Abstract
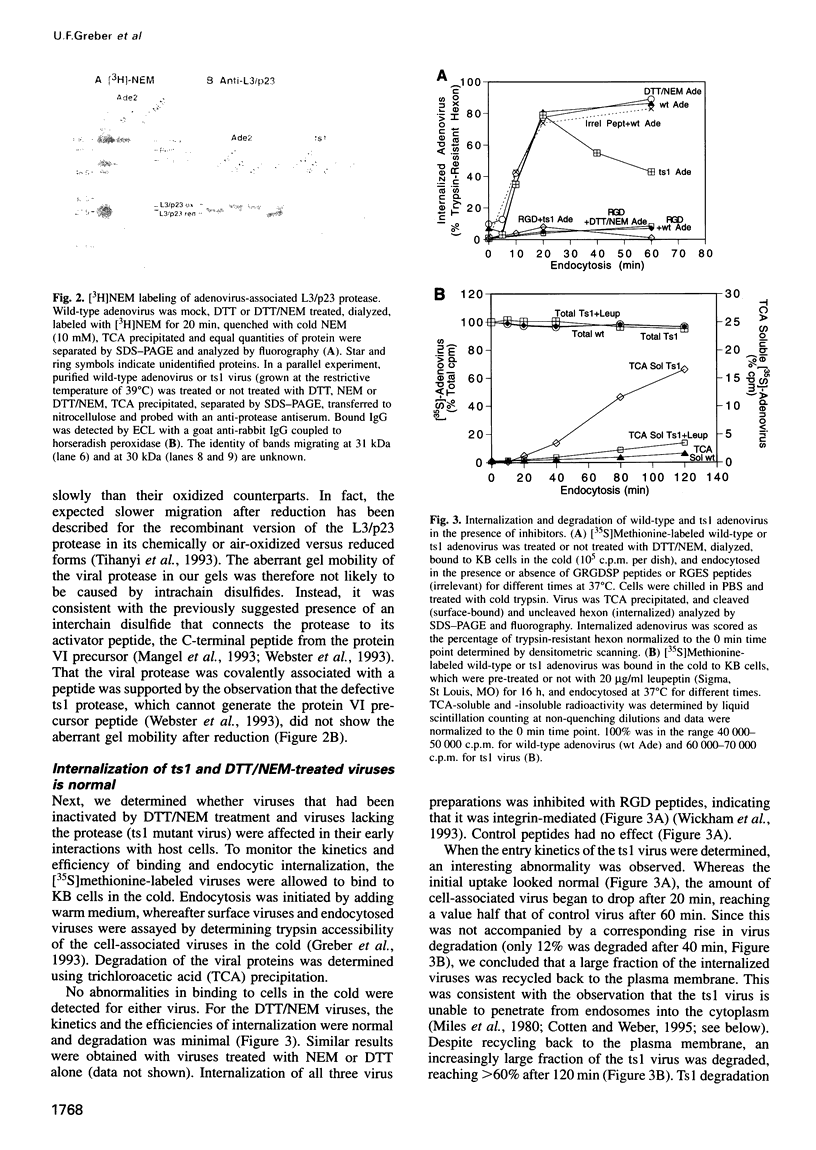
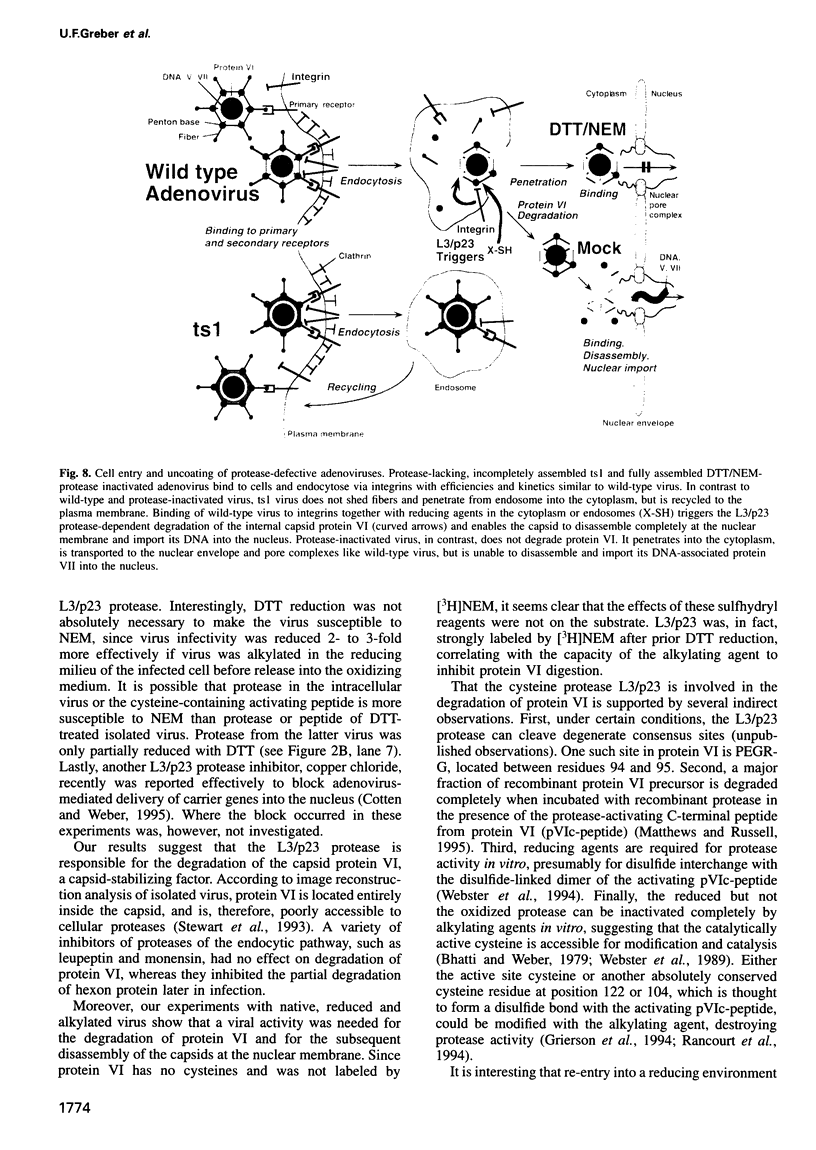
Adenovirus uncoating is a stepwise process which culminates in the release of the viral DNA into the nucleus through the nuclear pore complexes and dissociation of the capsid. Using quantitative biochemical, immunochemical and morphological methods, we demonstrate that inhibitors of the cystine protease, L3/p23, located inside the capsid block the degradation of the capsid-stabilizing protein VI, and prevent virus uncoating at the nuclear membrane. There was no effect on virus internalization, fiber shedding and virus binding to the nuclear envelope. The viral enzyme (dormant in the extracellular virus) was activated by two separate signals, neither of which was sufficient alone; virus interaction with the integrin receptor (inhibited with RGD peptides) and re-entry of the virus particle into a reducing environment in the endosome or the cytosol. Incorrectly assembled mutant viruses that lack the functional protease (ts1) failed at releasing fibers and penetrating into the cytosol. The results indicated that L3/p23 is needed not only to assemble an entry-competent virus but also to disassemble the incoming virus.
Full text
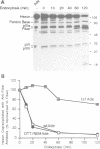
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Persson H. Gene and mRNA for precursor polypeptide VI from adenovirus type 2. J Virol. 1981 May;38(2):469–482. doi: 10.1128/jvi.38.2.469-482.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M., Harfe B., Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993 Sep;67(9):5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin M. T., Boulanger P. Involvement of cellular adhesion sequences in the attachment of adenovirus to the HeLa cell surface. J Gen Virol. 1993 Aug;74(Pt 8):1485–1497. doi: 10.1099/0022-1317-74-8-1485. [DOI] [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2: partial characterization. Virology. 1979 Jul 30;96(2):478–485. doi: 10.1016/0042-6822(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970 Mar;40(3):462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- Collins D. S., Unanue E. R., Harding C. V. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991 Dec 15;147(12):4054–4059. [PubMed] [Google Scholar]
- Cotten M., Weber J. M. The adenovirus protease is required for virus entry into host cells. Virology. 1995 Nov 10;213(2):494–502. doi: 10.1006/viro.1995.0022. [DOI] [PubMed] [Google Scholar]
- Dales S., Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973 Dec;56(2):465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994 Jun 1;4(6):506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B., Cheresh D. A. Vitronectin and its receptors. Curr Opin Cell Biol. 1993 Oct;5(5):864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Gerace L., Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Goldman M. J., Wilson J. M. Expression of alpha v beta 5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995 Oct;69(10):5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U. F., Singh I., Helenius A. Mechanisms of virus uncoating. Trends Microbiol. 1994 Feb;2(2):52–56. doi: 10.1016/0966-842x(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Greber U. F., Willetts M., Webster P., Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993 Nov 5;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Grierson A. W., Nicholson R., Talbot P., Webster A., Kemp G. The protease of adenovirus serotype 2 requires cysteine residues for both activation and catalysis. J Gen Virol. 1994 Oct;75(Pt 10):2761–2764. doi: 10.1099/0022-1317-75-10-2761. [DOI] [PubMed] [Google Scholar]
- Hannan C., Raptis L. H., Déry C. V., Weber J. Biological and structural studies with an adenovirus type 2 temperature-sensitive mutant defective for uncoating. Intervirology. 1983;19(4):213–223. doi: 10.1159/000149363. [DOI] [PubMed] [Google Scholar]
- Hellen C. U., Wimmer E. The role of proteolytic processing in the morphogenesis of virus particles. Experientia. 1992 Feb 15;48(2):201–215. doi: 10.1007/BF01923512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. Formation of disulfides with diamide. Methods Enzymol. 1987;143:264–270. doi: 10.1016/0076-6879(87)43050-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis N., Fender P., Barge A., Kitts P., Chroboczek J. Cell-binding domain of adenovirus serotype 2 fiber. J Virol. 1994 Jun;68(6):4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., McGrath W. J., Toledo D. L., Anderson C. W. Viral DNA and a viral peptide can act as cofactors of adenovirus virion proteinase activity. Nature. 1993 Jan 21;361(6409):274–275. doi: 10.1038/361274a0. [DOI] [PubMed] [Google Scholar]
- Martin K., Helenius A. Transport of incoming influenza virus nucleocapsids into the nucleus. J Virol. 1991 Jan;65(1):232–244. doi: 10.1128/jvi.65.1.232-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. A., Russell W. C. Adenovirus protein-protein interactions: hexon and protein VI. J Gen Virol. 1994 Dec;75(Pt 12):3365–3374. doi: 10.1099/0022-1317-75-12-3365. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Russell W. C. Adenovirus protein-protein interactions: molecular parameters governing the binding of protein VI to hexon and the activation of the adenovirus 23K protease. J Gen Virol. 1995 Aug;76(Pt 8):1959–1969. doi: 10.1099/0022-1317-76-8-1959. [DOI] [PubMed] [Google Scholar]
- Miles B. D., Luftig R. B., Weatherbee J. A., Weihing R. R., Weber J. Quantitation of the interaction between adenovirus types 2 and 5 and microtubules inside infected cells. Virology. 1980 Aug;105(1):265–269. doi: 10.1016/0042-6822(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Panetti T. S., McKeown-Longo P. J. The alpha v beta 5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem. 1993 Jun 5;268(16):11492–11495. [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L. Structure and assembly of adenoviruses. Curr Top Microbiol Immunol. 1984;109:1–52. doi: 10.1007/978-3-642-69460-8_1. [DOI] [PubMed] [Google Scholar]
- Pisoni R. L., Park G. Y., Velilla V. Q., Thoene J. G. Detection and characterization of a transport system mediating cysteamine entry into human fibroblast lysosomes. Specificity for aminoethylthiol and aminoethylsulfide derivatives. J Biol Chem. 1995 Jan 20;270(3):1179–1184. doi: 10.1074/jbc.270.3.1179. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U. Structural proteins of adenoviruses. VII. Purification and properties of an arginine-rich core protein from adenovirus type 2 and type 3. Virology. 1971 Aug;45(2):364–373. doi: 10.1016/0042-6822(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Rancourt C., Keyvani-Amineh H., Sircar S., Labrecque P., Weber J. M. Proline 137 is critical for adenovirus protease encapsidation and activation but not enzyme activity. Virology. 1995 May 10;209(1):167–173. doi: 10.1006/viro.1995.1240. [DOI] [PubMed] [Google Scholar]
- Rancourt C., Tihanyi K., Bourbonniere M., Weber J. M. Identification of active-site residues of the adenovirus endopeptidase. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):844–847. doi: 10.1073/pnas.91.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Precious B. Nucleic acid-binding properties of adenovirus structural polypeptides. J Gen Virol. 1982 Nov;63(Pt 1):69–79. doi: 10.1099/0022-1317-63-1-69. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D. J., Willingham M. C., Pastan I. Role of a low-pH environment in adenovirus enhancement of the toxicity of a Pseudomonas exotoxin-epidermal growth factor conjugate. J Virol. 1984 Sep;51(3):650–655. doi: 10.1128/jvi.51.3.650-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaei M., Zimmermann B., Scheller M. Endocytosis of integrin alpha 5 beta 1 (fibronectin receptor) of mouse peritoneal macrophages in vitro: an immunoelectron microscopic study. J Struct Biol. 1993 Nov-Dec;111(3):180–189. doi: 10.1006/jsbi.1993.1048. [DOI] [PubMed] [Google Scholar]
- Singh I., Helenius A. Role of ribosomes in Semliki Forest virus nucleocapsid uncoating. J Virol. 1992 Dec;66(12):7049–7058. doi: 10.1128/jvi.66.12.7049-7058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow C. M., Senior A., Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987 May;104(5):1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. L., Fuller S. D., Burnett R. M. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993 Jul;12(7):2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tihanyi K., Bourbonnière M., Houde A., Rancourt C., Weber J. M. Isolation and properties of adenovirus type 2 proteinase. J Biol Chem. 1993 Jan 25;268(3):1780–1785. [PubMed] [Google Scholar]
- Varga M. J., Weibull C., Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991 Nov;65(11):6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. M. Adenovirus endopeptidase and its role in virus infection. Curr Top Microbiol Immunol. 1995;199(Pt 1):227–235. doi: 10.1007/978-3-642-79496-4_12. [DOI] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A., Hay R. T., Kemp G. The adenovirus protease is activated by a virus-coded disulphide-linked peptide. Cell. 1993 Jan 15;72(1):97–104. doi: 10.1016/0092-8674(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Webster A., Kemp G. The active adenovirus protease is the intact L3 23K protein. J Gen Virol. 1993 Jul;74(Pt 7):1415–1420. doi: 10.1099/0022-1317-74-7-1415. [DOI] [PubMed] [Google Scholar]
- Webster A., Leith I. R., Hay R. T. Activation of adenovirus-coded protease and processing of preterminal protein. J Virol. 1994 Nov;68(11):7292–7300. doi: 10.1128/jvi.68.11.7292-7300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A., Russell W. C., Kemp G. D. Characterization of the adenovirus proteinase: development and use of a specific peptide assay. J Gen Virol. 1989 Dec;70(Pt 12):3215–3223. doi: 10.1099/0022-1317-70-12-3215. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Wickham T. J., Filardo E. J., Cheresh D. A., Nemerow G. R. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994 Oct;127(1):257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Xia D., Henry L. J., Gerard R. D., Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure. 1994 Dec 15;2(12):1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura A. Adenovirus-induced leakage of co-endocytosed macromolecules into the cytosol. Cell Struct Funct. 1985 Dec;10(4):391–404. doi: 10.1247/csf.10.391. [DOI] [PubMed] [Google Scholar]