Abstract
Purpose
Aurora A kinase (AAK) is upregulated in highly proliferative lymphomas, suggesting its potential as a therapeutic target. Alisertib is a novel oral AAK inhibitor without adverse safety signals in early-phase studies that demonstrated preliminary activity in T-cell lymphoma. This phase II study was conducted to further investigate the efficacy of alisertib in relapsed or refractory peripheral T-cell non-Hodgkin lymphoma (PTCL).
Patients and Methods
Eligible patients with histologically confirmed relapsed/refractory PTCL or transformed Mycosis fungoides (tMF) received alisertib 50 mg twice a day for 7 days on 21-day cycles.
Results
Of 37 eligible patients, the histologic subtypes enrolled included PTCL not otherwise specified (n = 13), angioimmunoblastic T-cell lymphoma (n = 9), tMF (n = 7), adult T-cell lymphoma/leukemia (n = 4), anaplastic large-cell lymphoma (n = 2), and extranodal natural killer/T-cell lymphoma (n = 2). Grade 3 and 4 adverse events in ≥ 5% of patients included neutropenia (32%), anemia (30%), thrombocytopenia (24%), febrile neutropenia (14%), mucositis (11%), and rash (5%). Treatment was discontinued most commonly for disease progression. Among the PTCL subtypes, the overall response rate was 30%, whereas no responses were observed in tMF. Aurora B kinase was more commonly overexpressed than AAK in tumor specimens. Analysis of AAK, Aurora B kinase, MYC, BCL-2, phosphatidylinositol 3-kinase γ, and Notch1 expression revealed no association with response.
Conclusion
Alisertib has antitumor activity in PTCL, including heavily pretreated patients. These promising results are being further investigated in an ongoing international, randomized phase III trial comparing alisertib with investigator's choice in PTCL.
INTRODUCTION
Despite multiple agents demonstrating activity in peripheral T-cell lymphoma (PTCL), patient outcomes remain poor. For patients with relapsed or refractory disease who did not proceed with stem-cell transplantation, a population-based study reported a median overall survival time of 5.5 months and a median progression-free survival time of 3.1 months.1 Even patients with favorable prognostic factors, including a performance status of 0 or 1, a complete response with previous treatment, or the ability to receive combination chemotherapy, demonstrated median overall survival times ranging from 6 to 13 months. A registry analysis demonstrated that in relapsed patients with chemosensitive disease eligible for stem-cell transplantation, only 62%, 43%, and 58% remained alive 1 year after autologous, myeloablative allogeneic, and reduced-intensity allogeneic transplantation, respectively.2 These data underscore the need for additional therapeutic options for this patient population.
The Aurora kinases are a highly conserved family of serine/threonine protein kinases that play essential regulatory roles throughout mitosis. Aurora A kinase (AAK) localizes to centrosomes and the spindle poles from prophase through metaphase and is required for assembly of the mitotic spindle, committing the cell to mitosis. Aurora B kinase (ABK) localizes to the centromeres, where it plays a prominent role in the metaphase-to-anaphase transition, being essential for mitotic progression and cytokinesis. Amplification of both AAK and ABK has been observed in a variety of malignancies and proposed to be oncogenic in some cases. Increased AAK expression has been demonstrated in non-Hodgkin lymphoma, where overexpression correlated with rapidly dividing lymphoma subtypes.3 AAK upregulation has been demonstrated in T-cell histologies, warranting evaluation as a therapeutic target in the proliferative PTCLs.4
Alisertib is a selective small-molecule inhibitor of AAK demonstrating G2/M arrest, abnormal mitotic spindle formation, the appearance of tetraploidy, and subsequent apoptosis in vitro and in vivo.5,6 Phase I evaluation demonstrated dose-limiting myelosuppression. Additional toxicities, including mucositis and somnolence, related to γ-aminobutyric acid A α-1 benzodiazepine receptor binding, were ameliorated with use of the recommended phase II dose of 50 mg twice a day for 7 of 21 days.7,8 A phase II study using this dose and schedule of alisertib demonstrated clinical responses in a variety of aggressive B- and T-cell lymphomas. A 27% overall response rate was observed, including four of eight patients with T-cell histologies.9
On the basis of the supporting laboratory data and early clinical efficacy, we conducted a single-arm phase II clinical trial of alisertib in PTCL and transformed Mycosis fungoides (tMF) through the US Intergroup. The results suggest reversible toxicities in this patient population and confirm clinical activity of alisertib in PTCL.
PATIENTS AND METHODS
Study Design and Objectives
The primary end point of this multicenter phase II trial was the response rate in patients with relapsed or refractory PTCL after administration of alisertib. Secondary end points included safety, progression-free survival, overall survival, and correlative studies intended to identify biomarkers predictive of clinical activity. Institutional review boards approved the protocol at each participating site, and informed, written consent was obtained from all patients before enrollment. All authors had access to the primary clinical trial data. The study was registered before enrolling patients (ClinicalTrials.gov NCT01466881).
Eligibility Criteria
Patients age 18 years and older were eligible if they had a diagnosis of PTCL or tMF. An Eastern Cooperative Oncology Group performance status of ≤ 2 was required. Acceptable WHO-defined PTCL histologies included PTCL not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma, anaplastic large-cell lymphoma (ALCL), adult T-cell lymphoma/leukemia, entranodal natural killer/T-cell lymphoma, and subcutaneous panniculitis-like T-cell lymphoma, hepatosplenic T-cell lymphoma, and enteropathy-type T-cell lymphoma.10 Patients must have received at least one prior systemic therapeutic regimen for lymphoma and have bidimensionally measurable disease. Treatment refractoriness was defined as no response to the most recent treatment regimen or disease progression in ≤ 6 months. Baseline laboratory parameters included absolute neutrophil count ≥ 1500 cells/μL, platelet count ≥ 75,000 cells/μL, and adequate renal and hepatic function. Patients who had received antibody therapy or chemotherapy in the preceding 3 weeks, had undergone allogeneic stem-cell transplantation, or had other active malignancies, central nervous system lymphoma, HIV positivity, or other active infection were excluded.
Protocol Treatment and Clinical Protocol Assessments
Baseline evaluation included history and physical examination, laboratory evaluations, bone marrow biopsy, and imaging by computed tomography (CT) and positron emission tomography. Alisertib was administered at 50 mg orally twice a day for the first 7 days of each 21-day treatment cycle. Treatment continued for up to 1 year or until disease progression or unacceptable toxicity. Adverse events were assessed at baseline, throughout the treatment, and during the 28-day period after treatment discontinuation and were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0.
Alisertib was held for grade 4 hematologic toxicities, including anemia, thrombocytopenia, or ≥ 7 days of neutropenia, and for grade 3 or 4 nonhematologic toxicity. Treatment resumption was allowed on recovery to ≤ grade 1 with mandated dose adjustments to 40 mg orally twice a day and subsequently 30 mg twice a day if necessary. If treatment was delayed for > 3 weeks, patients were removed from protocol. Granulocyte-colony–stimulating factor was permitted at the discretion of the treating physician. Antiemetic and antidiarrheal prophylaxis was also allowed but not mandated.
CT and positron emission tomography scans for the assessment of objective tumor responses were performed at baseline and after cycle 4, with subsequent CTs being performed every four cycles thereafter until disease progression. If disease progression did not occur by the end of treatment, patients were evaluated with physical examination, laboratory studies, and imaging studies every 4 months until progression.
Measurement of Immunohistochemical Markers
Immunohistochemistry was performed with validated antibodies to Aurora A (Cell Signaling Technology, Danvers, MA), Aurora B (EMD Millipore, Billerica, MA), BCL-2 (Ventana Medical Systems, Tucson, AZ), MYC (Ventana Medical Systems), Notch1 (Spring Bioscience, Pleasanton, CA), and phosphatidylinositol 3-kinase γ (PI3K-γ; Santa Cruz Biotechnology, Dallas, TX) on pretreatment biopsy specimens. Hematopathologic review identified lymphoma involvement in representative biopsy specimens. The Aperio high-capacity ScanScope XT/XT2 system was used for image analysis. Nuclear positivity was quantified by Aperio's version 9 algorithm (Aperio Technologies, Vista, CA) for Aurora A, Aurora B, and MYC stains. Aperio's Positive Pixel Count algorithm was used to quantify BCL-2, Notch1, and PI3K-γ. Each algorithm distinguishes between strong (3+), moderate (2+), weak (1+), and negative (0) staining.
Cytokine Analysis
The RayBio Human Cytokine Antibody Array G-Series 2000 kit (RayBiotech, Norcross, GA) was used for detection of 174 cytokines disseminated among three different glass array slides. Patient serum was collected on day 1 (pretreatment) and day 8 (post-treatment with alisertib) and stored (−80°C). While glass array slides were thawing to room temperature, serum was diluted five-fold with 1× blocking buffer. Slides were blocked with 1× blocking buffer and incubated overnight at 4°C with 80 μL of serum. Slides were washed and then incubated with 70 μL of biotin-conjugated anticytokines overnight at 4°C. Slides were washed again, as done previously, and incubated at room temperature for 2 hours with Streptavidin-Fluor reagent. Each glass slide contained positive and negative internal controls. Fluorescence was detected by RayBiotech (Sunnyvale, CA) with the GenePix 4000 scanner using the Cy3 channel, and signal intensity was obtained with the scanning software. The RayBio Analysis Tool software (RayBiotech) was used to evaluate signal intensities after normalizing to the positive control and subtracting background. Changes in individual cytokines, including a ≥ 1.25-fold increase and a ≥ 0.75-fold decrease, were considered significant, as recommended by the manufacturer.
Statistical Analysis
The primary objective of this trial is to assess the objective response probability in patients with relapsed or refractory PTCL treated with alisertib. The trial used a two-stage design with 93% power to detect a response rate of 30% versus 10% using a one-sided significance level of 5.5% test. Twenty evaluable patients were enrolled onto the first stage. With two or more observed responses, 22 additional patients were enrolled to include at least 35 eligible patients.
Standard response criteria were used for classification of objective tumor responses.11 Progression-free survival was defined as the time from date of registration to date of the first documentation of disease progression or death regardless of cause, whichever occurred first. Patients who were alive and progression free at the time of final data analysis were censored at the time of their last assessment. Overall survival was defined as the time from date of registration to date of death as a result of any cause. Patients last known to be alive were censored at date of last contact. Median and 1-year estimates of progression-free survival and overall survival, and their 95% CIs, were estimated using the Kaplan-Meier method. Exact binomial CIs were calculated for response outcomes. Measurement of immunohistochemical markers were descriptively summarized by the mean, standard deviation, median, minimum, and maximum by response. Differences in expression levels of cytokines between post-treatment (day 8) and pretreatment were tested using a paired t-test.
RESULTS
Patient Characteristics
Forty-two patients were enrolled from 18 sites between October 28, 2011, and June 6, 2013. Five patients were deemed ineligible because of inability to confirm T-cell non-Hodgkin lymphoma diagnosis on central pathologic review in four cases and inadequate renal function in one case. Baseline clinical characteristics and prior therapies for the 37 eligible patients are detailed in Table 1. The median age was 62 years, and 35% of patients were female. PTCL subtypes included PTCL NOS (n = 13), angioimmunoblastic T-cell lymphoma (n = 9), adult T-cell lymphoma/leukemia (n = 4), ALCL, anaplastic lymphoma kinase negative (n = 2), and entranodal natural killer/T-cell lymphoma (n = 2). Seven patients with tMF were enrolled as well. The median number of prior therapies was 3 (range, 1 to 18), and these included cyclophosphamide, doxorubicin, vincristine, prednisone–like regimens (n = 30), folate antagonists (n = 15), histone deacetylase inhibitors (n = 12), brentuximab (n = 3), and autologous stem-cell transplantation (n = 3). Twenty patients (54%) were refractory to their most recent treatment regimen. Eleven patients (30%) had bone marrow involvement with lymphoma at study entry, and 24 patients (65%) had an elevated lactate dehydrogenase level at study entry.
Table 1.
Patient Demographics and Baseline Clinical Characteristics
| Characteristic | Value (N = 37) |
|---|---|
| Median age (range), years | 62 (21-86) |
| Female sex, No. (%) | 13 (35) |
| Hispanic race, No. (%) | 4 (11) |
| ECOG performance status 0/1/2 (%) | 19/57/24 |
| Lymphoma histology, No. (%) | |
| Peripheral T-cell NOS | 13 (35) |
| Angioimmunoblastic | 9 (24) |
| Transformed Mycosis fungoides | 7 (19) |
| Adult T-cell lymphoma/leukemia | 4 (11) |
| Anaplastic, ALK negative | 2 (5) |
| Extranodal NK/T cell | 2 (5) |
| Median time from diagnosis to enrollment (range), months | 10 (2-224) |
| Median No. of prior regimens (range) | 3 (1-18) |
| Refractory to most recent therapy, No. (%) | 20 (54) |
| Prior regimens, No. (%) | |
| CHOEP/CHOP-like | 30 (81) |
| Gemcitabine | 16 (43) |
| Pralatrexate/methotrexate | 15 (41) |
| Romidepsin/vorinostat | 12 (32) |
| Radiation therapy | 10 (27) |
| Bortezomib | 4 (11) |
| Brentuximab | 3 (8) |
| Autologous stem-cell transplantation | 3 (8) |
| Elevated LDH, No. (%) | 24 (65) |
Abbreviations: ALK, anaplastic lymphoma kinase; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; NK, natural killer; NOS, not otherwise specified.
Safety
Treatment-related adverse events are detailed in Table 2. Myelosuppression accounted for the most common events grade 3 or higher, with neutropenia, anemia, and thrombocytopenia occurring in 32%, 30%, and 24% of patients, respectively. There were five cases of febrile neutropenia that were considered treatment related. One treatment-related death occurred during protocol therapy. This patient died of sepsis 8 days after completing two cycles of therapy, in part related to treatment-related neutropenia. One patient with PTCL NOS, who received two separate multiagent chemotherapy regimens in addition to three cycles of alisertib, developed a leukocytosis most consistent with a Janus-activating kinase 2– and BCR/ABL-negative myeloproliferative neoplasm.
Table 2.
Drug-Related Adverse Events ≥ 10% (all grades) or Grade ≥ 3 in Two or More Patients
| Type of Adverse Event | All-Grade Adverse Events | Grade ≥ 3 Adverse Events* |
|---|---|---|
| Any drug-related adverse events | 32 (86) | 24 (65) |
| Anemia | 22 (59) | 11 (30) |
| Thrombocytopenia | 17 (46) | 9 (24) |
| Fatigue | 17 (46) | 1 (3) |
| Neutropenia | 16 (43) | 12 (32) |
| Leukopenia | 15 (41) | 7 (19) |
| Lymphopenia | 12 (32) | 8 (22) |
| Alopecia | 9 (24) | — |
| Mucositis | 8 (22) | 4 (11) |
| Alkaline phosphatase increased | 7 (19) | 2 (5) |
| Anorexia | 7 (19) | 1 (3) |
| Fever | 7 (19) | 1 (3) |
| Diarrhea | 6 (16) | 1 (3) |
| Febrile neutropenia | 5 (14) | 5 (14) |
| Bilirubin increased | 5 (14) | 1 (3) |
| AST increased | 5 (14) | — |
| Hyponatremia | 5 (14) | — |
| Rash | 4 (11) | 2 (5) |
| Creatinine increased | 4 (11) | — |
| Weight loss | 4 (11) | — |
| Pain | — | 2 (5) |
| CD4 lymphocytes decreased | — | 2 (5) |
NOTE: Data are given as number (percentage) of 37.
Grade ≥ 3 adverse events occurring in one patient each (3%) and not mentioned above were as follows: abdominal pain, back pain, hypercalcemia, hyperglycemia, hypotension, leukocytosis, pneumonia, sepsis, skin infection, gastrointestinal hemorrhage, and toxic epidermal necrolysis.
The most common nonhematologic adverse event of any grade was fatigue, occurring in approximately half of patients. Other common drug-related toxicities included alopecia and mucositis, occurring in 24% and 22% of patients, respectively. Treatment was discontinued most commonly for lymphoma progression. Nine patients underwent a dose reduction to 40 mg twice a day because of myelosuppresion, five of which were required after cycle 1. Four patients required further dose reduction to 30 mg twice a day.
Efficacy
At the end of the first stage, two partial responses were observed, meeting criteria to continue the trial. The overall response rate among the PTCL subtypes was 30% (95% CI, 9% to 61%), including 7% (2 of 30) complete responses and 23% (7 of 30) partial responses. Of the responding patients, 44% were refractory to prior therapy. Furthermore, responses were independent of the number of prior therapies (P = .36). An additional 17% (5 of 30) of patients achieved stable disease. No patient with tMF demonstrated an objective response. Combining the PTCL and tMF patients, the overall response rate for all 37 patients receiving treatment was 24% (95% CI, 12% to 41%), with response by histologic subtype detailed in Table 3. The median time to response was 12 weeks, consistent with the first response assessment. Seven patients for whom response assessment was inadequate were considered nonresponders. The estimated median progression-free survival time was 3 months (95% CI, 2.2 to 4.3 months), and the estimated 1-year progression-free survival rate was 8% (95% CI, 2.1% to 19.6%). Two patients (5%), both of whom attained complete responses, remain free of progression after receiving 16 and 17 cycles of therapy, respectively. The Kaplan-Meier estimate of progression-free survival for all patients is shown in Figure 1. The median duration of response is 3 months (range, 1 to 18 months). The estimated median overall survival time was 8 months (95% CI, 4.5 to 9.5 months), and the estimated 1-year overall survival rate was 30% (95% CI, 16.1% to 44.7%). A median of 4 cycles (range, 1 to 17) of alisertib were administered per patient. Nine patients (24%) received more than six cycles and two patients (5%) completed 1 year of therapy with alisertib.
Table 3.
Response by Histology
| Histology | PTCL NOS | AITL | Transformed MF | ATLL | ALCL | ENKTL | Total |
|---|---|---|---|---|---|---|---|
| Total | 13 | 9 | 7 | 4 | 2 | 2 | 37 |
| CR/PR | 1/3 | 0/3 | 0/0 | 1/0 | 0/1 | 0/0 | 2/7 |
| SD | 1 | 2 | 2 | 0 | 1 | 1 | 7 |
| PD/NA | 8 | 4 | 5 | 3 | 0 | 1 | 21 |
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ATLL, adult T-cell lymphoma; CR, complete response; ENKTL, extranodal natural killer/T-cell lymphoma; MF, Mycosis fungoides; NA, not accessible; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma; PD, progressive disease; PR, partial response; SD, stable disease.
Fig 1.
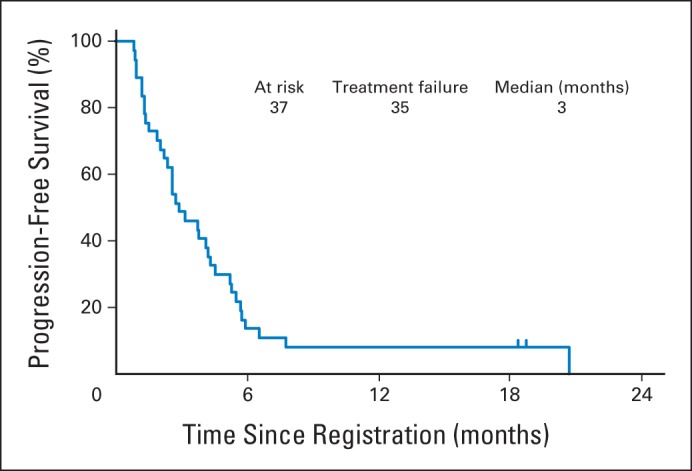
Kaplan-Meier estimate of progression-free survival.
Correlative Studies
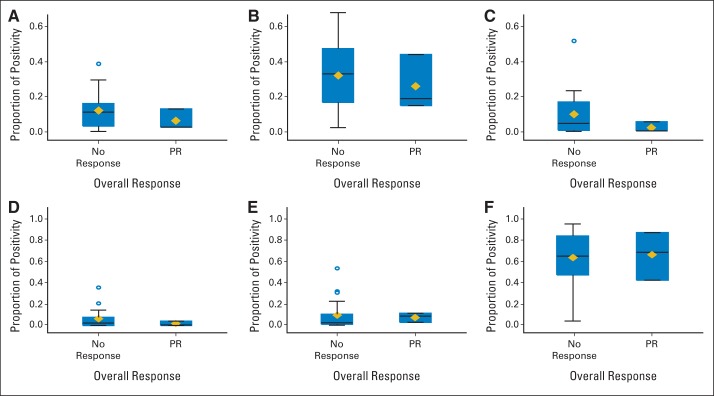
To determine whether AAK or ABK expression in tumor was associated with response to therapy, immunohistochemistry for AAK and ABK was performed on pretreatment tumor specimens (n = 22). In addition, samples were tested for BCL-2, MYC, Notch1, and PI3K-γ protein expression to better understand expression levels in T-cell lymphoma and in attempt to identify a predictive biomarker. No differences in responses were observed for the six biomarkers with alisertib therapy (Fig 2). Notably, median ABK expression was increased compared with AAK (P = .0004). In addition, PI3K-γ was more highly expressed in every specimen compared with the other markers tested (P < .0001).
Fig 2.
Box plots of immunohistochemical markers in pretreatment lymphoma biopsy specimens from 22 patients. (A) Aurora A, (B) Aurora B, (C) BCL-2, (D) MYC, (E) Notch1, and (F) phosphatidylinositol 3-kinase γ. The bottom and top edges of the boxes indicate the intraquartile ranges (IQRs). The horizontal lines within the boxes represent the medians; diamonds, means; whiskers, 1.5× the IQRs; and circles, outliers. PR, partial response.
Cytokine profiles, including 174 nonredundant cytokines, were evaluated on days 1 and 8 in 11 patients as an exploratory pharmacodynamic end point. There was no association with clinical response with the observed changes in these cytokines.
DISCUSSION
Frequent drug resistance and overall poor outcomes complicate the management of relapsed and refractory PTCL; the need for effective new agents with favorable toxicity profiles is clear. Our multicenter phase II trial confirms antitumor activity of single-agent alisertib in relapsed refractory PTCL. The 30% response rate observed in our study is broadly consistent with response rates observed with other single agents in the PTCLs, including romidepsin, belinostat, pralatrexate, and brentuximab.12–16 The responses in our study were independent of the number or type of previous therapies; notably, 24% of our patients had prior pralatrexate and 32% had prior histone deacetylase (HDAC) inhibitor therapy. Nearly half of all responses occurred in patients having demonstrated previously refractory disease, including patients who were refractory to prior novel therapies. In contrast, we did not observe activity in patients with tMF. The high incidence of myelosuppression, along with frequent cutaneous lesions, led to neutropenic fever and infectious complications precluding adequate dosing in this high-risk subgroup.
Similar to previously described phase I data with alisertib, myelosuppression was common and constituted the predominant indication for dose reduction. Nonetheless, two responding patients in our trial received alisertib for 1 year. Mucositis, anorexia, and diarrhea occurred in less than one quarter of patients and were largely grade 1 or 2 in severity. Grade 1 or 2 fatigue was also common, being observed in nearly half of patients. These toxicities compare favorably with other novel agents recently developed for T-cell lymphomas.15 On the basis of these results, a global phase III, randomized trial (NCT01482962) designed for US Food and Drug Administration registration has been initiated, comparing alisertib with investigator's choice (gemcitabine, pralatrexate, or romidepsin) in patients with relapsed/refractory PTCL.
Although there are now four agents approved for relapsed/refractory PTCL, there is a wide range of responses, perhaps related to underlying biologic heterogeneity. The identification of a predictive biomarker, accompanying an effective agent, would allow for the personalization of therapy similar to the experience with brentuximab in ALCL.17 We therefore evaluated several immunohistochemical markers on pretreatment biopsy specimens. No correlation was observed between responses to alisertib and AAK expression in our study, similar to previous investigations.9,18 This may relate to degree and depth of mitotic arrest and proapoptotic factors acting independently of the degree of AAK expression.19 In addition, in vitro data using T-cell lines suggest that ABK inhibition does occur with clinically achievable doses of alisertib, despite the reported selectivity to AAK.20 Although ABK expression was not associated with clinical responses in the subset of patients with sufficient biopsy material for analysis, levels of ABK in PTCL appeared higher than AAK, consistent with previous observations.20
The MYC oncogene regulates Aurora gene expression functioning to control mitotic entry, a response that appears critical for the maintenance of MYC-driven lymphomas.21,22 MYC-overexpressing cells may be particularly susceptible to Aurora kinase inhibition. AAK-overexpressing cells have been reported to upregulate BCL-2.23 In addition, significant cross-talk between AAK activation and the PI3K–AKT axis may function as a mechanism to promote cell survival.24,25 We did not observe any association between alisertib response and MYC, BCL-2, and PI3K expression, although our study had limited power to examine these factors. Increased PI3K-γ was noted in the available samples, suggesting combined PI3K and Aurora kinase inhibition may be worthy of further study.26 Serum cytokine profiling before and after alisertib identified a significant decrease in six cytokines (leptin, macrophage inhibition factor, macrophage inflammatory protein-1β [chemokine ligand 4], tissue inhibitor of metalloproteinases-1, latency-associated peptide, and Siglec-5) in more than half of patients. These cytokines are implicated in antiapoptosis, proinflammation, cell survival, metastasis, and promotion of an aggressive tumor microenvironment. Furthermore, in more than 50% of patients, two serum cytokines (BLC [CXC chemokine ligand 13] and monocyte chemoattractant protein-1 [chemokine ligand 2]) implicated in cytokinesis and lymphocyte homing demonstrated a significant increase.
The novel mechanism of alisertib lends itself to rational combination strategies. Zullo et al27 have demonstrated the combination of alisertib with romidepsin, an HDAC inhibitor approved in relapsed/refractory PTCL, to be highly synergistic in T-cell lines. In this model, it was postulated that AAK inhibition may prevent signal transducer and activator of transcription 3 phosphorylation, preventing activation of MYC and BCL-XL. The romidepsin-induced accumulation of acetylated proteins may further impede this pathway. Additional work demonstrates that vorinostat, another approved HDAC inhibitor for patients with PTCL, upregulates the expression of several proapoptotic genes, sensitizing cells to AAK inhibition.28 On the basis of these data, two ongoing clinical trials are testing alisertib plus vorinostat and romidepsin (NCT01567709 and NCT01897012, respectively).
In conclusion, our data confirm the antilymphoma activity of alisertib, providing a single-agent benchmark for its efficacy in PTCL. Given these results, an international phase III trial and studies of rational combinations have been initiated. Future work should build on our correlative studies to determine a biomarker for response to this agent, and other agents in PTCL.
Acknowledgment
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
We thank the patients who participated in this study and their families as well as the clinical trial staff who made the study possible.
Footnotes
Supported by National Institutes of Health/National Cancer Institute Cooperative Group Grants No. CA32102, CA38926, CA11083, CA13612, CA46368, CA128567, CA46282, CA46113, CA22433, CA21115, and CA21946, and National Clinical Trials Network Grants No. CA180888 and CA180819. P.M.B. is a Lymphoma Research Foundation Clinical Investigator. B.D.H. received a West Cancer Center/University of Tennessee junior investigator cancer research grant.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01466881.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Paul M. Barr, Daruka Mahadevan, Michael LeBlanc, Richard I. Fisher, Jonathan W. Friedberg
Provision of study materials or patients: Paul M. Barr, Nina D. Wagner-Johnston, Steven M. Horwitz, Bruce D. Cheson, Nancy L. Bartlett, Jonathan W. Friedberg
Collection and assembly of data: Paul M. Barr, Hongli Li, Daruka Mahadevan, Mansoor Ul Haq, Bryan D. Huber
Data analysis and interpretation: Paul M. Barr, Hongli Li, Catherine Spier, Daruka Mahadevan, Michael LeBlanc, Christopher R. Flowers, Nina D. Wagner-Johnston, Steven M. Horwitz, Bruce D. Cheson, Sonali M. Smith, Brad S. Kahl, Nancy L. Bartlett, Jonathan W. Friedberg
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Paul M. Barr
Consulting or Advisory Role: Millennium, Pharmacyclics, Gilead Sciences, TG Therapeutics
Research Funding: Pharmacyclics (Inst)
Hongli Li
No relationship to disclose
Catherine Spier
No relationship to disclose
Daruka Mahadevan
Honoraria: Pharmacyclics
Speakers' Bureau: Pharmacyclics
Michael LeBlanc
Consulting or Advisory Role: GSK Biologics
Mansoor Ul Haq
No relationship to disclose
Bryan D. Huber
No relationship to disclose
Christopher R. Flowers
Consulting or Advisory Role: OptumRx, Algeta, Seattle Genetics, Celgene
Research Funding: Acerta (Inst), Infinity (Inst), Onyx (Inst), Janssen Oncology (Inst), Gilead Sciences (Inst), Spectrum (Inst), Celgene (Inst), TG Therapeutics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), Abbvie (Inst)
Nina D. Wagner-Johnston
Consulting or Advisory Role: Gilead Sciences
Speakers' Bureau: Gilead Sciences
Research Funding: Celgene
Steven M. Horwitz
Honoraria: Amgen, Bristol-Myers Squibb, Celgene, Jannsen Pharmaceutica Products, LP, Millennium
Consulting or Advisory Role: Celgene, Amgen, Bristol-Myers Squibb, Janssen Pharmaceuticals, Millennium
Research Funding: Celgene, Millennium, Infinity, Kiowa-Kirin, Seattle Genetics, Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: Infinity Pharmaceuticals, ADC Therapeutics, RAND Corporation, Janssen Pharmaceuticals
Richard I. Fisher
Consulting or Advisory Role: Johnson & Johnson, MorphoSys, Celgene
Bruce D. Cheson
Consulting or Advisory Role: Celgene, Gilead Sciences, Pharacyclics, Astellas Pharma, Astra-Zeneca, Pfizer, Roche-Genentech, Amgen
Research Funding: Celgene (Inst), Pharmayclics (Inst), Gilead Sciences (Inst), Seattle Genetics (Inst), Roche/Genentech (Inst), Acerta (Inst), AstraZeneca/MedImmune (Inst)
Sonali M. Smith
Honoraria: Celgene, Janssen Oncology
Consulting or Advisory Role: Genentech/Roche, Celgene, Onyx, Seattle Genetics, TG Therapeutics, Gilead Sciences, Seattle Genetics, Immunogenix, Pharmacyclics
Speakers' Bureau: Spectrum, Janssen Oncology
Brad S. Kahl
Consulting or Advisory Role: Genentech, Pharmacyclics, Celgene, Millennium, Seattle Genetics
Research Funding: Abbott Laboratories
Nancy L. Bartlett
Consulting or Advisory Role: Seattle Genetics, Gilead Sciences
Research Funding: Seattle Genetics (Inst), Millennium (Inst), Pfizer (Inst), Pharmacyclics (Inst), Novartis (Inst), MedImmune (Inst), Celgene (Inst), ImaginAb (Inst), Genentech (Inst), Janssen Research Foundation (Inst), AstraZeneca (Inst)
Jonathan W. Friedberg
Consulting or Advisory Role: Trubion, Bayer, Kite Pharmaceuticals
Research Funding: Seattle Genetics (Inst), Genentech (Inst), Millennium (Inst), Janssen Pharmaceuticals (Inst)
REFERENCES
- 1.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: Spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 2.Smith SM, Burns LJ, van Besien K, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 2013;31:3100–3109. doi: 10.1200/JCO.2012.46.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yakushijin Y, Hamada M, Yasukawa M. The expression of the aurora-A gene and its significance with tumorgenesis in non-Hodgkin's lymphoma. Leuk Lymphoma. 2004;45:1741–1746. doi: 10.1080/10428190410001683615. [DOI] [PubMed] [Google Scholar]
- 4.Mahadevan D, Spier C, Della Croce K, et al. Transcript profiling in peripheral T-cell lymphoma, not otherwise specified, and diffuse large B-cell lymphoma identifies distinct tumor profile signatures. Mol Cancer Ther. 2005;4:1867–1879. doi: 10.1158/1535-7163.MCT-05-0146. [DOI] [PubMed] [Google Scholar]
- 5.Manfredi MG, Ecsedy JA, Chakravarty A, et al. Characterization of alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17:7614–7624. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 6.Palani S, Patel M, Huck J, et al. Preclinical pharmacokinetic/pharmacodynamic/efficacy relationships for alisertib, an investigational small-molecule inhibitor of Aurora A kinase. Cancer Chemother Pharmacol. 2013;72:1255–1264. doi: 10.1007/s00280-013-2305-8. [DOI] [PubMed] [Google Scholar]
- 7.Cervantes A, Elez E, Roda D, et al. Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora a kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4764–4774. doi: 10.1158/1078-0432.CCR-12-0571. [DOI] [PubMed] [Google Scholar]
- 8.Dees EC, Cohen RB, von Mehren M, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: Safety, pharmacokinetics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18:4775–4784. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 9.Friedberg JW, Mahadevan D, Cebula E, et al. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol. 2014;32:44–50. doi: 10.1200/JCO.2012.46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. ed 4. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 11.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 12.Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123:3095–3100. doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connor OA, Masszi T, Savage KJ, et al. Belinostat, a novel pan-histone deacetylase inhibitor (HDACi), in relapsed or refractory peripheral T-cell lymphoma (R/R PTCL): Results from the BELIEF trial. J Clin Oncol. 2013;31:519s. (abstr 8507) [Google Scholar]
- 15.O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: Results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: Results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 18.Kelly KR, Shea TC, Goy A, et al. Phase I study of MLN8237–investigational Aurora A kinase inhibitor–in relapsed/refractory multiple myeloma, non-Hodgkin lymphoma and chronic lymphocytic leukemia. Invest New Drugs. 2014;32:489–499. doi: 10.1007/s10637-013-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilton JF, Shapiro GI. Aurora kinase inhibition as an anticancer strategy. J Clin Oncol. 2014;32:57–59. doi: 10.1200/JCO.2013.50.7988. [DOI] [PubMed] [Google Scholar]
- 20.Qi W, Spier C, Liu X, et al. Alisertib (MLN8237) an investigational agent suppresses Aurora A and B activity, inhibits proliferation, promotes endo-reduplication and induces apoptosis in T-NHL cell lines supporting its importance in PTCL treatment. Leuk Res. 2013;37:434–439. doi: 10.1016/j.leukres.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.den Hollander J, Rimpi S, Doherty JR, et al. Aurora kinases A and B are up-regulated by MYC and are essential for maintenance of the malignant state. Blood. 2010;116:1498–1505. doi: 10.1182/blood-2009-11-251074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Liu H, Goga A, et al. Therapeutic potential of a synthetic lethal interaction between the MYC proto-oncogene and inhibition of aurora-B kinase. Proc Natl Acad Sci U S A. 2010;107:13836–13841. doi: 10.1073/pnas.1008366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XX, Liu R, Jin SQ, et al. Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res. 2006;16:356–366. doi: 10.1038/sj.cr.7310046. [DOI] [PubMed] [Google Scholar]
- 24.Yao JE, Yan M, Guan Z, et al. Aurora-A down-regulates IkappaBalpha via Akt activation and interacts with insulin-like growth factor-1 induced phosphatidylinositol 3-kinase pathway for cancer cell survival. Mol Cancer. 2009;8:95. doi: 10.1186/1476-4598-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Shi Y, Woods KW, et al. Akt inhibitor a-443654 interferes with mitotic progression by regulating aurora a kinase expression. Neoplasia. 2008;10:828–837. doi: 10.1593/neo.08408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahadevan D, Morales C, Huber BD, et al. Novel targeted combination therapies for peripheral T-cell lymphoma. Presented at the 56th ASH Annual Meeting and Exposition; December 6-9, 2014; San Francisco, CA. [Google Scholar]
- 27.Zullo K, Guo Y, Cooke L, et al. The investigational aurora A kinase inhibitor alisertib exhibits broad activity in preclinical models of T-cell lymphoma and is highly synergistic with romidepsin. Presented at the 56th ASH Annual Meeting and Exposition; December 6-9, 2014; San Francisco, CA. [Google Scholar]
- 28.Kretzner L, Scuto A, Dino PM, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-MYC, hTERT, and microRNA levels. Cancer Res. 2011;71:3912–3920. doi: 10.1158/0008-5472.CAN-10-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]