Abstract
The optimal management of local recurrences after primary resection of pancreatic cancer still remains to be clarified. A 58-year-old woman developed an isolated recurrence of pancreatic cancer six year after distal pancreatectomy. Re-resection was attempted but the lesion was deemed unresectable at surgery. Then chemotherapy was administrated without obtaining a reduction of the tumor size nor an improvement of the patient’s symptoms. Thus the patient underwent percutaneous cryoablation under computed tomography (CT)-guidance obtaining tumor necrosis and a significant improvement in the quality of life. A CT scan one month later showed a stable lesion with no contrast enhancement. While the use of percutaneous cryoblation has widened its applications in patients with unresectable pancreatic cancer, it has never been described for the treatment of local pancreatic cancer recurrence after primary resection. Percutaneous cryoablation deserves further studies in the multimodality treatment of local recurrence after primary pancreatic surgery.
Key words: Pancreatic cancer, recurrence, percutaneous cryoablation, computed tomography scan
Introduction
Pancreatic cancer represents the fourth leading cause of cancer-related death in the Western world and still has a poor prognosis, in spite of the progresses achieved in cancer treatment over the last decades.1-3 Surgical resection is considered the only curative treatment for localized pancreatic adenocarcinoma, although it is known that only 15 to 20% of patients are candidates for resection surgery.4 Local and distant recurrences are common, even after radical surgery and adjuvant treatment. Local recurrences are linked with poor prognosis and possible onset of severe symptoms, such as excruciating pain, jaundice and hemorrhage.5 The management of these lesions is challenging due to both the objective difficulties of redoing surgical resection with a curative intent and the common coexistence of distant metastases.2,6,7
Case Report
A 58-year-old year lady underwent open distal pancreatectomy for a moderately differentiated pancreatic adenocarcinoma measuring 20x10 mm with no peripancreatic lymph node metastases, followed by adjuvant chemotherapy. She had been well without recurrence of cancer for six years following primary treatment, when she started to complain of moderate to severe abdominal pain, dyspepsia and weight loss. An ultrasound scan revealed a hypoechoic lesion measuring 16x18 mm in the pancreatic head. The patient was then sent for a contrast-enhanced computed tomography (CT) scan that showed an irregular mass located in the head of the pancreas (Figure 1). A CT-guided fine-needle aspiration cytology confirmed the lesion to be a cancer recurrence. A fluorodeoxyglucose positron emission tomography/CT study showed intense radiotracer accumulation in the pancreatic head. Since no signs of distant metastases were present at metastatic work-up, the patient underwent an exploratory celiotomy for salvage surgery. However, the cancer recurrence was deemed unresectable at the time of surgery because of tight adhesions and the lack of any cleavage planes with the hepatic artery and the portal vein. Therefore, first-line chemotherapy with cisplatin-gemcitabine combination was administered. Since the lesion in the pancreatic head had increased to 25x30 mm at the follow-up CT scan, a second-line chemotherapy with oxaliplatine was given. Nonetheless, a CT scan revealed a progression of disease (tumor mass maximum diameter 30x30 mm). Fourteen months after the diagnosis of cancer recurrence the patient underwent CT-guided percutaneous cryoablation (PCA) under local anesthesia and conscious sedation, using a tabletop argon gas-based cryoablation system (Galil Medical, Yokeneam, Israel). Two cryoprobes (Icesed®) were inserted under CT-guidance (Figure 2) into the tumor for double intraprocedurally freezing thawing cycles. Under CT guidance, the needle’s position and the ice ball dimensions (Figure 3) were assessed. There were no procedure-related complications and the postoperative period was uneventful. We only observed a low (and asymptomatic) increase of serum amylase and lipase the day after the procedure, which returned to normal in five days. The patient was discharged on postoperative day 2. One month after the treatment the patient was asymptomatic and contrast-enhanced CT scan showed no enhancement of the treated lesion (Figure 4). Three months after the PCA procedure the patient was lost to follow-up.
Figure 1.
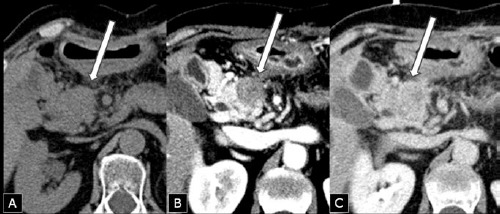
Computed tomography scan after six years from primary distal pancreatectomy for ductal adenocarcinoma showing an enlargement of the pancreatic head containing a mass (arrow) measuring 28x23 mm, isodense at baseline examination (A), relatively hypodense after rapid contrast medium injection (arterial phase) (B), and isodense in the portal phase (C).
Figure 2.
Patient in the computed tomography-room during the percutaneous cryoablation procedure.
Figure 3.
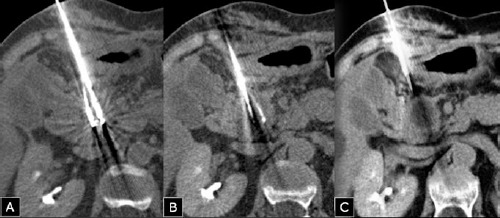
Two cryoprobes (Icesed®) with active tip positioned within the tumor were inserted under computed tomography (CT)-guidance with the patient in the supine position (A, B). The CT scan performed during the freezing phase showed an hypodense area located in the site of the tumor, corresponding to the ice ball (C).
Figure 4.
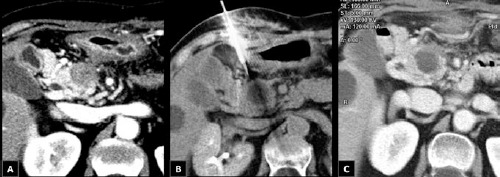
Modifications of the tumor seen at computed tomography (CT) scan at the time of initial diagnosis (A), during the percutaneous cryoablation (PCA) procedure (B), and after one month (C). Repeat CT scan after one month showed a well-defined and homogeneously hypodense area of 3x3 cm in the pancreatic head, corresponding to the coagulation necrosis obtained by PCA.
Discussion
Local recurrence following pancreatic resection for cancer occurs in 35-86% of patients operated on with curative intent.8,9 While local relapses are often detected in conjunction with disseminated disease, there is a smaller group of patients (approximately 30%), where isolated local recurrence is observed without evidence of distant metastasis.6,10
To date, no standard treatment or supportive care guidelines exist for the management of this severe condition.6,10 Systemic chemotherapy has historically resulted in a median survival rate of 5-11 months,4,11 and also radiation therapy has been shown to result in significant local control and to have some survival effects in selected patients.10
Some authors suggest that redo surgery could be effective and prolong survival in selected patients; however morbidity rates for repeated pancreatic resection can be associated to high morbidity rates due to adhesions, anatomical complexities and post-radiation fibrosis.9 Moreover, patients with local recurrences are frequently not good surgical candidates because of the poor general conditions.6 In the case described herein, a re-resection was attempted, but unfortunately it was not possible to remove the recurrence located in the pancreatic head because of the adhesions and possible infiltration of the hepatic artery and portal vein.
During the last ten years different minimally invasive ablative methods have been emerging for the palliative treatment of pancreatic cancer.2,12 Cryoablation is an ablative method which has been widely used for the treatment of several benign and malignant solid tumors.4,5,12-14 In essence, its effectiveness is based on the specific cytotoxic effects of cold on tissue that produces both instant and delayed destruction of cellular ultrastructure at temperatures lower than –40°C.2,4,13 Cryoablation has been predominantly performed during either open or laparoscopic surgery.12 Ultrasound or CT-guided PCA is a relatively new method used for the treatment of various solid malignancies.12 Acceptance of PCA for pancreatic malignancies has been delayed by concerns regarding both technical difficulties and risk of complications, in that the pancreas has a soft texture, has a profound location in the abdomen, and is close to structures such as the duodenum, portal vein, superior mesenteric artery, hepatic artery and common bile duct. However, recent advances in image-guided percutaneous techniques and instruments have led to a surge in the application of PCA in patients with pancreatic cancer.2,3 In fact, some studies and literature reviews have shown the safety and feasibility of PCA for unresectable or not amenable to surgery pancreatic cancer, although its use remains restricted to very selected cases in expert hands. The risk of vascular injuries from cold temperatures is hampered by the fact that the rapid blood flow in the large vessels close to the pancreas has a hot pool effect.3,4
Optimal tumor freezing and prevention of injuries to adjacent organs can be obtained with intraprocedural monitoring using ultrasonography, CT, or magnetic resonance imaging.2,4 Ultrasound can be used to guide PCA, but posterior acoustic shadowing limits visualization. At CT-scan the frozen lesion appears as an ice ball that is hypodense with respect to unfrozen tissue. For these reasons, we used CT guidance to assess the size and the location of the ice ball during the procedure.
Possible minor and major adverse effects have been described after cryoablation of pancreatic tumor, the main ones being abdominal pain, fever, increased serum amylase, bleeding, and leakage of pancreatic juice.2-4 However, rates of severe complications due to cryoablation appear to be lower than observed in other ablative techniques.12
In our patient, the postoperative period was uneventful and the patient was discharged 2 days after the treatment. The majority of authors report the patients undergoing PCA for pancreatic cancer under general anesthesia.4,5 It should be noted that we performed PCA on this patient under local anesthesia and conscious sedation without any intraoperative complications. Unfortunately, the patient was lost to follow-up after 3 months, thus it was not possible to evaluate further the clinical course and response to treatment. We were just able to notice that she was asymptomatic at the time of the one-month CT scan, hence we can assume that the procedure had a role in ameliorating her initial symptoms (moderate to severe pain and dyspepsia). Nonetheless, we believe that this case is worthy of attention, since PCA, as well as other ablative procedures, has been gaining popularity as a minimally invasive palliative procedure in patients with unresectable locally advanced pancreatic cancer, and several authors reported its effectiveness and safety in selected patients.2,3,5,12 To the best of our knowledge, however, ours is the first description of the use of PCA in the setting of an isolated local recurrence of pancreatic cancer. Besides, the patient underwent PCA with no complications and was discharged on postoperative day 2.
Conclusions
The case described herein, together with the evidence of the feasibility of cryoablation in locally advanced pancreatic tumors, suggests that PCA under CT-guidance is feasible and safe, and deserves further attention in the multimodality treatment of patient with local recurrence after primary surgery for pancreatic carcinoma.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [DOI] [PubMed] [Google Scholar]
- 2.Niu L, Zhou L, Xu K, Mu F. The role of cryosurgery in palliative care for cancer. Ann Palliat Med 2013;2:26-34. [DOI] [PubMed] [Google Scholar]
- 3.Tao Z, Tang Y, Li B, et al. Safety and effectiveness of cryosurgery on advanced pancreatic cancer: a systematic review. Pancreas 2012;41:809-11. [DOI] [PubMed] [Google Scholar]
- 4.Xu K, Niu L, Yang D. Cryosurgery for pancreatic cancer. Gland Surg 2013;2:30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu L, Wang Y, Yao F, et al. Alleviating visceral cancer pain in patients with pancreatic cancer using cryoablation and celiac plexus block. Cryobiology 2013;66:105-11. [DOI] [PubMed] [Google Scholar]
- 5.Kyriazanos ID, Tsoukalos GG, Papageorgiou G, et al. Local recurrence of pancreatic cancer after primary surgical intervention: how to deal with this devastating scenario? Surg Oncol 2011;20:e133-e42. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto D, Chikamoto A, Ohmuraya M, et al. Pancreatic cancer in the remnant pancreas following primary pancreatic resection. Surg Today 2014;44:1313-20. [DOI] [PubMed] [Google Scholar]
- 7.Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009;35:600-4. [DOI] [PubMed] [Google Scholar]
- 8.Hishinuma S, Ogata Y, Tomikawa M, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg 2006;10:511-8. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura A, Itasaka S, Takaori K, et al. Radiotherapy for patients with isolated local recurrence of primary resected pancreatic cancer. Prolonged disease-free interval associated with favorable prognosis. Strahlenther Onkol 2014;190:485-90. [DOI] [PubMed] [Google Scholar]
- 10.Strobel O, Hartwig W, Hackert T, et al. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol 2013;20:964-72. [DOI] [PubMed] [Google Scholar]
- 11.Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol 2014;20:2267-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pusceddu C, Melis L, Fancellu A, et al. Feasibility and safety of percutaneous radiofrequency, microwave or cryoablation for unresectable thoracic malignancies in close proximity to heart and large vessels. Proc. 66th Symp. Society of Surgical Oncology, 2013 March 6-9 National Harbor, MD (USA). Ann Surg Oncol 2013;20:S107. [Google Scholar]
- 13.Pusceddu C, Sotgia B, Amucano G, Fele RM, et al. Breast cryoablation in patients with bone metastatic breast cancer. J Vasc Interv Radiol 2014;25:1225-32. [DOI] [PubMed] [Google Scholar]


