arm was not statistically significantly different from that observed with single-agent bevacizumab.
Table 2.
Objective Response Rates
| Response | Arm A (bevacizumab alone) | Arm B (bevacizumab plus temsirolimus) | Arm C (bevacizumab plus sorafenib) | Arm D (sorafenib plus temsirolimus) |
|---|---|---|---|---|
| No. assessed | 83 | 79 | 82 | 84 |
| CR, % | 1.2 | — | 2.4 | — |
| PR, % | 12.0 | 31.6 | 28.0 | 20.2 |
| SD, % | 50.6 | 51.9 | 43.9 | 51.2 |
| PD, % | 24.1 | 6.3 | 14.6 | 15.5 |
| NE, % | 12.0 | 10.1 | 11.0 | 13.1 |
| ORR (pairwise comparison; Fisher's exact P) | ||||
| A v B | .008 | |||
| A v C | .009 | |||
| A v D | .30 | |||
Abbreviations: CR, complete response; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
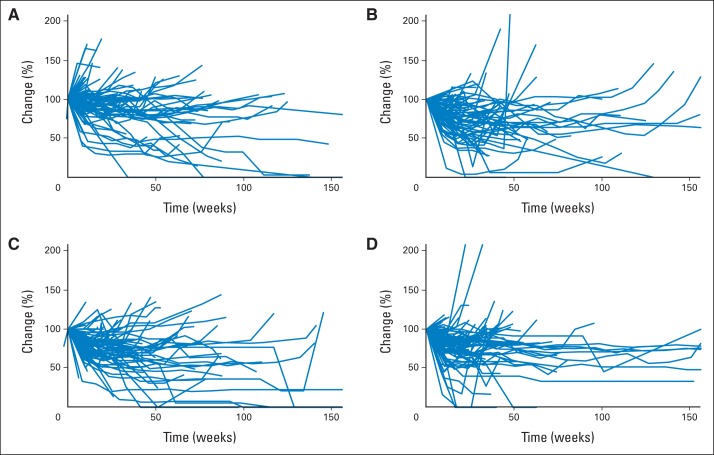
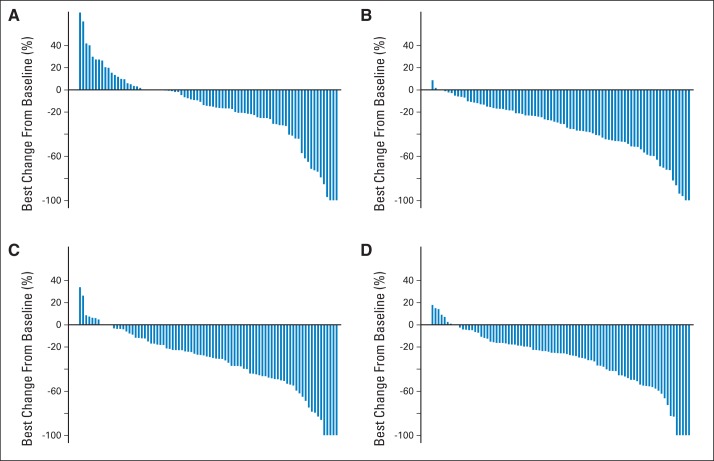
Appendix Figure A1 (online only) shows spider plots depicting the percent change from baseline in tumor burden over time by treatment arm, considering the change in the sum of the longest diameter of target lesions among 326 patients with measurable disease at baseline. It seems that a higher proportion of patients randomly assigned to arm C (bevacizumab plus sorafenib) had favorable tumor size/regression trajectories. This is consistent with the nonsignificantly prolonged PFS and higher response rate observed in this arm. Appendix Figure A2 (online only) shows each patient's maximum percent change in target lesions.
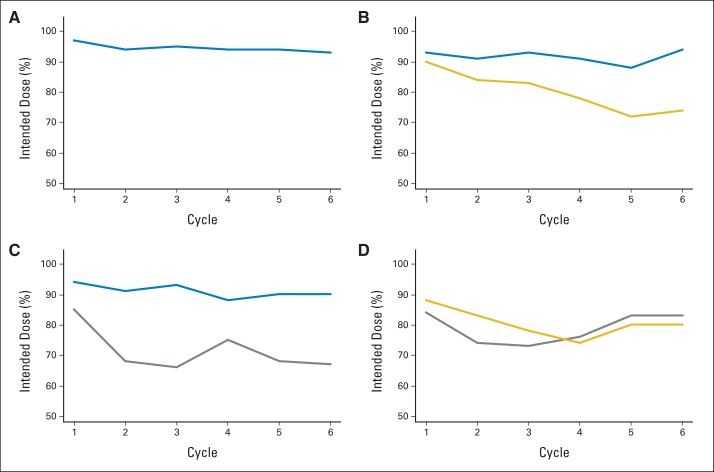
Treatment and Dose-Intensity
To understand if observed treatment effects were affected by dose reductions resulting from toxicity, we explored dose-intensity for each arm. Dose-intensity at selected intervals by agent and arm is shown in Figure 4. This was the proportion of planned dose actually administered in a cycle. Doses administered were determined based on pill counts and diaries for sorafenib, an oral agent. All other agents were administered by infusion, and doses were reported on case report forms. The dose-intensity remained high for bevacizumab when administered as monotherapy or in combination. However, sorafenib and temsirolimus dose-intensity was notably attenuated in the early cycles of therapy. Only arms A and B initiated each agent at their standard single-agent doses per protocol. Thus, the doses of sorafenib and temsirolimus administered in arms C and D were the furthest from the single-agent doses for which phase III trials demonstrated single-agent benefit in ccRCC.
Fig 4.
Dose-intensity. (A) Arm A (bevacizumab); (B) arm B (bevacizumab plus temsirolimus); (C) arm C (bevacizumab plus sorafenib); (D) arm D (sorafenib plus temsirolimus). Bevacizumab is shown in blue, temsirolumus in gold, and sorafenib in gray.
Adverse Events
All treated patients were included in the analysis of adverse events. Table 3 lists rates of all adverse events of grade ≥ 3 that were considered possibly, probably, or definitely related to treatment. The rates of grade 3 to 5 toxicities were 44% (arm A), 77% (arm B), 82% (arm C), and 84% (arm D), with the lower rate in arm A being statistically significant (Fisher's exact P < .001 comparing grades 3 to 5 with 0 to 2). There were five treatment-related deaths (included in grade 3 to 5 toxicity rates) and seven other deaths that were unlikely to have been related or were unrelated to treatment but that occurred within 30 days of the end of treatment (distributed across all four arms). Nineteen patients died as a result of disease progression within 30 days of receiving protocol therapy and were similarly distributed among the treatment cohorts.
Table 3.
Severe Adverse Events Possibly, Probably, or Definitely Related to Treatment
| Adverse Event | Treatment Arm (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| A (bevacizumab alone; n = 88) |
B (bevacizumab plus temsirolimus; n = 86) |
C (bevacizumab plus sorafenib; n = 90) |
D (sorafenib plus temsirolimus; n = 91) |
|||||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Hemoglobin | — | — | 2 | — | 2 | — | 9 | — |
| Lymphopenia | — | — | 3 | — | 2 | — | 11 | — |
| Platelets | — | — | 2 | — | 1 | — | 9 | — |
| Hypertension | 20 | — | 17 | — | 34 | 3 | 8 | — |
| Fatigue | 2 | — | 15 | — | 10 | 1 | 13 | 1 |
| Rash/desquamation | 1 | — | 2 | — | 3 | — | 8 | — |
| Hand-foot reaction | — | — | 1 | — | 22 | — | 3 | — |
| Dehydration | — | — | 6 | — | 3 | — | 1 | — |
| Diarrhea | — | — | 7 | — | 7 | — | 10 | — |
| Hypercholesterolemia | — | — | 5 | — | — | — | 3 | 2 |
| Hyperglycemia | — | — | 10 | — | 2 | — | 18 | — |
| Hypophosphatemia | 2 | — | 8 | — | 11 | — | 33 | — |
| Hypokalemia | — | — | 2 | — | 3 | — | 5 | — |
| Proteinuria | 10 | — | 23 | 2 | 9 | — | 1 | — |
| Hyponatremia | — | — | 3 | — | 8 | 1 | 4 | — |
| Hypertriglyceridemia | — | — | 5 | — | — | — | 4 | 1 |
| Abdominal pain | 1 | — | 3 | — | 7 | — | 3 | — |
| Head/headache | 2 | — | 6 | — | 4 | — | 2 | — |
| Dyspnea | — | — | 7 | — | 1 | — | 1 | 1 |
NOTE. Occurring at rate ≥ 5% in any arm.
DISCUSSION
ECOG 2804 was one of the first examinations of combination targeted therapy doublets in patients with ccRCC. We observed PFS ranging from 7 to 9 months for the combination regimens, which did not differ from the single-agent activity of bevacizumab seen in our trial or previous investigations in similar patient populations.3,20
The demonstration of benefit with sequential therapy mandates that combination regimens produce large improvements in disease control, which motivated setting the targeted improvement in PFS at 67%. Response rates by RECIST were highest on arms B and C, in which bevacizumab was combined with another agent. It is interesting to note that this impact on tumor regression (with statistical significance) did not translate into a statistically significant difference in PFS, although the arm with the highest response rate (sorafenib plus bevacizumab) also had the longest median PFS. Because the default approach to administration of these agents has been sequential monotherapy (including bevacizumab, which is commonly administered alone, despite its label with IFN), these data do not support the hypothesis that these agents can produce a synergistic effect on tumor in the initial course of therapy or delay the emergence of resistance.
The rate of serious, life-threatening, or lethal adverse events with the combination therapies in this study was substantial. Overall, grade 3 to 5 toxicities ranged from 77% to 84% in the combination arms compared with 44% in the single-agent bevacizumab arm and aligned with the observed toxicities in the previous phase Ib trials. Notably, this rate of severe toxicity was observed despite reduced doses of sorafenib in both arms in which it was included and temsirolimus in the sorafenib plus temsirolimus arm. The difficulty in combining these agents is further underscored by a higher rate of less serious toxicities, which are well described for each agent. Because dose reductions are commonly considered for patients with intolerable grade 2, as well as grade 3 to 4, toxicities, it is clear that these combinations are difficult to administer. The precise nature of the toxicity interactions between VEGF, VEGFR/PDGF receptor, and mTOR inhibitors is not known, but increases in the rates of hand-foot syndrome and hypertension support on-target mechanistic interactions as opposed to purely pharmacokinetic effects, such as interference with drug clearance or higher resultant drug exposure. Although we did not examine pharmacokinetics in this trial, those analyses performed in the phase Ib trials did not reveal significant drug-drug interactions.16–18
Other combinations of bevacizumab have demonstrated additional toxicities. The combination of bevacizumab and sunitinib caused a particularly marked pattern of toxicity consistent with microangiopathy, taken to reflect severe endothelial cell injury as a consequence of presumably on-target effects in normal tissues.28 Since the launch of our study, preclinical investigations have shown that acquired resistance to VEGFR is at least in part the result of angiogenic escape via the production of alternative endothelial growth factors capable of restoring angiogenesis despite continued VEGFR blockade. A dominant mechanism underlying tyrosine kinase inhibitor resistance is the hyperactivation of HIF-2 in response to tumor hypoxia and the enhanced production of interleukin-8 and other HIF-inducible angiogenic factors. Although combining different US Food and Drug Administration–approved agents has proven disappointing thus far (mainly because of toxicity concerns), new targets (eg, HIF-2α antagonists, sphingosine kinase, CXCR4, p53, angiopoietin-2, c-Me,t and activin-like kinase 1) have emerged and await validation in ongoing clinical trials. It is also hypothesized that the greatest therapeutic index might be achieved by employing agents with entirely distinct mechanisms of action.
There is also increasing interest in combining antiangiogenic agents with novel immunotherapies, a concept that is motivated in part by the observations in preclinical models of immunosuppressive effects mediated by proangiogenic growth factors.29 The now-standard single-agent therapies—sunitinib and pazopanib—each produce response rates of 30% to 40% and median PFS of ≥ 10 months.30–32 These agents are inhibitors of multiple proangiogenic cytokine receptors, including VEGFR and PDGF receptor. Although sorafenib shares this basic mechanism of action, the objective response rate with this agent in ccRCC is notably lower (10% to 20%).13 It is possible that agents such as sunitinib and pazopanib represent combination therapies in and of themselves, by intercepting multiple pathways important in ccRCC pathogenesis.
Our results, combined with other recent targeted therapy combination investigations, suggest that combinations with overlapping toxicities that cannot be combined at their individual full recommended phase II doses should only be investigated in a cautious stepwise fashion through phases I, II, and III. For this reason, our trial involved a cautiously designed phase II randomized design to pick the potential regimen for future study if sufficient activity and tolerable toxicity were observed. A phase III trial testing the bevacizumab plus temsirolimus combination in comparison with bevacizumab plus IFN (ClinicalTrials.gov identifier NCT00631371) was launched in parallel with this study, and its potential value might have been assessed differently if the results of this study were available.
Pursuing therapeutic strategies with the doublets examined in this study is, in our view, not warranted. Evidence has accumulated to support the application of sequential single-agent therapy. Additional advances in immunotherapy for ccRCC using agents that are likely to be more active and less toxic than high-dose interleukin-2 (eg, programmed death–1 pathway blockade) have also changed the field and may need to be taken into account in defining the optimal sequence and choice of treatment for ccRCC.
Appendix
Fig A1.
Change in size of target lesions over time. (A) Arm A (bevacizumab); (B) arm B (bevacizumab plus temsirolimus); (C) arm C (bevacizumab plus sorafenib); (D) arm D (sorafenib plus temsirolimus).
Fig A2.
Patients' maximum percent change in target lesions. (A) Arm A (bevacizumab); (B) arm B (bevacizumab plus temsirolimus); (C) arm C (bevacizumab plus sorafenib); (D) arm D (sorafenib plus temsirolimus).
Support information appears at the end of this article.
Presented in abstract form (first analysis of trial) at the Kidney Cancer Association Annual Meeting, Chicago, IL, October 5-6, 2012.
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00378703.
Support
Coordinated by the Eastern Cooperative Oncology Group (ECOG) –American College of Radiology Imaging Network Cancer Research Group (co-chairs Robert L. Comis, MD, and Mitchell D. Schnall, MD, PhD) and supported in part by Public Health Service Grants No. CA180820, CA180794, CA180867, CA32102, CA20319, CA180888, CA47577, and CA31946 from the National Cancer Institute, National Institutes of Health, and the US Department of Health and Human Services. Biospecimens were provided by the ECOG Pathology Coordinating Office and Reference Laboratory.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Keith T. Flaherty, Judith B. Manola, Michael Pins, David F. McDermott, Michael B. Atkins, Janice J. Dutcher, Robert S. DiPaola
Administrative support: Judith B. Manola
Provision of study materials or patients: Keith T. Flaherty, David F. McDermott, Michael B. Atkins, Janice J. Dutcher, Daniel J. George, Kim A. Margolin
Collection and assembly of data: Keith T. Flaherty, Judith B. Manola, David F. McDermott, Michael B. Atkins, Janice J. Dutcher, Daniel J. George, Kim A. Margolin, Robert S. DiPaola
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
BEST: A Randomized Phase II Study of Vascular Endothelial Growth Factor, RAF Kinase, and Mammalian Target of Rapamycin Combination Targeted Therapy With Bevacizumab, Sorafenib, and Temsirolimus in Advanced Renal Cell Carcinoma—A Trial of the ECOG–ACRIN Cancer Research Group (E2804)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Keith T. Flaherty
Consulting or Advisory Role: Genentech
Judith B. Manola
No relationship to disclose
Michael Pins
Speakers' Bureau: Genentech/Roche, Pfizer
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech/Roche, Pfizer
Research Funding: Prometheus
Michael B Atkins
Honoraria: Bristol-Myers Squibb, Amgen
Consulting or Advisory Role: Merck, Genentech, Pfizer, Novartis, GlaxoSmithKline, C-Cam, X4, Neostem, Amgen, Eli Lilly
Janice J. Dutcher
Honoraria: Prometheus, Pfizer, Novartis
Consulting or Advisory Role: Prometheus, Bristol-Myers Squibb, Merck, TRACON Pharma, Celgene
Speakers' Bureau: Prometheus, Pfizer, Novartis
Travel, Accommodations, Expenses: Prometheus, Pfizer, Novartis, Merck, Celgene, Bristol-Myers Squibb
Daniel J. George
Honoraria: Dendreon, Novartis, sanofi-aventis
Consulting or Advisory Role: Bayer, Dendreon, Exelixis, Medivation, Novartis, Pfizer, BIND Biosciences, sanofi-aventis, GlaxoSmithKline, Astellas Pharma, Progenics, Innocrin, Genentech
Speakers' Bureau: Dendreon, Novartis, sanofi-aventis
Research Funding: Exelixis (Inst), Genentech/Roche (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst), Progenics (Inst), Astellas Pharma (Inst), Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), Millennium Pharmaceuticals (Inst), Innocrin
Travel, Accommodations, Expenses: Astellas Pharma, AVEO, Algeta/Bayer, Dendreon, Pfizer, sanofi-aventis, Progenics, Bristol-Myers Squibb
Kim A. Margolin
No relationship to disclose
Robert S. Dipaola
Research Funding: AbbVie (Inst)
REFERENCES
- 1.Seizinger BR, Rouleau GA, Ozelius LJ, et al. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988;332:268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- 2.Gnarra JR, Zhou S, Merrill MJ, et al. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci U S A. 1996;93:10589–10594. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 10.Hudson CC, Liu M, Chiang GG, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 13.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 14.Norden-Zfoni A, Desai J, Manola J, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 15.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: Modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies MA, Fox PS, Papadopoulos NE, et al. Phase I study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. Clin Cancer Res. 2012;18:1120–1128. doi: 10.1158/1078-0432.CCR-11-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchan JR, Pitot HC, Qin R, et al. Phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma (RCC): Safety and activity in RTKI refractory RCC patients. J Clin Oncol. 2009;27(suppl):244s. abstr 5039. [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25:4536–4541. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR. The Analysis of Binary Data. London, United Kingdom: Methuen; 1970. [Google Scholar]
- 23.Wilcoxon F. Individual comparisons of grouped data by ranking methods. J Econ Entomol. 1946;39:269. doi: 10.1093/jee/39.2.269. [DOI] [PubMed] [Google Scholar]
- 24.Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York, NY: Wiley; 1973. [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Cox DR. Regression models and life tables. J Royal Stat Soc B. 1972;34:181–220. [Google Scholar]
- 27.Grambsch PM, Therneau TM, Fleming TR. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 28.Rini BI, Garcia JA, Cooney MM, et al. Toxicity of sunitinib plus bevacizumab in renal cell carcinoma. J Clin Oncol. 2010;28:e284–e285. doi: 10.1200/JCO.2009.27.1759. author reply e286-e287. [DOI] [PubMed] [Google Scholar]
- 29.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 31.Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. Eur J Cancer. 2013;49:1287–1296. doi: 10.1016/j.ejca.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]