Abstract
Aims
It has been proposed that competitive sport increases the risk of ventricular tachyarrhythmias (VTA) and death in patients with arrhythmogenic right-ventricular cardiomyopathy (ARVC). However, it is unknown whether this only applies to competitive sport or if recreational sports activity also increases the risk of VTA/death.
Methods and results
Probands diagnosed with ARVC according to the 2010 task force criteria for ARVC (n = 108) were included in the current analysis. At the time of enrolment, study participants were questioned about exercise level prior to and after ARVC diagnosis, within three categories of sports participation: competitive (n = 41), recreational (n = 48), and inactive (n = 19). Competitive sport was associated with a significantly higher risk of VTA/death when compared with both recreational sport [HR = 1.99 (1.21–3.28), P = 0.007] and inactive patients [HR = 2.05 (1.07–3.91), P = 0.030]. No increased risk of VTA/death was associated with recreational sport when compared with patients who were inactive [HR = 1.03 (0.54–1.97), P = 0.930]. Symptoms developed at an earlier age in patients who participated in competitive sport (30 ± 12 years), when compared with patients who participated in recreational sport (38 ± 17 years) (P = 0.015) and inactive patients (41 ± 11 years) (P = 0.002). No difference in age at first symptom was seen between patients who participated in recreational sport and inactive patients (P = 0.651).
Conclusion
Competitive sport was associated with a two-fold increased risk of VTA/death, and earlier presentation of symptoms, when compared with inactive patients, and to patients who participated in recreational sport. When compared with inactive patients, recreational sport was not associated with earlier onset of symptoms or increased risk of VTA/death.
Clinical trials.gov identifier
Keywords: Arrhythmogenic right ventricular cardiomyopathy, ARVC, Ventricular arrhythmia, Exercise, Sport, Presentation
See page 1708 for the editorial comment on this article (doi:10.1093/eurheartj/ehv183)
Introduction
Arrhythmogenic right-ventricular dysplasia or cardiomyopathy (ARVC) is an inherited cardiac disease that predominantly affects the right ventricle (RV) and results in ventricular tachyarrhythmias (VTAs) and sudden cardiac death (SCD). Arrhythmogenic right-ventricular cardiomyopathy typically presents in adolescence or early adulthood, and although it only affects 0.02–0.10% of the general population, it is thought to account for approximately 5–20% of SCD cases in the young with geographical variations.1–7 The genetics of the disease include mutations in the desmosomal proteins of the heart, causing cell-to-cell binding abnormalities in the myocytes. In recent years, evidence has emerged suggesting that participation in competitive/endurance sports may be a trigger of the phenotypical penetrance of the ARVC disease, and be related to a worse prognosis.8–10 The general hypothesis is that increased cardiac output, as seen with vigorous exercise, causes stretching of the RV and myocardial wall stress, which results in higher tension of the cell-to-cell adherences, leading to myocardial damage, inflammation, and cell death. The damaged myocardium is subsequently replaced by fibrofatty scar tissue predisposing the patients to VTA. Current recommendations for ARVC patients advise against competitive sport participation,11 and generally against all high-intensity/high-dynamic recreational sport participation due to the potentially increased risk of VTA and/or SCD. However, there is a paucity of studies investigating the effects of recreational sport participation in ARVC patients.
The current study was designed to assess the effects of competitive and recreational sport participation on age at first onset of ARVC symptoms, and risk of VTA/death in ARVC probands. We hypothesize that participation in competitive sport is associated with earlier presentation of the disease and increased risk of VTA/death, whereas participation in recreational sports is not associated with earlier presentation of the disease or increased risk of VTA/death, when compared with ARVC probands who are inactive. We further aimed to investigate whether a change in exercise level after ARVC diagnosis is associated with a reduction in the risk of subsequent VTA/death.
Methods
North American Multidisciplinary Study
The North American Multidisciplinary Study was a multicentre study aimed to create a North American ARVC Registry with ARVC probands and their family members, prospectively gathering information on genetics, clinical outcome, and diagnostic measures associated with the disease. The design and protocol of the study has previously been published.12
Briefly, ARVC patients and family members were enrolled from 18 centres in the USA and Canada from September 2001 to July 2010.
Patients suspected of ARVC were screened with ECG, ambulatory Holter monitoring, echocardiography, and MRI. Based on the 1994 Task Force Criteria (TFC),13 a specialist at the enrolling centre evaluated whether or not patients fulfilled the ARVC diagnostic criteria.
Patients were eligible for enrolment in the registry up to 2 years after their original diagnosis. Patients with confirmed ARVC underwent additional invasive tests including electrophysiology testing, blood testing, myocardial biopsy, and RV angiography. First-degree relatives of patients diagnosed with ARVC underwent non-invasive tests and blood testing for genetics, and full diagnostic evaluation with invasive tests if the non-invasive tests were confirmatory of ARVC diagnosis. Non-invasive and invasive tests were analysed at core-laboratories in the USA and Europe.12
Genetic testing was conducted in all patients who gave informed consent. As previously described, DNA was amplified by polymerase chain reaction, and the presence of the specific desmosome-encoding genes: Plakophilin-2, Desmoplakin, Junctional plakoglobin, Desmocollin-2, Desmoglein-2, Plakophilin-4, and the intermediate filament-encoding gene Desmin was investigated.14 The presence of other non-desmosomal genes was not evaluated.
Patients were excluded from the registry if they were under 12 years of age or had been implanted with an implantable cardioverter defibrillator (ICD) more than 2 years prior to enrolment. Furthermore, four patients were excluded from the registry due to confirmed sarcoidosis.
Following the publication of the updated 2010 TFC,15 a blinded committee re-evaluated all ARVC probands in the registry and reassessed whether they were affected, borderline affected, or unaffected. The committee did not have access to specific information regarding sports participation prior to the reassessment.
Study population
The North American ARVC Registry contains 322 ARVC probands or family members. For the current study, all family members (n = 197) were excluded, as were probands with unknown exercise level before diagnosis (n = 17), leaving us with a study population of 108 ARVC probands, who were affected or borderline affected according to the 2010 TFC for ARVC.15
Endpoints
The primary endpoint of the current study was defined as a combined endpoint of VTA or death (whichever came first). Ventricular tachyarrhythmia events were defined as centre-reported documented clinical sustained VTA episodes with a duration of more than 30s, or appropriate ICD therapy for VTA occurring both before and after enrolment in the registry.
By study protocol, centres were required to report and document all episodes of VTA, occurring prior to enrolment or during follow-up, with the specific date of the event. Although it was not a requirement, centres were encouraged to report the type and cycle length of the VTA.
Exercise level
Exercise level, type of sport participation most commonly practiced, and age at sport initiation was obtained from a patient questionnaire at enrolment in the ARVC registry. Patients were asked what type of exercise level they participated in both before and after diagnosis, with the following pre-specified fixed options: (i) inactive; (ii) recreational; (iii) competitive/professional; or (iv) unknown.
Patients were further asked what type of sport they most often practiced, and at what age they first initiated the sport.
Based on the recommendations for sports participation in patients with cardiovascular disease, we defined a variable of high dynamic sport including basketball, soccer, hockey, skiing, running, biking, and tennis, and a variable of low to moderately dynamic sport defined as bowling, golf, weight lifting, wrestling, baseball, or softball.11
Statistics
Clinical characteristics at enrolment were compared between the groups using χ2 and Fishers exact test for dichotomous variables and Kruskal–Wallis test for continuous variables.
The time-specific cumulative probability of the endpoint with different exercise levels, were displayed by the method of Kaplan–Meier using log-rank test to test for differences in risk between groups. Cox proportional hazard regression models were employed to test the relative difference in the risk of the endpoint between the groups, reporting hazard ratios (HR) with 95% confidence intervals (CI) and P-values. Adjustment factors were determined by best subset regression, setting the limit for entry into the model at P < 0.05. At this limit, negative T-waves in V1–V3 was the only variable significantly associated with the endpoint.
Kaplan–Meier plots were used to illustrate the time relationship between first sport initiation to the earliest presentation of an ARVC symptom.
A two-tailed P-value below 0.05 was considered statistically significant. Analyses were performed using SAS statistical software 9.3 version (SAS Institute, Cary, NC, USA).
Results
Of the 108 ARVC probands with known exercise level, 19 patients (17.6%) reported that they were inactive, 48 patients (44.4%) participated in recreational sport, and 41 patients (38%) participated in competitive sport prior to being diagnosed with ARVC.
As shown in Table 1, patients who participated in competitive sports prior to their diagnosis were younger at the time of their ARVC diagnosis, when compared with patients who participated in recreational sports and to inactive patients. Competitive sports participants were more often male, more often had negative T-waves in V1–V3, and had significantly larger right- and left-ventricular volumes, when compared with both recreational sports participants and inactive patients (Table 1). Furthermore, 71% of the competitive sports participants had inducible ventricular tachycardia (VT) or ventricular fibrillation (VF) at electrophysiological study, whereas only 32% of inactive patients and 44% of recreational sports participants had inducible arrhythmias (Table 1). Importantly, no difference in ICD implantation or right-ventricular ejection fraction was evident between the groups at baseline (Table 1).
Table 1.
Clinical characteristics of arrhythmogenic right-ventricular cardiomyopathy probands with different levels of sport participation before diagnosis
| Clinical characteristics | Inactive, n = 19 | Recreational, n = 48 | Competitive, n = 41 | Overall P-value |
|---|---|---|---|---|
| Affected probands (2010 TFC15) | 17 (89) | 39 (81) | 38 (93) | 0.261 |
| Borderline affected probands (2010 TFC15) | 2 (11) | 9 (19) | 3 (7) | 0.261 |
| Age at enrolment (years) | 44 ± 10 | 42 ± 15 | 36 ± 14 | 0.087 |
| Female | 11 (58) | 23 (48) | 14 (34) | 0.184 |
| Height (cm) | 171 ± 12 | 172 ± 10 | 175 ± 10 | 0.394 |
| Weight (kg) | 88 ± 28 | 75 ± 16 | 74 ± 15 | 0.098 |
| Body surface area (m2) | 2.0 ± 0.3 | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.516 |
| Age at earliest symptom (years) | 41 ± 11 | 38 ± 17 | 30 ± 12*,§ | 0.005 |
| Age at diagnosis (years) | 43 ± 10 | 41 ± 15 | 34 ± 13§ | 0.040 |
| Age at first VT/VF (years) | 43 ± 10 | 40 ± 15 | 33 ± 13§ | 0.024 |
| Diagnostic criteria points | 4.1 ± 1.2 | 4.2 ± 1.1 | 4.2 ± 0.9 | 0.645 |
| Genotype positivea | 9 (53) | 14 (36) | 12 (33) | 0.307 |
| Left-ventricular ejection fraction (echo)b | 63 ± 12 | 60 ± 9 | 61 ± 8 | 0.496 |
| Arrhythmic events prior to enrolment (VT/VF) | 13 (68) | 33 (69) | 33 (80) | 0.404 |
| Sustained VT prior to enrolment | 6 (32) | 10 (21) | 18 (44)* | 0.065 |
| Non sustained VT prior to enrolment | 2 (11) | 10 (21) | 7 (17) | 0.671 |
| Syncope or VT/VF prior to enrolment | 14 (74) | 36 (75) | 35 (85) | 0.409 |
| Cycle length of VT/VF prior to enrolment (ms) | 266 ± 19 | 262 ± 44 | 294 ± 55 | 0.389 |
| ICD implanted | 16 (84) | 38 (79) | 35 (85) | 0.798 |
| Follow-up (years) | 2.7 ± 1.9 | 2.8 ± 1.3 | 3.4 ± 2.1 | 0.293 |
| Pharmacotherapy | ||||
| Antiarrhythmics | 5 (31) | 24 (60) | 23 (64)§ | 0.076 |
| Beta-blockers | 15 (94) | 33 (83) | 27 (75) | 0.291 |
| MRIc | ||||
| Right-ventricular end-diastolic volume (mL) | 157 ± 87 | 153 ± 48 | 205 ± 65* | 0.012 |
| Right-ventricular end-diastolic volume indexed by body surface area (mL/m2) | 83 ± 57 | 84 ± 27 | 108 ± 29* | 0.007 |
| Left-ventricular end-diastolic volume (mL) | 104 ± 16 | 129 ± 27 | 153 ± 31*,§ | 0.001 |
| Left-ventricular end-diastolic volume indexed by body surface area (mL/m2) | 52 ± 8¶ | 70 ± 14 | 80 ± 17*,§ | 0.004 |
| Right-ventricular ejection fraction (%) | 41 ± 18 | 45 ± 11 | 42 ± 10 | 0.383 |
| ECG | ||||
| Negative T wave in V1–V3d | 13 (68) | 32 (67) | 36 (88)* | 0.044 |
| Negative T wave in II, III, aVFd | 10 (53) | 19 (40) | 24 (59) | 0.193 |
| Premature ventricular beats >1000 on Holter | 4 (29) | 16 (47) | 19 (53) | 0.304 |
| EP Study | ||||
| Any induced VT or VF | 6 (32) | 21 (44) | 29 (71)*,§ | 0.006 |
Continuous variables are presented in mean ± standard deviation. Dichotomized variables are presented in crude numbers with percentages in parenthesis.
VT, ventricular tachycardia; VF, ventricular fibrillation; ICD, implantable cardioverter defibrillator.
aGenetic analyses were only conducted in probands who gave informed consent. Consequently, we have information regarding genotype in 17 out of 19 (89%) inactive, 38 of 48 (79%) recreational, and 38 of 41 (93%) competitive.
bOut of the 108 probands, 30 patients (28%) did not have an echocardiographic data available on LVEF, with 6 (32%) out of 19 inactive, 19 (40%) out of 48 recreational, and 5 (12%) out of 41 competitive, respectively.
cOut of the 108 probands, 52 patients (48%) did not have an MRI performed, with 12 (63%) out of 19 inactive, 24 (50%) out of 48 recreational, and 16 (39%) out of 41 competitive, respectively.
dOne or more leads.
*P < 0.05 between patients who participated in recreational and competitive sports.
§P < 0.05 between inactive patients and patients who participated in competitive sports.
¶P < 0.05 between inactive patients and patients who participated in recreational sports.
No significant differences were evident between patients who were inactive and those who participated in recreational sport, except for significantly larger right-ventricular volume indexed by BSA in patients who participated in recreational sports (Table 1).
Risk of ventricular tachyarrhythmia/death by exercise level and type before arrhythmogenic right-ventricular cardiomyopathy diagnosis
From birth to end of follow-up, 83 of 108 patients (76.9%) experienced a VTA event, with 79 of these events (95.2%) occurring prior to ICD implantation, and four events (4.8%) following ICD implantation. Only two patients died during follow-up. One patient in the competitive group died of unspecified cardiac causes at age 61 years, and one patient in the inactive group died following complications related to heart transplant surgery at age 43 years.
Competitive sport participation prior to ARVC diagnosis was associated with earlier presentation of events (34 ± 13 years), and with a significantly increased risk of VTA/death over time, when compared with both patients who participated in recreational sport (40 ± 15 years) and inactive patients (43 ± 10 years), whereas no significant difference was evident between patients who participated in recreational sport and inactive patients (Table 1, Figure 1A). At 40 years after birth the absolute risk of VTA/death was 61, 33, and 22%, respectively, in patients who participated in competitive sport, recreational sport, or who were inactive (Figure 1A).
Figure 1.
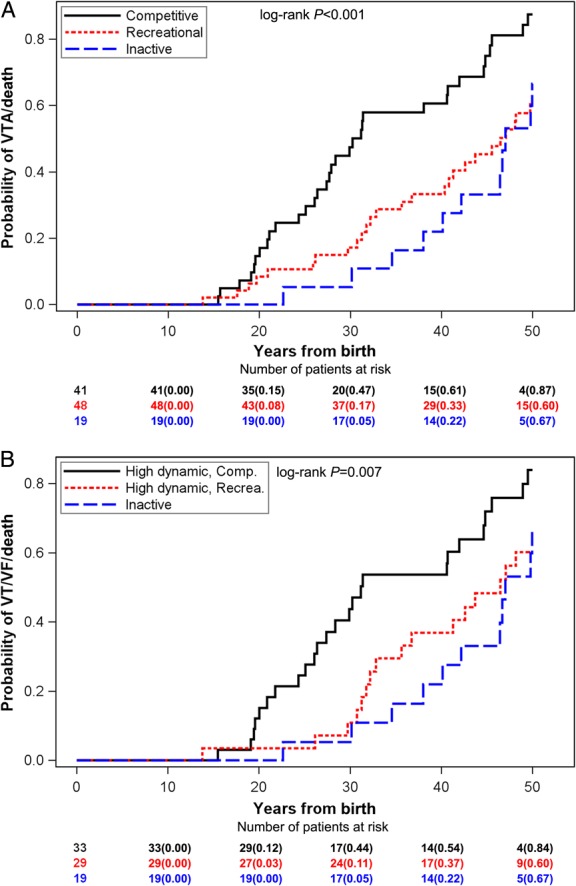
Kaplan–Meier graph showing the cumulative probability of VTA/death from birth in ARVC probands by sports level (A) and by sports type and level (B) before diagnosis. Sports level defined as competitive (black), recreational (red), or inactive (blue). The x-axis shows years from birth. (A) Probability of VTA/death from birth by sports level. (B) Probability of VTA/death from birth by sports level in patients who participated specifically in high-dynamic sports or were inactive. VTA, ventricular tachyarrhythmia; ARVC, arrhythmogenic right-ventricular cardiomyopathy.
No differential risk of VTA/death was found between patients who participated in recreational sports and patients who were inactive (Table 2). Competitive sport participation was, however, associated with a significant two-fold increased risk of VTA/death, when compared with both recreational sport participants and inactive patients (Table 2). The results were consistent when analysed in affected probands only (Supplementary material online, Appendix).
Table 2.
Relative risk of ventricular tachyarrhythmia/death from birth to end of follow-up in patients with different level of sports participation before diagnosis
| Events/patients | Hazard ratio | 95% confidence intervals | P-value | |
|---|---|---|---|---|
| Overall | ||||
| Recreational vs. inactive | 34/48 | 1.03 | 0.54–1.97 | 0.930 |
| Competitive vs. inactive | 36/41 | 2.05 | 1.07–3.91 | 0.030 |
| Competitive vs. recreational | 36/41 | 1.99 | 1.21–3.28 | 0.007 |
| High-dynamic sport | ||||
| Recreational, high dynamic vs. inactive | 20/29 | 0.93 | 0.45–1.90 | 0.835 |
| Competitive, high dynamic vs. recreational, high dynamic | 28/33 | 1.89 | 1.03–3.48 | 0.041 |
The Cox models were adjusted for negative T-waves in V1–V3.
Of the 83 VTA events, data on the type of VTA was present in 31 patients (37.3%). Nine of these 31 patients (29.0%) experienced sustained VT lasting 15–30 s, and 22 patients (71.0%) experienced sustained VT lasting more than 30 s, with equal distribution among the competitive, recreational, and inactive patients (P = 0.771).
Cycle length of the VTA episode was significantly different between patients who participated in competitive sports (305.4 ± 55.4 ms) and patients who participated in recreational sports (250.1 ± 30.0 ms) (P = 0.030). We were unable to show a significant difference between the cycle length of the VTA episode in inactive patients (263.0 ± 19.4 ms) and patients who participated in competitive sports (305.4 ± 55.4 ms) (P = 0.122). However, information on cycle length was only present in 6 of 19 (31.6%) inactive patients, 7 of 48 (14.6%) recreational patients, and 12 of 41 (29.3%) competitive patients.
Type of sport and risk of ventricular tachyarrhythmia/death
To investigate whether the lower risk of VTA/death found in patients who participated in recreational sport was associated with a higher prevalence of less strenuous sports types, we investigated the percentage of patients in each exercise level who participated in high-dynamic sports as previously defined. As shown in Table 3, no significant difference was evident in the percentage of patients who participated in high-dynamic sports types, with 94% in the recreational group and 89% in the competitive group (P = 0.68). The sports most often practiced in both the recreational and the competitive group were running and/or biking, followed by basketball, soccer, hockey, and swimming (Table 3.)
Table 3.
Type of most practiced sport
| Recreational, n = 31a | Competitive, n = 37a | P-value | |
|---|---|---|---|
| Age at sport initiation | 21.1 ± 10.2 | 14.8 ± 6.8 | 0.007 |
| Low/moderate dynamic (%) | 2 (6.5) | 4 (10.8) | 0.681 |
| High dynamic (%) | 29 (93.5) | 33 (89.2) | 0.681 |
| Basketball/soccer/hockeyb (%) | 7 (24.1) | 10 (30.3) | 0.776 |
| Tennisb (%) | 3 (9.1) | 3 (10.3) | 1.00 |
| Skiingb (%) | 2 (6.9) | 4 (12.1) | 0.676 |
| Swimmingb (%) | 6 (20.7) | 12 (36.4) | 0.263 |
| Running/bikingb (%) | 23 (79.3) | 25 (75.8) | 0.771 |
Age at sport initiation provided in mean with standard deviations.
Type of most practiced sports participation is in crude numbers with percentages in parenthesis. Information on type of sports most often practiced was available in 31 of 48 (65%) of the patients who participated in recreational sports, and in 37 of 41 (90%) of the patients who participated in competitive sports.
High-dynamic sports and low/moderate dynamic sports are defined in the Methods.
aNumber of patients with specified type of sport.
bPatients could practice more than one sport.
We found no difference in the risk of VTA/death between patients who participated in high-dynamic recreational sport and inactive patients, whereas high-dynamic competitive sport was associated with a significant two-fold increased risk when compared with high-dynamic recreational sport (Table 2, Figure 1B).
Influence of exercise level and age at sport initiation on onset of symptoms
Patients who participate in competitive sports before their diagnosis were significantly younger at first presentation of ARVC symptom (30 ± 12) when compared with both patients who participated in recreational sport (38 ± 17, P = 0.018) and inactive patients (41 ± 11, P = 0.003) (P-value: inactive vs. recreational = 0.66) (Table 1).
Patients who participated in competitive sports initiated sport participation at a significantly younger age when compared with patients who participated in recreational sport (15 vs. 21 years, P = 0.007) (Table 3). Furthermore, competitive sport was associated with the development of earlier symptoms after first sport initiation when compared with patients who participated in recreational sport, with 40 vs. 7% having experienced symptoms 10 years after first sport initiation and 67 vs. 29%, 20 years after first sport initiation (Figure 2).
Figure 2.

Kaplan–Meier graph showing the time relationship between first sport initiation and the time of first ARVC symptom in probands who participated in sports on a competitive (black) or recreational (red) level. The x-axis shows years from first sport initiation, and the y-axis shows the cumulative probability of earliest ARVC symptom. ARVC, arrhythmogenic right-ventricular cardiomyopathy.
Influence of exercise level on the right ventricle
Right-ventricular volume was measured by MRI in 56 of 108 patients (51.9%), with non-significant differences in distribution between the groups (P = 0.10–0.42).
Patients who participated in competitive sports had larger RV volumes at enrolment (204.6 ± 64.9 mL), compared with patients who participated in recreational sports (152.8 ± 48.0 mL) (P = 0.005), and to inactive patients (157.0 ± 86.8 mL) (P = 0.072), although in the latter we had too few patients to reach significance. The difference persisted after we indexed the measurements by body surface area (Table 1). However, right-ventricular ejection fraction was similar at baseline between all three groups (Table 1). In a linear regression model, participation in competitive sports was the only significant factor predictive of higher RV volumes (P = 0.003).
Influence of changes in exercise level on the risk of subsequent ventricular tachyarrhythmia/death
The majority of patients who were inactive or participated in recreational sport before their diagnosis did not change exercise level after diagnosis (inactive: 94%, recreational: 71%). None of the patients who were inactive or participated in recreational sport before their diagnosis went on to participate in competitive sports after ARVC diagnosis.
Of the patients who participated in competitive sport before ARVC diagnosis, 82% reduced their exercise level after diagnosis (31% to inactive and 51% to recreational); however, seven patients (18%) continued to participate in competitive sports after diagnosis, and four of these patients developed an event of VTA/death during follow-up.
Reducing exercise level from competitive to recreational or inactive lowered the subsequent risk of VTA/death to the level of patients who were inactive or participated in recreational sports both before and after diagnosis, with similar cumulative probabilities of VTA/death 4-years after diagnosis (38 and 31%, respectively) (Figure 3, Table 4). However, the event rates of VTA after ICD implantation were still significantly higher in patients who participated in competitive sport before diagnosis, but reduced their exercise level to recreational or inactive after diagnosis, when compared with patients who were inactive/recreational both before and after diagnosis (166.2 vs. 119.9 VTAs per 100 patient years).
Figure 3.

Kaplan–Meier graph showing the cumulative probability of VTA/death from the time of ARVC diagnosis in probands who participated in sports on a competitive level, both before and after diagnosis (black), who participated in sports on a competitive level before diagnosis and changed sport level to either inactive or recreational after diagnosis (red), or who were inactive or participated in sports on a recreational level both before and after diagnosis (blue). The x-axis shows years from diagnosis. VTA, ventricular tachyarrhythmia; ARVC, arrhythmogenic right-ventricular cardiomyopathy.
Table 4.
Relative risk of ventricular tachyarrhythmia/death after diagnosis in patients with different level of sports participation before and after diagnosis
| Events/patients | Hazard ratio | 95% confidence intervals | P-value | |
|---|---|---|---|---|
| Competitive before-competitive after | 4/7 | 1.77 | 0.59–5.29 | 0.307 |
| Competitive before-inactive/recreational after | 12/32 | 0.98 | 0.45–2.10 | 0.950 |
Model adjusted for negative T-waves in V1–V3.
Reference group: Inactive/recreational both before and after diagnosis.
The risk of VTA/death after ARVC diagnosis remained high in patients who continued to participate in competitive sport after diagnosis (4-year cumulative probability: 57%), however, due to limited number of patients in this group the results did not reach significance (Figure 3, Table 4). The event rate of VTA after ICD implantation was higher in the patients who continued to participate in competitive after diagnoses, when compared with those who reduced their exercise level to inactive/recreational (266.8 vs. 166.2 VTAs per 100 patient years).
No significant differences in clinical characteristics were evident between patients who continued to participate in competitive sport after ARVC diagnosis and those who reduced their exercise level after diagnosis. However, patients who continued to participate in competitive sport after ARVC diagnosis were slightly older at first sport initiation (17 ± 8 vs. 14 ± 6 years, P = 0.55), first ARVC symptom (34 ± 10 vs. 29 ± 13 years, P = 0.28), and at first VT (37 ± 12 vs. 32 ± 13 years, P = 0.35).
Discussion
In this study, we explored the influence of competitive and recreational sport participation on symptom development and risk of VTA/death in ARVC probands. Our findings support that participation in competitive sport is associated with earlier onset of ARVC symptoms and an increased risk of ventricular arrhythmias. Furthermore, the findings suggested that recreational sport participation was not associated with earlier presentation of symptoms or increased risk of VTA/death when compared with inactive ARVC probands. However, the absolute risk in both recreational sports participant and inactive patients was not negligible, with a 23% probability of VTA/death 2-years after diagnosis and a 22–33% probability of VTA/death at 40 years of age. In addition, our results suggested that reducing exercise level from competitive to inactive or recreational after diagnosis might lead to a decreased subsequent risk of VTA/death.
Arrhythmogenic right-ventricular cardiomyopathy and exercise
The pathogenesis of ARVC is related to mutations in the genes coding for the desmosomal proteins of the myocytes, causing dysfunctional cell binding, myocytes death, and subsequent fibrofatty replacement of the affected RV. However, not all mutations carriers experience penetrance of the phenotypical ARVC disease with cardiac symptoms of syncope, VTAs, and/or cardiac arrest. It has been proposed that the phenotypical presentation of ARVC is dependent on several factors including desmosomal mutations, and environmental factors like exercise. La Gerche et al.16 previously showed that endurance exercise in healthy athletes is associated with an isolated pronounced acute dilation of the RV and reduction in RV function. Similarly, Heidbuchel et al.8 found a high prevalence of RV involvement in high-level endurance athletes with ventricular arrhythmias, indicating that endurance/competitive exercise might promote RV changes and symptoms compatible with the ARVC diagnosis. In another publication from La Gerche et al.,17 they investigated desmosomal mutations in endurance athletes with ventricular arrhythmias and RV structural changes, and found a low frequency of genotype positive individuals, indicating that competitive/endurance exercise may promote ARVC even in the absence of genetic mutations. Sawant et al.18 recently published similar findings in both genotype positive and negative ARVC patients, further supporting exercise as an essential environmental factor in the pathogenesis of ARVC. Heidbuchel et al.8 did not conduct genetic testing, so it is unclear to which extent the athletes had desmosomal mutations. In the current study, we found a similar distribution of genotype positive patients among those who were inactive or participated in recreational activity, and those who participated in competitive sports. However, we only studied patients with established ARVC, and further, we did not investigate non-desmosomal mutations. Furthermore, the genetic field is still evolving, and there might be several mutations predisposing to ARVD that has yet to be discovered. Our data can therefore not be used to investigate the hypothesis of purely exercise-induced ARVC. Dilation of the RV, associated with competitive/endurance exercise, may lead to excessive myocardial damage and subsequent fibrofatty replacement of the affected RV, thereby triggering the disease, especially in patients with abnormal cell-to-cell binding of the myocytes. Animal studies support this theory. Kirchhof et al.10 showed that genetically altered ARVC mice developed the phenotypical presentation of ARVC much earlier if subjected to vigorous exercise. Recently, James et al.9 investigated the penetrance of ARVC in 87 desmosomal mutation carriers, and found endurance exercise to be associated with a higher penetrance of disease, earlier onset of symptoms, and increased risk of VTA and heart failure. Further supporting this, Saberniak et al.19 recently investigated myocardial function in ARVC patients, and found reduced RV and LV function in athletes with ARVC, when compared with their non-athletes counterpart. Furthermore, studies have shown a significant RV involvement in endurance athletes with VTAs,8 and a significantly higher rate of SCD due to ARVC in young athletes when compared with non-athletes.1 Corresponding to these findings, current recommendations for ARVC patients, with phenotypical presentation of the disease, strongly advise against participation in competitive sports.11,20
In the current study, we investigated the risk of VTA/death in ARVC probands with different levels of sport participation. We found that competitive sport was associated with a significantly increased risk of VTA/death and earlier presentation of symptoms, when compared with both patients who participated in recreational sport and those who were inactive. These findings are consistent with the finding from James et al.,9 and supportive of the hypothesis that vigorous sports activity can aggravate the presentation of the disease. We further found that age at sport initiation was highly associated with age at first symptom in patients who participated in competitive sports.
There is a paucity of studies investigating the effects of recreational sport participation on RV function in healthy people and specifically in ARVC patients. However, due to the high risk of VTA and SCD in ARVC patients, it is currently not recommended that ARVC patients participate in most forms of recreational sports.21
The strain on the RV associated with recreational exercise is not expected to be as pronounced as with competitive exercise, and therefore recreational exercise may not be associated with aggravation of the disease penetrance. To the best of our knowledge, no other study has investigated the impact of recreational sport on ARVC disease presentation and prognosis. In the current study, we found similar RV volumes in inactive patients and in patients who participated in recreational sports, whereas significantly larger RV volumes were found in patients who participated in competitive sports. Furthermore, competitive exercise was the only factor predictive of higher RV volumes. No difference was found in age at first ARVC symptom or in the risk of VTA/death between inactive patients and patients who participated in recreational sport. Importantly, there was no difference in the amount of patients in the competitive and recreational group that participated in high-impact sport types (89 vs. 94%). The sports most often practiced in the high-dynamic category were running and/or biking, followed by basketball, soccer, hockey, and swimming, which is consistent with the results from James et al.9 Furthermore, all of these exercise types are considered to have a high strain on the heart and especially the right ventricle,16 and therefore participation in these sports types, on any level, is not recommended for ARVD at the present time.11,20,21 However, our results showed no difference in the risk of VTA/death between inactive patients and patients who participated specifically in high-dynamic sport types on a recreational level, while there was a significantly increased risk in patients who participated in high-dynamic sport types on a competitive level.
Our results suggest that ARVC patients who participate in recreational sports have similar risk of VTA/death as inactive patients. However, the absolute risk in ARVC patients who were either inactive or participate in recreational sports was non-negligible, these data suggest that in ARVC patients, recreational sport participation might be just as ‘safe’ as remaining inactive. Therefore, recreational exercise may not accelerate the progression of the disease as previously suggested/feared.
After diagnosis, ARVC patients will be provided with recommendations regarding future exercise and prognosis. Most patients who participated in competitive sport before diagnosis will reduce their exercise level after diagnosis. This was also the case in our study, where 82% reduced their exercise level, resulting in a reduction in the subsequent risk of VTA/death. These findings are consistent with previous findings.9
Although the current study's findings are consistent with the findings from James et al., there are several differences between the studies. James et al.9 investigated a cohort of 87 patients with desmosomal mutations, however, only 41% were probands and only 64% met the TFC. Furthermore, they did not specifically investigate the effects of recreational exercise on penetrance of the disease and risk of VTA. Therefore, the current study is to date the largest study investigating the effect of both competitive and recreational sports participation on presentation of symptoms and risk of VTA/death in a population solely consisting of ARVC probands.
Limitations
The current study was conducted in 108 ARVC probands, and even though this is one of the higher number of ARVC proband populations in the literature to date, we are still limited in sample size. Furthermore, given the retrospective nature of the study, unmeasured confounders might have affected our results. However, given the hypothesis that competitive exercise is a trigger of the phenotypical presentation of ARVC, and the fact that all patients in this study were classified as either affected or borderline affected probands, we find it unlikely that any unmeasured confounders would have changed our results significantly.
The exercise level was self-reported through an enrolment patient questionnaire. We therefore do not know the actual hours of sport participation per day, and cannot eliminate the possibility of recollection bias. Furthermore, patients might have different definitions of competitive/recreational sports participation, although we find it unlikely that patients would have been prone to characterize themselves as competitive sports participant unless they were in fact that. The definition of recreational sports participation might be more fluctuating, which could have affected our results.
Lastly, this was a register study, and therefore we do not have uniform data on type and cycle length of the VTA, echocardiographically measured LVEF, and MRI measurements on RVEF, RV, and LV volume, which could potentially have distorted the comparison of the groups.
Conclusion
In the current study involving affected or borderline affected (2010 TFC15) ARVC probands from the North American ARVC register, we found competitive sports participation to be associated with earlier presentation of the disease and increased risk of VTA/death. Participation in recreational sport was not associated with earlier presentation of the disease or increased risk of VTA/death, when compared with inactive ARVC probands, although the absolute risk of VTA/death was high in both groups. This study suggests that affected or borderline affected ARVC probands may be able to participate in recreational sports with the same risk of VTA/death as ARVC patients who are inactive, although the absolute risk of VTA/death remains high.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The North American Multidisciplinary Study was financed by the National Institute of Health. Grant: NIH U01-HL65594.
Conflict of interest: W.Z. and A.J.M. reports receiving grant support from Boston Scientific. N.A.M.E. III reports receiving consultant fees from Medtronic, St Judes Medical and Boston Scientific. H.C. reports receiving support for ARVD-related projects from St Jude Medical and Medtronic. No other conflicts of interest were reported.
Acknowledgements
This research was performed while A.C.R. was a Mirowski-Moss Awardee. A.C.R. has received unrestricted travel grants from The Denmark-America Foundation, Falck Denmark, The Lundbeck-Foundation, Bønnelykkefonden, Carl, and Ellen Hertz Grant, and Torben and Alice Frimodts Foundation. The authors would like to express their gratitude to each of the patients who participated in the North American Multidisciplinary ARVD registry study, the enroling centres, core laboratories, genetic centre, and the data and coordinating centre. Without their work, the current study would not have been possible.
Appendix
North American Multidisciplinary ARVD Registry Study Genetic Center
Jeffrey A. Towbin, MD, The Phoebe Willingham Muzzy Molecular Cardiology Research Laboratory, Baylor College of Medicine, Houston, TX, USA
Gian Antonio Danieli, PhD, University of Padua, Padova, Italy
Data and Coordination Center
Wojciech Zareba, MD, PhD, University of Rochester, Rochester, NY, USA
Mary Brown, MS, University of Rochester, Rochester, NY, USA
Patricia Severski, BS, University of Rochester, Rochester, NY, USA
Scott McNitt, MS, University of Rochester, Rochester, NY, USA
Bronislava Polonsky, MS, University of Rochester, Rochester, NY, USA
Core Laboratories
David A. Bluemke, MD, PhD, (MRI) Johns Hopkins Hospital, Baltimore, MD, USA
Estes, N A Mark, MD (ICD) Tufts-New England Medical Center, Boston, MA, USA
Michael H. Picard, MD, Danita Y. Sanborn, MD (Echocardiography) Massachusetts General Hospital, Boston, MA, USA
Gaetano Thiene, MD, Cristina Basso, MD, (Pathology) Istituto di Anatomia Patologica, Padua, Italy
Thomas Wichter, MD, (Angiography) Medical Klinik C. University Muenster, Muenster, Germany
Wojciech Zareba, MD, (ECG, Holter, SAECG) University of Rochester, Rochester, NY, USA
Enrolling Centers
(Numbers in parentheses include number enrolled, n = 108)
Center Director, location, and research coordinator
Hugh Calkins, MD, Johns Hopkins Hospital, Baltimore, MD, USA (n = 19)
Julie Rutberg, MS, Crystal Tichnell, MGC, Cindy James, PhD.
David Cannom, MD, Los Angeles Cardiology Associates, Los Angeles, CA, USA (n = 14)
Young Park, RN
David Wilber, MD, Loyola University Medical Center, Maywood, IL, USA (n = 12)
Cynthia Finn, RN
Frank Marcus, MD, University of Arizona, Tucson, AZ, USA (n = 10)
Kathy Gear, RN
Henry Duff, MD, University of Calgary HSC, Calgary, Canada (n = 9)
Kandice Schroeder, RN
Melvin Scheinman, MD, University of California San Francisco, San Francisco, CA, USA (n = 9)
Nilita Escobar-Nicoud, RN
Mark Estes, III, MD, Tufts New England Medical Center, Boston, MA, USA (n = 8)
Melanie Marshall, RN
James Daubert, MD, University of Rochester, Rochester, NY, USA (n = 7)
Kathryn Pyykkonen, RN
Mario Talajic, MD, Montreal Heart Institute, Montreal, Quebec, Canada (n = 6)
Vitalie Perreault, RN, MSc, Martine Vaillancourt, RN
Andrew Krahn, MD, London Health Science Center-University Campus, London Ontario, Canada (n = 4)
Bonnie Spindler, RN
Michael Sweeney, MD, Brigham and Women's Hospital, Boston, MA, USA (n = 3)
Victoria Fox, MD, Lisa M. Andruszkiewicz
Hasan Garan, MD, New York Presbyterian Medical Center, New York, NY, USA (n = 2)
Maurita Baumeister, RN
Scott Sakaguchi, MD, University of Minnesota, Minneapolis, MN, USA (n = 1)
Julie Dicken, RN
Jack Kron, MD, Oregon Health and Science University, Portland, OR, USA (n = 1)
Kathy Kearns, RN
Bruce Lerman, MD, New York Hospital, Cornell Medical Center, New York, NY, USA (n = 1)
Tracey Shannon, RN
Charles Kerr, MD, St Paul's Hospital, Vancouver, B.C. (n = 1)
Carol Honeyman, RN
Jonathan S. Steinberg, MD, St Luke's-Roosevelt Hospital Center, New York, NY, USA (n = 1)
Robin Knox, RN
References
- 1.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol 2003;42:1959–1963. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D, Thiene G. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: clinical impact of molecular genetic studies. Circulation 2006;113:1634–1637. [DOI] [PubMed] [Google Scholar]
- 3.Tabib A, Loire R, Chalabreysse L, Meyronnet D, Miras A, Malicier D, Thivolet F, Chevalier P, Bouvagnet P. Circumstances of death and gross and microscopic observations in a series of 200 cases of sudden death associated with arrhythmogenic right ventricular cardiomyopathy and/or dysplasia. Circulation 2003;108:3000–3005. [DOI] [PubMed] [Google Scholar]
- 4.Saguner AM, Duru F, Brunckhorst CB. Arrhythmogenic right ventricular cardiomyopathy: a challenging disease of the intercalated disc. Circulation 2013;128:1381–1386. [DOI] [PubMed] [Google Scholar]
- 5.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988;318:129–133. [DOI] [PubMed] [Google Scholar]
- 6.Risgaard B, Winkel BG, Jabbari R, Behr ER, Ingemann-Hansen O, Thomsen JL, Ottesen GL, Gislason GH, Bundgaard H, Haunso S, Holst AG, Tfelt-Hansen J. Burden of sudden cardiac death in persons aged 1 to 49 years: nationwide study in Denmark. Circ Arrhythm Electrophysiol 2014;7:205–211. [DOI] [PubMed] [Google Scholar]
- 7.Winkel BG, Risgaard B, Sadjadieh G, Bundgaard H, Haunso S, Tfelt-Hansen J. Sudden cardiac death in children (1–18 years): symptoms and causes of death in a nationwide setting. Eur Heart J 2014;35:868–875. [DOI] [PubMed] [Google Scholar]
- 8.Heidbuchel H, Hoogsteen J, Fagard R, Vanhees L, Ector H, Willems R, Van Lierde J. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J 2003;24:1473–1480. [DOI] [PubMed] [Google Scholar]
- 9.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchhof P, Fabritz L, Zwiener M, Witt H, Schafers M, Zellerhoff S, Paul M, Athai T, Hiller KH, Baba HA, Breithardt G, Ruiz P, Wichter T, Levkau B. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation 2006;114:1799–1806. [DOI] [PubMed] [Google Scholar]
- 11.Pelliccia A, Fagard R, Bjornstad HH, Anastassakis A, Arbustini E, Assanelli D, Biffi A, Borjesson M, Carre F, Corrado D, Delise P, Dorwarth U, Hirth A, Heidbuchel H, Hoffmann E, Mellwig KP, Panhuyzen-Goedkoop N, Pisani A, Solberg EE, van-Buuren F, Vanhees L, Blomstrom-Lundqvist C, Deligiannis A, Dugmore D, Glikson M, Hoff PI, Hoffmann A, Horstkotte D, Nordrehaug JE, Oudhof J, McKenna WJ, Penco M, Priori S, Reybrouck T, Senden J, Spataro A, Thiene G. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2005;26:1422–1445. [DOI] [PubMed] [Google Scholar]
- 12.Marcus F, Towbin JA, Zareba W, Moss A, Calkins H, Brown M, Gear K. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C): a multidisciplinary study: design and protocol. Circulation 2003;107:2975–2978. [DOI] [PubMed] [Google Scholar]
- 13.McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 1994;71:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu T, Yang Z, Vatta M, Rampazzo A, Beffagna G, Pilichou K, Scherer SE, Saffitz J, Kravitz J, Zareba W, Danieli GA, Lorenzon A, Nava A, Bauce B, Thiene G, Basso C, Calkins H, Gear K, Marcus F, Towbin JA, Multidisciplinary Study of Right Ventricular Dysplasia I. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2010;55:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbuchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 2012;33:998–1006. [DOI] [PubMed] [Google Scholar]
- 17.La Gerche A, Robberecht C, Kuiperi C, Nuyens D, Willems R, de Ravel T, Matthijs G, Heidbuchel H. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart 2010;96:1268–1274. [DOI] [PubMed] [Google Scholar]
- 18.Sawant AC, Bhonsale A, Te Riele AS, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H, James CA. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J Am Heart Assoc 2014;3:e001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ, Ribe M, Holst AG, Edvardsen T, Haugaa KH. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelliccia A, Corrado D, Bjornstad HH, Panhuyzen-Goedkoop N, Urhausen A, Carre F, Anastasakis A, Vanhees L, Arbustini E, Priori S. Recommendations for participation in competitive sport and leisure-time physical activity in individuals with cardiomyopathies, myocarditis and pericarditis. Eur J Cardiovasc Prev Rehabil 2006;13:876–885. [DOI] [PubMed] [Google Scholar]
- 21.Maron BJ, Chaitman BR, Ackerman MJ, Bayes de Luna A, Corrado D, Crosson JE, Deal BJ, Driscoll DJ, Estes NA, III, Araujo CG, Liang DH, Mitten MJ, Myerburg RJ, Pelliccia A, Thompson PD, Towbin JA, Van Camp SP. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 2004;109:2807–2816. [DOI] [PubMed] [Google Scholar]


