Abstract
Background
Lucid dreams are frequently pleasant and training techniques have been developed to teach dreamers to induce them. In addition, the induction of lucid dreams has also been used as a way to ameliorate nightmares. On the other hand, lucid dreams may be associated with psychiatric conditions, including Post-Traumatic Stress Disorder (PTSD) and Reward Deficiency Syndrome-associated diagnoses. In the latter conditions, lucid dreams can assume an unpleasant and frequently terrifying character.
Case Presentations
We present two cases of dramatic alleviation of terrifying lucid dreams in patients with PTSD. In the first case study, a 51-year-old, obese woman, diagnosed with PTSD and depression, had attempted suicide and experienced terrifying lucid nightmares linked to sexual/physical abuse from early childhood by family members including her alcoholic father. Her vivid “bad dreams” remained refractory in spite of 6 months of treatment with Dialectical Behavioral Therapy (DBT) and standard pharmaceutical agents which included prazosin, clonidie and Adderall. The second 39-year-old PTSD woman patient had also suffered from lucid nightmares.
Results
The medication visit notes reveal changes in the frequency, intensity and nature of these dreams after the complex putative dopamine agonist KB220Z was added to the first patient’s regimen. The patient reported her first experience of an extended period of happy dreams. The second PTSD patient, who had suffered from lucid nightmares, was administered KB220Z to attenuate methadone withdrawal symptoms and incidentally reported dreams full of happiness and laughter.
Conclusions
These cases are discussed with reference to the known effects of KB220Z including enhanced dopamine homeostasis and functional connectivity of brain reward circuitry in rodents and humans. Their understanding awaits intensive investigation involving large-population, double-blinded studies.
Keywords: putative complex dopamine agonist, KB220Z, parasomnia, functional brain connectivity, lucid nightmares, Post-Traumatic Stress Disorder (PTSD)
“The implication is that fantasy and dreams are part of a single continuing fantasy process which is subject to certain transformations imposed by physiological and stimulus events. It is unnecessary to sleep in order to generate dream-like ideation, and, apparently, it is unnecessary to be awake in order to produce relatively coherent, undream-like ideation.”
Eric Klinger (1971, p. 57)
Introduction
Throughout the 20th century, dreaming was central to leading psychotherapeutic approaches to mental disorders. Psychodynamic models of the mind were inspired by the work of Sigmund Freud’s Interpretation of Dreams (Schredl, Bohusch, Kahl, Mader & Somesan, 2000). Based on his experience, Freud suggested that the unconscious foundations of most symptom understanding could be illuminated by an accurate evaluation of reported dreams. At the dawn of the 21st century these conceptualizations were challenged by neuroscientists who favored mechanistic models of brain dysfunction.
A generation of psychiatrists has turned to psychiatric biology for a more scientific, rigorous, and reliable phenotype to understand mental disorders. A new psychological techniques as well as pharmacotherapy’s emerged as the current cornerstone of treatment for mental disorders (Fusar-Poli et al., 2007). Importantly, nightmares are a prevalent parasomnia associated with a range of psychiatric conditions, and many psychiatrists continue to embrace neo-psychoanalytic speculations concerning their meaning. However, new advances in neuroscience, especially neuroimaging techniques and progress in neurogenetics, are helping to supplement the quest for answers regarding this complex phenomenon.
Empirical research has over the years stipulated some type of emotionally adaptive function for dreaming, e.g., image-contextualization, affect de-somatization, mood regulation, or fear extinction. Specifically, nightmares have been considered by many to be an intensified expression of an emotionally adaptive function. Moreover, Kovachy et al. (2013) discussed the role of sleep disturbances in Post-Traumatic Stress Disorder (PTSD), suggesting that nightmares in children and adolescents exposed to trauma and/or diagnosed with PTSD have significant loading. Nielsen and Levin (2007) suggested an affective network dysfunction (AND) model that integrates the tenets of prior models in proposing that nightmares reflect problems with the fear extinction function of dreaming. According to the authors, this new model accounts for a wide range of dysphoric dream imagery that includes bad dreams, idiopathic nightmares and post-traumatic nightmares. As such, it seems to incorporate recent findings from brain imaging in the areas of sleep physiology, PTSD, anxiety disorders and the consolidation and extinction of fear memories.
Spoormaker, Schredl and van den Bout (2005) correctly pointed out that it is important to discriminate nightmares as PTSD and non-PTSD related in terms of a patient’s history or other neurobiological defined parameters. Specifically, it is necessary to distinguish idiopathic nightmares from posttraumatic nightmares, which are part of a posttraumatic stress reaction or disorder that may result from experiencing a traumatic event. Both types of nightmares have been associated with an elevated level of periodic limb movements, although only posttraumatic nightmares seem to be related to more frequent and longer nocturnal awakenings. Furthermore, Mellman, David, Kulick-Bell, Hebding and Nolan (1995) believe that bad dreams may be the result of previous psychiatric problems such as depression when exposed to a traumatic event, indicating pre-existing conditions such as neurogenetic antecedents to these psychiatric conditions as has been recently observed for depression (Pearson-Fuhrhop et al., 2014).
To determine if depression per se is a major factor in terms of frequent nightmares compared to victims of extraordinary stress such as Nazi Holocaust survivors, Rosen, Reynolds, Yeager, Houck and Hurwitz (1991) evaluated 42 survivors, 37 depressed patients, and 54 healthy subjects of about the same age in terms of sleep impairment. Survivors had significantly more frequent awakenings due to bad dreams and had less loss of enthusiasm than the depressed subjects. Sleep disturbances and frequency of nightmares were significantly and positively correlated with the duration of the survivors’ internment in concentration camps. Importantly, the Nazi Holocaust survivors’ frequent nightmares remained a considerable problem even 45 years after liberation.
Nightmares as a function of neurotransmission
Currently, we do not understand fully the mechanism(s) of nightmares, why people have them and what the neurological causes of this sleep impairment are, despite a significant literature on the neurobiology of dreams. One theory suggests that the hippocampus is involved, and contextual memory is impaired in dreams occurring during paradoxical sleep. Accordingly, this impairment is caused by increased concentrations of acetylcholine, dopamine, and cortisol, as well as the absence of serotonin and noradrenaline (Sil’kis, 2010). Specifically, paradoxical sleep occurs via the activation of cholinergic neurons in the pedunculopontine nucleus as a result of suppression of their inhibition from the output basal ganglia nuclei. Sil’kis further suggested that any neurotransmitter or neuropeptide able to promote long-term potentiation in all components of the polysynaptic pathway through the hippocampus, especially at CA1 and CA3, can improve episodic memory and reduce nightmares. He also suggested that these disruptions involve variations in acetylcholine, dopamine, noradrenaline, serotonin, and glutamate concentrations. Importantly, this disinhibition is induced by activation of dopaminergic cells by pedunculopontine neurons. It was further suggested that there is a subsequent rise in dopamine concentration in the input basal ganglia structure striatum, and modulation of the efficacy of cortico-striatal inputs may be critical for the development of nightmares (Sil’kis, 2006). Moreover, in the absence of signals from the retina, a disinhibition of neurons in the pedunculopontine nucleus and superior colliculus allows them to excite neurons in the lateral geniculate body and other thalamic nuclei projecting to the primary and higher visual cortical areas, prefrontal cortex, and back into the striatum. Thus, dreams as visual images and motor hallucinations are the result of an increase in activity of specifically selected groups of thalamic and neocortical neurons. The involvement of dopamine is paramount in that its homeostasis provides synaptic transmission efficacy during the circulation of signals in closed interconnected loops, each of which includes one of the visual cortical areas, one of the thalamic nuclei, limbic areas and a region of the basal ganglia.
Additionally, Gottesmann (2006) suggested that in schizophrenia, the rapid eye movement (REM) dreaming sleep stage is characterized by common intracerebral disconnections, disturbed responsiveness, and sensory differentiation processes. Simply, disinhibition of superior colliculus neurons and their excitation by the pedunculopontine nucleus lead to an appearance of REM during paradoxical sleep. In fact, during REM sleep, the principal dreaming stage, the cortex is activated but significantly disinhibited, since all aminergic neurons are silent except the dopaminergic ones (Gottesmann & Joncas, 2000).
Solms (2000) in an attempt to unravel sleep disturbances, especially “bad dreams”, pointed out that dreaming and REM sleep are dissociable states, and that dreaming is controlled by forebrain mechanisms, that cholinergic brain stem activity controls the REM state and as such can generate the psychological phenomena of dreaming only through the mediation of a second, probably dopaminergic, forebrain mechanism. Moreover, dreaming can be manipulated by dopamine agonists and antagonists with no concomitant change in REM frequency, duration, and density.
In fact, dreaming is obliterated by focal lesions along a specific (probably dopaminergic) forebrain pathway and these lesions do not have any appreciable effect on REM frequency, duration, and density. As such, Solms’ suggested that the forebrain mechanism in question is the final common pathway to dreaming and that the brainstem oscillator that controls the REM state is just one of the many arousal triggers that can activate this forebrain mechanism.
Lucid dreams, the subject of these case histories, are a special form of dream life, in which the dreamer is frequently aware that he/she is dreaming, can stop/re-start the dreams, depending on the pleasantness or unpleasant nature of the dream, and experiences the dream as if he/she were fully awake.
Based on the clinical experience of one of us (TM), it was decided to incorporate a well-researched putative complex safe putative dopamine agonist known as KB220Z (Blum, Oscar-Berman et al., 2012) in a diagnosed PTSD patient’s treatment plan to determine if this complex would provide any beneficial effects to combat reoccurring lucid nightmares (“bad dreams”) refractive with other pharmaceutical agents.
Standard treatment options
In an attempt to provide medical guidelines to treat unwanted “bad dreams” especially in PTSD patients, Aurora et al. (2010) recommended the following treatment options:
-
–
Prazosin is recommended for treatment of PTSD-associated nightmares.
-
–
Image Rehearsal Therapy is recommended for treatment of nightmare disorder.
-
–
Systematic Desensitization and Progressive Deep Muscle Relaxation training are suggested for treatment of idiopathic nightmares.
-
–
Clonidine may be considered for treatment of PTSD-associated nightmares.
-
–
The following medications may be considered for treatment of PTSD-associated nightmares, but supporting data are sparse: trazodone, atypical antipsychotic medications, topiramate, low dose cortisol, fluvoxamine, triazolam and nitrazepam, phenelzine, gabapentin, cyproheptadine, and tricyclic antidepres sants. Nefazodone is not recommended as first line therapy for nightmare disorder because of the increased risk of hepatotoxicity (Level C).
-
–
The following behavioral therapies may be considered for treatment of PTSD-associated nightmares based on low-grade evidence: Exposure, Relaxation, and Rescripting Therapy; Sleep Dynamic Therapy; Hypnosis; Eye-Movement Desensitization and Reprocessing; and the Testimony Method.
-
–
The following behavioral therapies may be considered for treatment of nightmare disorder based on low-grade evidence: Lucid Dreaming Therapy and Self-Exposure Therapy.
-
–
Venlafaxine is not suggested for treatment of PTSD-associated nightmares. No recommendation is made regarding clonazepam and individual psychotherapy because of sparse data.
Case Presentation One
AC at the time of the initial evaluation was a 51-year-old woman allergic to both iodine and fish, living with her daughter, brother, and sister-in-law. She was receiving citalopram and seroquel for depression (10/10 highest rating) and was a thyroid ectomized obese individual. The patient stated she had been in psychotherapy for two years but never discussed any abuse in her marriage. She denied any psychiatric hospitalizations. The onset of her psychological symptoms began when she was living in Puerto Rico in 2008, while divorcing her second husband. Her house caught on fire, and she was involved in a motor vehicle accident without loss of consciousness. She did not receive any support from her company at that time. She also reported that her first husband physically, sexually, and verbally abused her.
Abuse history
The patient was sexually abused by two uncles involving sexual intercourse, as well as by a cousin during her childhood. With respect to physical abuse, she stated there was extreme violence in the home, and her mother was frequently beaten by her father while they lived in New York City. She herself was beaten at five years of age by her father and never told her mother. She was also emotionally abused by her cousins. The patient was the victim of physical violence by her first husband and verbal abuse by her second husband. The patient attempted suicide three times, once involving crashing her motorcycle into a car. She never revealed this in therapy. The patient denied any history of legal involvement.
Family psychiatric history
One cousin committed suicide, and there were numerous other suicides in the family. Her father was an alcoholic. (Later, it was determined that the father had presumed Tourette’s Syndrome, in view of his frequent throat-clearing and motor mannerisms). The patient’s mother was 73 years old and described as “quiet and submissive”. During her marriage, the mother was beaten and stabbed. She did not engage in any activities nor was she affectionate towards the patient, when the latter was 5–6 years of age. The patient’s father is deceased and was described as “mean, violent, and very scary”. AC had not had any contact with her father until three years prior to his death, when he apologized for his abuse. She stated that her father died of cancer. She has six siblings, with whom she has distant relationships. The patient later reported that her uncle had vocal tics and facial grimacing and blinking, consistent with Tourette’s Syndrome. She stated that her father beat her up and slapped her face on both sides at age six, because she told her mother that the father had a girlfriend. She stated her father was the “chief and the family were the tribe”. She added that, while drunk, he would carry arrows and play a game, where he would squeeze her hand very hard.
Social history
The patient is a high school graduate with some college courses. She admitted to being distractible during grade school and would frequently be dreamy and look out the window. She has been married twice and has three children, one of whom is “problematic”. She is supported by Social Security Disability Insurance benefits.
Past medical history
The patient is status-post thyroidectomy, maintained on synthroid. She also suffers from migraine headaches, terrifying lucid dreams, and lactose intolerance. The patient drank alcohol daily. She denied a history of alcohol dependence in the past. She denied drug abuse in the past, although she admitted to the use of marijuana and overusing prescription medications. The patient is a non-smoker, who drinks two to three cups of tea and two to three cups of coffee a day.
Mental status examination
The patient was alert and oriented in all three spheres. Her mood was described as extremely depressed. She had suicidal ideation, without plan. She had homicidal ideation, without plan. She denied auditory hallucinations, symptoms of OCD, delusions, or mania. She admitted to generalized anxiety as well as hyper-vigilance and daily lucid nightmares during which she felt “helpless”. She reported daily flashbacks of earlier traumas and admitted to a history of burning herself with hot water or a hot knife. She ate three meals a day and slept only 0 to 2 hours.
Diagnosis
Axis I: Post-Traumatic Stress Disorder; Major Depressive Disorder; ADHD, inattentive type
Axis II: N/A
Axis III: obesity, s/p thyroidectomy
Axis IV: severe – symptom management
Axis V: CGAF 40
Initial treatment plan and progress notes
The patient was referred for individual therapy and for group Dialectical Behavioral Therapy (DBT). She was started on prazosin at bedtime. Her zoloft and seroquel doses were increased.
Session 1
This session showed that the patient was alert, oriented and cooperative. She continued to complain of symptoms of PTSD. Her mood was extremely depressed, but her sleep and appetite were fair. She was being seen in DBT as well as individual psychotherapy. She had, for the first time, a dream about getting married again, which was interpreted by the psychiatrist as a possible sign of hopefulness, in contrast to her continuing dreams involving helplessness and hopelessness.
Session 2
The patient continued to complain of PTSD symptoms as well as those of depression. Her mood was only fair and there was no evidence of suicidal or homicidal ideation. Appetite and sleep were normal. During this interview, she had recollections of her father masturbating her at four years of age and touching her behind. She also stated that her uncle had been more aggressive from ages 7 to 14 and forced her to have intercourse. Another uncle abused her from ages 10 to 17. She reported feeling guilty about these experiences from the age of 7 onward.
Session 3
Her mood was normal, and there was no evidence of suicidal or homicidal ideation nor manic changes. Her appetite was normal, but she continued to complain of “nightmares”. She had been started on prazosin, with the dose increased from 2 mg to 6 mg at bedtime.
Session 4
She complained of problems with her children but reported that her mood was essentially normal, and there was no evidence of suicidality, homicidality, or nightmares. She did, in fact, report a decreased intensity in her dreams, which was coincident with the recently prescribed and increased adderall 20 mg in the morning.
Session 5
The patient complained of nightmares again, with a fair mood and no evidence of suicidal, homicidal, or manic symptoms. She continued individual and DBT. She thought that prazosin was making her nightmares worse. In particular, she described lucid dreams of an unpleasant nature. These dreams, upon further interrogation, were “as if I were fully awake” and had a very primal content involving rape and murder. Her prazosin, which she maintained was exacerbating her vivid, lucid, primal dreams was decreased to 4 mg at bedtime. Prior to the next session, her visiting nurse stated that there was a need to decrease her prazosin further because of an increase in her heart rate (reflex tachycardia). Xanax was given at bedtime in an attempt to alleviate the lucid dreams with traumatic content. The patient then reported a lucid dream, wherein she was trying to dig out from behind a thick wall with a pick ax. She had opened up a hole in the wall but could not get her entire body through this hole. Her psychiatrist interpreted the hole as a sign of hope and speculated that the self-built wall represented the patient’s mistrust and strong defense against expressing any feelings or impulses (a character trait adopted since early childhood). Additionally, the interpretation was advanced that the responsibility for the wall and its erection was a decision of the patient.
Session 6
The patient stated her mood was depressed, but there was no evidence of suicidal or homicidal ideation nor mania. Lithium was started 300 mg for two days and then 600 mg a day thereafter as an augmenting strategy for her depression and because of her past history of suicidal preoccupations. The patient had been complaining of a head tremor, which she described as “internal” rather than external, and it was decided to discontinue her adderall. Her mood remained depressed and she reported she had been dreaming about having sex as a little girl with an older man, who resembled one of her uncles. She stated that she awoke suddenly and that her younger daughter with whom she slept (a cultural habit) stated that the patient had struck her in the head, apparently during one of these dreams.
Contact with the patient’s visiting nurse indicated that the patient was still complaining of “nightmares”, but that they had decreased with the initiation of clonidine 0.1 mg at bedtime (HS). Clonidine was then increased after a two-week period to two pills at bedtime.
Session 7
The patient reported a depressed mood but no suicidal or homicidal ideation nor mania. She reported a lack of motivation (consistent with her probable diagnosis of ADHD). She stated that she was having lucid sexual dreams again, in which she was “helpless”. She stated that there was no change in her internal head tremors. She had been referred to a local teaching hospital with an extended waiting period. It was decided to discontinue her lithium, because she thought that these might be the source of her quasi-neurological symptoms.
Session 8
She had expressed a continued willingness to go for a neurologic evaluation at a local teaching hospital. Her lithium continued to be titrated. She reported ongoing lucid dreams but was not specific about their content, although the exploration of these was precluded by the limited time available during the visit.
Session 9
She reported that her youngest daughter (the one with whom she slept in a culturally familiar way) had been arrested and had to appear in court for attacking a fellow student, who had been bullying her. The patient reported a decrease in her head tremor and her mood was relatively normal. She reported that she was eating only one meal a day and had started taking KB220Z and noted decreased hunger on this nutraceutical. Off the adderall, her concentration had decreased somewhat.
Session 10
The patient reported anxiety, apparently related to the fact that the local police had come to discuss a sexual assault on her oldest daughter (not the one she was sleeping with). She noted that while taking the KB220Z, her blood sugar had increased to 200, and she was placed on metformin but that her bad lucid dreams had become somewhat happier. Her nutraceutical (KB220Z which contained chromium) theoretically should not have increased her blood sugar. Nevertheless, her KB220Z was decreased to one pill in the morning and two at bedtime.
Session 11
The patient reported being angry at a Motor Vehicle Agency representative but felt more motivated than she had after her adderall had been decreased. She stated that the combination of metformin and KB220Z was associated with a blood sugar decrease from 135 to 123 mg/ml. She stated at this point that she was only taking one KB220Z in the morning and one at bedtime and felt much calmer. She also reported that the content of her dreams had become “much happier”. In addition, she said that she was no longer having nightmares or lucid dreams at all and that this had been the case for two months, which was coincident with the initiation of KB220Z. Her lucid dreams appeared to have satisfied the criteria of such dreams, since they were as if she were fully awake, were in vivid color and could be, to a certain extent, controlled by her voluntarily. Interestingly, the patient had missed the next few appointments because of transportation issues. She stated that during this time she had missed taking the KB220Z for three days and noted worsening lucid dreams, involving someone being raped.
Session 12
The patient stated that her car had become more reliable as a means of transportation, and she no longer had to rely on the transportation service. She stated that her mood was normal and there was no evidence of suicidal or homicidal ideation nor mania. She stated that she was eating one meal a day (against medical advice but done by the patient in order to lose weight). Her weight was approximately 240 pounds. She was taking metformin twice a day. She had missed a few days of KB220Z and stated that her lucid and traumatically terrifying dreams had returned. When she resumed taking three KB220Z a day, her vivid dreams persisted, but the content was not unpleasant. At one point, when she took four a day, these dreams continued to be lucid and had an unpleasant content, which could not be explored because of the length of the time available for the visit, however, excessive dopamine (homeostasis is required), as suggested earlier in this article, could be the associated issue.
Session 13
The patient expressed frustration with her visiting nurse. Her KB220Z continued to be prescribed at two tablets twice a day from the previous visit, and the patient reported that on a lower dose she had dreams of being raped but that this constituted the first truly bad dream of a lucid nature on this regimen.
Session 14
The patient complained about problems with her youngest daughter who had been engaged in body piercing. She reported that her mood was depressed, that she had lost some weight, and that her dreams were normal on the four KB220Z a day, and that she was unable to remember any content, either pleasant or unpleasant. At this point, the patient wanted, because of an obsession about her weight and her resistance to following standard medical advice, to try a weight loss product known as Herbal Life. Because of possible but unknown interactions with the KB220Z, it was decided to discontinue the latter, for the time being. After a 6-month period without KB220Z the terrifying lucid dreams returned again. However, when she began the KB220Z again within 2 weeks she reported no more terrifying lucid dreams (see Figure 1).
Figure 1.
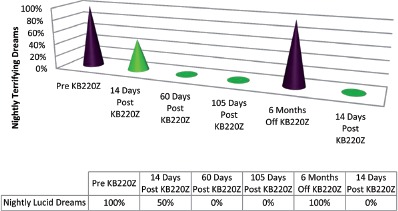
Case One
Case Presentation Two
Identifying information and chief complaint
The patient DR is a 39-year-old woman, mother of two daughters, who lives with her boyfriend. She was referred by her therapist for psycho-pharmacology treatment and was at the same time attending a local methadone clinic. Her principal complaints involved an eating disorder (purging), depression, and symptoms consistent with PTSD. She was also opioid-dependent. Her psychological symptoms included crying spells, depression, poor concentration, lack of appetite, restlessness, panic attacks, purging, and cravings for drugs.
Past history
When seen initially the patient was being maintained on methadone 85 mg a day for her opioid dependence. She had been hospitalized, in the remote past, for a suicide attempt, by hanging. She had also been hospitalized at 20 years of age, for depression and ideas of reference.
Physical abuse history
The patient was verbally abused by her mother. She was sexually abused, at age 6, by a male babysitter. The latter was 17 years old. The patient remembered the perpetrator had a tendency to tap his hands. She informed her mother about the sexual abuse (which involved intercourse), and the perpetrator was arrested and incarcerated. The patient stated that this early abuse triggered the almost nightly occurrence of nightmares (four to five times a week) as well as frequent flashbacks. She added that her 22-year-old daughter had also been kidnapped and raped.
Family psychiatric history
Mother was an alcoholic, who died two years prior to the most recent intake. The patient reported she mourned her mother’s death. Her father, who was currently living in Texas, had separated from her mother, when the patient was five years of age.
ADHD history
The patient states that, during grade school, she was easily distracted as well as shy. She was spacey and dreamy rather than restless. She admitted to vocal tics, involving throat-clearing. She reported a tendency to crack her bones, hands, ankles and wrists. She had a habit of blinking and would twist her neck and back frequently.
Social history
She was a high school graduate with an Associate’s Degree. She had worked as a phlebotomist and medical assistant. She had not worked for three years and was not married, but had two children. She was separated from her 11-year-old daughter.
Substance use history
The patient reported she had used cocaine daily for 11 years, totaling of between 3,000–4,000 hits. When asked how she felt after having taken cocaine, she stated “I felt level and normal”. This report appears to confirm the presence of RDS. The patient had used heroin for six or seven years as well. She denied the use of alcohol, angel dust, methamphetamine, LSD or ecstasy.
Short term memory assessment
The patient stated that, upon entering a room, she would forget why she went there, on a daily basis. A similar lapse in short-term memory would occur daily, when she approached the refrigerator and wondered why she had gone there. She was able to remember names of people whom she knew but would frequently need to re-read material, even when the subject matter was not too complex, like the newspaper. She admitted to olfactory hallucinations involving “burning rubber” (a frequently noted symptom, with chronic cocaine users, noted by TM). She reported no other symptoms consistent with temporal lobe epilepsy. She rated her concentration ability as 30%, with maximum concentration ability being 100%. Her procrastination tendency was 100% with maximum 100%. Her impulsiveness was 85% with maximum 100% and her boredom was 80% with maximum of 100% based on a short memory questionnaire.
Past medical history and reported allergies
The patient reported a history of Hepatitis C. Penicillin, macrobid, keflex, and trazodones were reported allergies (increased heart rate).
Mental status examination
The patient was alert and oriented in all three spheres. Her depression was rated as 10/10 (maximally depressed mood). She denied suicidal ideation, homicidal ideation, auditory or visual hallucinations. She admitted to olfactory hallucinations. She denied delusions but admitted to ideas of reference and a tendency towards obsessive compulsive disorder, wherein she felt compelled to wash the floor and bed continuously. She denied episodes of mania but reported a history of panic attacks and agoraphobia. She also reported that she slept three hours a night and would nap for three to four hours during the day. She admitted to almost nightly nightmares and daily flashbacks involving her sexual abuse. Her Dream Life is described in greater detail below.
Diagnostic impression
Axis I: Post-Traumatic Stress Disorder; Tourette’s Syndrome; ADHD; OCD, Panic Disorder, Major Depressive Disorder, Opioid Dependence, and Poly-substance Dependence, by History
Axis II: NA
Axis III: Hepatitis C
Axis IV: Severe-Symptom management
Axis V: CGAF 35
Treatment course
The patient was started on dextroamphetamine for her ADHD and Tourette’s Syndrome, prazosin for her nightmares and flashbacks, sertraline for her depression, zolpidem for her insomnia and gabapentin for her anxiety. On the next visit, her depression had been reduced to 7/10. She had stopped the dextroamphetamine 10 mg, because of feelings of agitation. She denied any drug use and was continuing her methadone treatment. There was some decrease in her flashbacks and nightmares. The antidepressants were increased and her mood continued to improve. Although she had been using cocaine intermittently during her methadone treatment, she stated she had been able to stop its use for six weeks. She agreed to re-challenge with dextroamphetamine, which was slowly titrated. Clonidine was added for on-going nightmares. She became gradually less depressed, denying suicidal or homicidal ideation. Her concentration, procrastination, impulsiveness and boredom also improved on dextroamphetamine. She was maintained on methadone 85 mg, at the local clinic.
Subsequent sessions
She reported that she had developed pyelonephritis, which was successfully treated with antibiotics. Her concentration, procrastination, boredom, impulsiveness and mood continued to improve. The patient missed three sessions. During her next session, her mood was more depressed, and she admitted to auditory hallucinations. She had inexplicably stopped the dextroamphetamine for three weeks and was staying in bed, without any motivation. She reported extreme boredom and procrastination. She stated that she had started using cocaine again and felt “more normal” when using it. She had been using cocaine for about ten days. Following her cessation of cocaine use, she felt “more depressed”. She continued on methadone, 85 mg, at seven days a week. After a long hiatus, the patient reported that she had stopped taking her sertraline, dextroamphetamine, prazosin, clonidine, and gabapentin for six or seven weeks. Her mood had become severely depressed. She was eating half a meal a day and sleeping eight hours at a time, mostly out of boredom. She smoked 60% of the time, out of boredom, and reported a return of her nightmares.
Medications were re-instituted, and she soon reported her mood had improved significantly. She admitted cravings for drugs but denied any use. She was receiving weekly psychotherapy. Her concentration, procrastination, impulsiveness and boredom were relatively improved. Because of side effects from the dextroamphetamine, she was eventually changed to the initial dose of lisdexamfetamine 30 mg. She continued on methadone 85 mg. Her mood was moderately depressed. Concentration and impulsiveness were improved but procrastination and boredom remained poor during the lisdexamfetamine titration. She continued on clonidine 0.1 mg at bed time, prazosin 2 mg at bed time, quetiapine 100 mg at bed time, sertraline 50 mg every morning, gabapentin 800mg four times a day, risperidone 1 mg at HS, alprazolam 0.5 mg three times a day and lisdexamfetamine 30 mg a day.
Because of her frequent non-compliance and relapse with illicit drugs, she continued to attend her appointments irregularly. She had to be frequently re-started on her regimen, which had proven somewhat effective as long as she was compliant with it and avoided illicit drug use. At a later point, even though the patient had been off cocaine for two weeks, because of multiple, positive toxic urine screens for cocaine, her methadone was rapidly tapered from 75 mg a day by her clinic. At this point, she was sleeping only three hours a day and eating less than one meal a day.
She indicated she wanted to detoxify with subutex, maintaining she was allergic to suboxone. She stated that she was staying in bed and had no drive or motivation. She reported panic as well as ideas of reference. She was having nightmares three to four times a week. Her concentration was dismal, with boredom and procrastination rated as maximally poor.
During the next session, her insurance company required a prior approval for her clonidine. She was now also being denied lisdexamfetamine, pending a prior approval. She appealed to the state Department of Public Health to prevent the rapid tapering of her methadone from 70 mg. She cited mental health reasons in her appeal. At this point, she was being seen on a weekly basis for medication management. She reported panic attacks as well as a lack of appetite but denied cravings for opiates or any use of other illicit drugs.
A letter was written to the state Department of Public Health in support of her appeal for a milder tapering of her methadone. The dopamine agonist, ropinorole, was then initiated along with lisdexamfetamine, which had been approved by the insurance company, so it was now possible to be increased to 70 mg a day.
The patient was refused an extension by the Department of Mental Health and her rapid methadone tapering continued. During this period, her mood was moderately depressed. She denied suicidal or homicidal ideation as well as hallucinations but complained of insomnia, involving six hours of sleep a night. She was eating only half a sandwich a day, despite urgings to increase per oral intake with supplements like Ensure Plus.
The patient re-appeared after another six-week hiatus, stating that she had been now tapered to methadone 5 mg and had, in the intervening period, also been hospitalized for Methicillin-resistant Staphylococcus Aureus (MRSA). She stated that she was going through an agonizing withdrawal, which involved thrashing her legs, vomiting, and severe diarrhea.
Because of the refusal to obtain a slower tapering schedule of methadone, the patient was offered KB220Z to assist in her withdrawal, while she awaited in-patient detoxification with subutex.
It has been reported that KB220Z can mitigate severe opiate withdrawal symptoms, in addition to improving other symptoms of Reward Deficiency Syndrome, as seen in ADHD and Tourette’s syndrome (Blum, Oscar-Berman et al., 2012). The patient was also urged to change insurance companies so that she could gain admission to an in-patient detoxification unit. This change was undertaken but would not be effective for another two weeks after her most recent visit. The patient stated, as had been her custom, that she had stopped the lisdexamfetamine, two weeks prior to the last visit, for unclear reasons.
She had been taking the KB220Z for two months and stated that she was now sleeping five hours a night as vs. one hour prior to that. She stated that her withdrawal symptoms had been improved by 40% and that she no longer had diarrhea or vomiting. She reported that she also no longer had nocturnal urinary incontinence, which she had suffered, during her rapid, methadone withdrawal. She self-increased her KB220Z from the initial dose of two tablets twice a day to five tablets a day and had done this two weeks prior to the last visit.
Dream life
At this point, the patient spontaneously reported a change in the nature, content, and emotional tone of her dreams. With more pointed questioning, she reported that she had an onset of vivid (lucid), life-like dreams (“as if I am fully awake”) about three or four years prior to her current medication clinic intake. She stated that these dreams had been occurring on a three- to four-times-a-week basis. She reported that the prazosin had diminished her flashbacks and nightmares but that the addition of KB220Z made her “dreams almost fun!” She stated that her lucid dreams were “much happier now, with the exception of an occasional drug dream”. She stated that they remained lucid but that prior to the addition of the KB220Z, the dreams had been of a terrifying and Post-Traumatic-Stress-Disorder nature, involving, primarily, her sexual abuse. In addition, they had been uniformly terrifying and were almost always accompanied by an increased heart rate. The patient reported that, since the KB220Z had been added, “During my dreams, all I am doing is laughing!”
This dream life, “Happiness Effect” had been going on for two months, since KB220Z had been added to her regimen. She even referred to nostalgic dreams of her mother, who had passed away two years prior, in which she would be affectionately calling her “Mommy”, as she had done as a little girl. There was no hint of sadness or grief in these reports of dreams about her mother.
This case represents the second of two, serendipitous reports in the change in the nature of lucid dreams of unpleasant and terrifying content to dreams filled with happiness, laughter and nostalgia.
Figure 2.
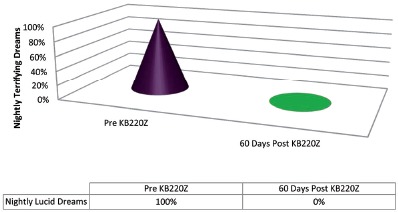
Case Two
Limitations
The primary limitation to this present work is the very small number of patients. The other limitation is that this is not a double-blind randomized controlled placebo study and as such the results must be considered preliminary at best. Another caveat is that there was no standard evaluation of timing following the administration of KB220Z. Finally, because the patients were taking other known prescribed pharmaceuticals, albeit relief only followed the addition of KB220Z, we must caution these interesting results.
Discussion and Conclusion
Both these patients were diagnosed with a form of RDS displaying ADHD symptomatology (Blum, Gardner et al., 2012). They had a markedly improved effect on their almost lifelong, unpleasant, traumatic, lucid dreams. Such dreams were described as being extremely life-like (“as if I’m fully awake”). Other terms used were “in color”. As noted in the literature, such dreams can be re-initiated, if they are pleasant, after, for example, getting up at night to go to the bathroom or can be, on some occasions, voluntarily turned off by the dreamer, when they become extremely terrifying or traumatic.
These descriptions of the patients’ dreams seemed to fit the standard characteristics of lucid dreams. What is note-worthy in both cases is that, at various times, because of the Reward Deficiency Syndrome-associated ADHD, the administered pharmaceuticals had no impact on the frequency, vividness or unpleasantness of their lucid dreams. It was only when the KB220Z was added to her regimen that the frequency, intensity and nature of these dreams changed for more than 90% of the time in case 1; as noted above, the patient even stated that she had never had an extended period of happy dreams in her life and that one, in particular, involved a re-union with former office mates, where they were all partying joyfully together. Similar results were obtained in case 2, whereby her dreams also became joyful and laughter-filled.
It would appear that neither powerful antagonist(s) to norepinephrine/epinephrine; benzodiazepine agonist(s) & antagonist(s), anti-depressant(s); stimulant(s); dopamine D2 antagonist(s); noradrenergic-alpha2 agonist(s) in these two cases were responsible for this dramatic effect, nor their combination had any apparent effect on the lucidity or unpleasant content of their nightly dreams. A PubMed search of the combination of terms “dopamine and lucid dreams” produced no retrieved references (3-1-15).
There are a number of published papers that describe the ingredients in the KB220Z complex consisting of various precursor amino-acids for synthesis of serotonin, a chromium salt; GABA and dopamine; a known natural enkephalinase inhibitor; a natural benzodiazepine stimulant and an herbal substance known to inhibit COMT and mitochondrial MAO-A (Blum, Femino et al., 2013). Following 27 clinical trials with KB220 variants, since 1982, there is strong evidence that this complex, which was designed to mimic the “Brain Reward Cascade”, appears to increase dopamine release and activation across the Brain Reward Circuitry leading dopamine homeostasis (Blum et al., 2008).
In fact, in human, neuro-imaging studies, KB220Z, compared to placebo, demonstrated unequivocal evidence of activation of the nucleus accumbens in heroin addicts (Blum, Oscar-Berman et al., 2013), using fMRI and quantitative EEG (qEEG) demonstrated regulation of widespread theta activity within the cingulate gyrus in abstinent, psychostimulant addicts and increased alpha waves and low beta waves (Blum et al., 2010). Moreover, a recent experiment, using resting state fMRI, demonstrated functional connectivity in rats clearly showing a selective robust activation of KB220Z over placebo. We found that connectivity with regions, such as the nucleus accumbens, the anterior cingulate, pre-limbic and infra-limbic structures, was significantly increased with KB220Z treatment. There was also evidence of recruitment of additional brain structures, such as the hippocampus, anterior thalamus, and somatosensory regions. We found similar results in abstinent heroin addicts where KB220Z also enhanced resting state functional connectivity (Blum et al., 2015). These co-activated regions could constitute a statistical analogue of a neural network (McLaughlin et al., 1992), which, combined with specific, cortical loci, may be involved in the genesis of “nightmares” (Febo, Blum, Liu & Gold, 2014).
In conclusion, the discovery that KB220Z increased functional connectivity, has enormous implications for treatment of psychiatric diseases including RDS. The involvement of the above brain regions may imply induced dopaminergic homeostasis (redeeming joy) required to reduce unwanted lucid “bad dreams” in PTSD patients like AC and DR or from other causes (Hinton, Peou, Joshi, Nickerson & Simon, 2013; Thompson & Pierce, 1999).
In an unpublished work we have now observed relief of terrifying lucid dreams in twelve out of thirteen patients (92.3%) following KB220z at various time intervals. While these findings are encouraging they require cautious interpretation and must await more intensive investigation involving double-blinded experiments in a large population.
Authors’ contribution
The concept and design of this experiment was perceived by TM and KB. The collection of data was obtained by TM. The development of each case was first presented by TM and then reworked for the manuscript by KB. The actual writing of the first draft was achieved by KB and TM. The clinical interpretation of the collected data included TM, KB, TS, MSG, MOB and MF. The development of the literature search and clinical input was based on the work of JF, GA, TM and KB. The graphic design and execution was achieved by GA, JF, TM and KB. All authors approved the final draft.
Conflict of interests
Kenneth Blum, PhD holds US and Foreign patents issued and pending on KB220Z and receives royalties based on its commercialization from various sources. Dr. Blum is also an officer and stock holder of IGENE, LLC, Victory Nutrition, RDSolutions, LLC and is a paid consultant of Dominion Diagnostics, LLC, and Malibu Recovery Center. Dr. Blum is a member of the scientific advisory board of Dominion Diagnostics, LLC. Gozde Agan and James Fratantonio are employed by Dominion Diagnostics. Dr. Gold is a paid consultant of Rivermend Health LLC. There are no other conflicts.
Acknowledgements
The authors appreciate the editorial assistance of Margaret A. Madigan. KB220Z was purchased from Nupathways, Indianapolis, Indiana.
Funding Statement
The writing of this paper was supported in part by funds from the National Institutes of Health, NIAAA (RO1-AA07112 and K05-AA00219) and the Medical Research Service of the US Department of Veterans Affairs (MOB). MF is the recipient of R01DA019946. KB is the recipient of a grant to PATH FOUNDATION NY, by Life Extension Foundation, Ft/Lauderdale, Florida.
References
- Aurora R. N., Zak R. S., Auerbach S. H., Casey K. R., Chowduri S., Krippot A., Maganti R. K., Ramar K., Kristo D. A., Bista S. R., Lamm C. I. & Morgenthaler T. I. (2010). Standards of practice committee on treatment of nightmares. Journal of Clinical Sleep Medicine, 6, 389–401. [PMC free article] [PubMed] [Google Scholar]
- Blum K., Chen A. L., Braverman E. R., Comings D. E., Chen T. J., Arcuri V., Blum S. H., Downs B. W., Waite R. L., Notaro A., Lubar J., Williams L., Prihoda T. J., Palomo T. & Oscar-Berman M. (2008). Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Journal of Neuropsychiatric Disease and Treatment, 4, 893–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Chen T. J., Morse S., Giordano J., Chen A. L., Thompson J., Allen C., Smolen A., Lubar J., Stice E., Downs B. W., Waite R. L., Madigan M. A., Kerner M., Fornari F. & Braverman E. R. (2010). Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D₂ agonist therapy: Part 2. Postgraduate Medical Journal, 122, 214–226. [DOI] [PubMed] [Google Scholar]
- Blum K., Femino J., Teitlebaum S., Oscar-Berman M., Giordano J. & Gold M. S. (2013). 12 steps program & fellowship: Neurobiology of recovery. New York: SpringerBriefs. [Google Scholar]
- Blum K., Gardner E., Oscar-Berman M. & Gold M. (2012). “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): Hypothesizing differential responsivity in brain rewardcircuitry. Current Pharmaceutical Design, 18, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Liu Y., Wang W., Wang Y., Zhang Y., Oscar-Berman M., Smolen A., Febo M., Han D., Simpatico T., Cronjé, F. J., Demetrovics Z. & Gold M. S. (2015). rsfMRI effects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgraduate Medical Journal, 127(2), 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Oscar-Berman M., Femino J., Waite R. L., Benya L., Giordano J., Borsten J., Downs W. B., Braverman E. R., Loehmann R., Dushaj K., Han D., Simpatico T., Hauser M., Barh D. & McLaughlin T. J. (2013). Withdrawal from buprenorphine/naloxone and maintenance with a natural dopaminergic agonist: A cautionary note. Journal of Addiction Research and Therapy, 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Oscar-Berman M., Stuller E., Miller D., Giordano J., Morse S., McCormick L., Downs W. B., Waite R. L., Barh D., Neal D., Braverman E. R., Lohmann R., Borsten J., Hauser M., Han D., Liu Y., Helman M. & Simpatico T. (2012). Neurogenetics and nutrigenomics of neuro-nutrient therapy for Reward Deficiency Syndrome (RDS): Clinical ramifications as a function of molecular neurobiological mechanisms. Journal of Addiction Research and Therapy, 3(5), 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M., Blum K., Liu Y. & Gold M. S. (2014). Putative dopamine agonist (KB220Z) enhances resting state functional connectivity in brain reward circuitry. Presentation at Addictions Conference 2014, Aug 4–6, Chicago, USA. [Google Scholar]
- Fusar-Poli P., Perez J., Broome M., Borgwardt S., Placentino A., Caverzasi E., Cortesi M., Veggiotti P., Politi P., Barale F. & McGuire P. (2007). Neurofunctional correlates of vulnerability to psychosis: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 31, 465–484. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. (2006). The dreaming sleep stage: A new neurobiological model of schizophrenia? Neuroscience, 140, 1105–1115. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. & Joncas S. (2000). Letter to the editor: Hypothesis for the neurophysiology of dreaming. Journal of Sleep Reseach, 3, 1–4. [PubMed] [Google Scholar]
- Hinton D. E., Peou S., Joshi S., Nickerson A. & Simon N. M. (2013). Normal grief and complicated bereavement among traumatized cambodian refugees: Cultural context and the central role of dreams of the dead. Culture, Medicine, and Psychiatry, 37, 427–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger E. (1971). Structure and functions of fantasy. 1st edition (p. 57). New York: Wiley Interscience. [Google Scholar]
- Kovachy B., O’Hara R., Hawkins N., Gershon A., Primeau M. M., Madej J. & Carrion V. (2013). Sleep disturbance in pediatric PTSD: Current findings and future directions. Journal of Clinical Sleep Medicne, 9, 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T., Steinberg B., Christensen B., Law I., Parving A. & Friberg L. (2014) Potential language and attentional networks revealed through factor analysis of rCBF data measured with SPECT. Journal of Cerebral Blood Flow & Metabolism, 12(4), 535–545. [DOI] [PubMed] [Google Scholar]
- Mellman T. A., David D., Kulick-Bell R., Hebding J. & Nolan B. (1995). Sleep disturbance and its relationship to psychiatric morbidity after Hurricane Andrew. The American Journal of Psychiatry, 152, 1659–1663. [DOI] [PubMed] [Google Scholar]
- Nielson T. & Levin R. (2007). Nightmares: A new neurocognitive model. Sleep Medicine Reviews, 11, 295–310. [DOI] [PubMed] [Google Scholar]
- Pearson-Fuhrhop K. M., Dunn E. C., Mortero S., Devan W. J., Falcone G. J., Lee P., Holmes A. J., Hollinshead M. O., Roffman J. L., Smoller J. W., Rosand J. & Cramer S. C. (2014) Dopamine genetic risk score predicts depressive symptoms in healthy adults and adults with depression. PLoS One, 9(5), e93772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J., Reynolds C. F. 3rd, Yeager A. L., Houck P. R. & Hurwitz L. F. (1991). Sleep disturbances in survivors of the Nazi Holocaust. The American Journal of Psychiatry, 148, 62–66. [DOI] [PubMed] [Google Scholar]
- Schredl M., Bohusch C., Kahl J., Mader A. & Somesan A. (2000). The use of dreams in psychotherapy: A survey of psychotherapists in private practice. The Journal of Psychotherapy Practice and Research, 9, 81–87. [PMC free article] [PubMed] [Google Scholar]
- Sil’kis I. G. (2006). Role of the basal ganglia in the occurrence of paradoxical sleep dreams (hypothetical mechanism). Zh Vyssh Nerv Deiat Im I P Pavlova, 56, 5–21. [PubMed] [Google Scholar]
- Sil’kis I. G. (2010) Paradoxical sleep as a tool for understanding the hippocampal mechanisms of contextual memory. Neuroscience and Behavioral Physiology, 40, 5–19. [DOI] [PubMed] [Google Scholar]
- Solms M. (2000). Dreaming and REM sleep are controlled by different brain mechanisms. Behavioral and Brain Sciences, 23, 843–850. [DOI] [PubMed] [Google Scholar]
- Spoormaker V. I., Schredl M. & van den Bout J. (2006). Nightmares: from anxiety symptom to sleep disorder. Sleep Medicine Reviews, 10, 19–31. [DOI] [PubMed] [Google Scholar]
- Thompson D. F. & Pierce D. R. (1999). Drug-induced nightmares. Annals of Pharmacotherapy, 33, 93–98. [DOI] [PubMed] [Google Scholar]


