Abstract
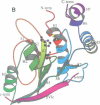
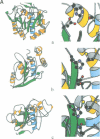
The three-dimensional structure of the human adenovirus-2 proteinase complexed with its 11 amino acid cofactor, pVIc, was determined at 2.6 A resolution by X-ray crystallographic analysis. The fold of this protein has not been seen before. However, it represents an example of either subtly divergent or powerfully convergent evolution, because the active site contains a Cys-His-Glu triplet and oxyanion hole in an arrangement similar to that in papain. Thus, the adenovirus proteinase represents a new, fifth group of enzymes that contain catalytic triads. pVIc, which extends a beta-sheet in the main chain, is distant from the active site, yet its binding increases the catalytic rate constant 300-fold for substrate hydrolysis. The structure reveals several potential targets for antiviral therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaire M., Chernaia M. M., Malcolm B. A., James M. N. Picornaviral 3C cysteine proteinases have a fold similar to chymotrypsin-like serine proteinases. Nature. 1994 May 5;369(6475):72–76. doi: 10.1038/369072a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. W. Expression and purification of the adenovirus proteinase polypeptide and of a synthetic proteinase substrate. Protein Expr Purif. 1993 Feb;4(1):8–15. doi: 10.1006/prep.1993.1002. [DOI] [PubMed] [Google Scholar]
- Anderson C. W. The proteinase polypeptide of adenovirus serotype 2 virions. Virology. 1990 Jul;177(1):259–272. doi: 10.1016/0042-6822(90)90479-b. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Cai F., Weber J. M. Organization of the avian adenovirus genome and the structure of its endopeptidase. Virology. 1993 Sep;196(1):358–362. doi: 10.1006/viro.1993.1489. [DOI] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Wolthers B. G. The structure of papain. Adv Protein Chem. 1971;25:79–115. doi: 10.1016/s0065-3233(08)60279-x. [DOI] [PubMed] [Google Scholar]
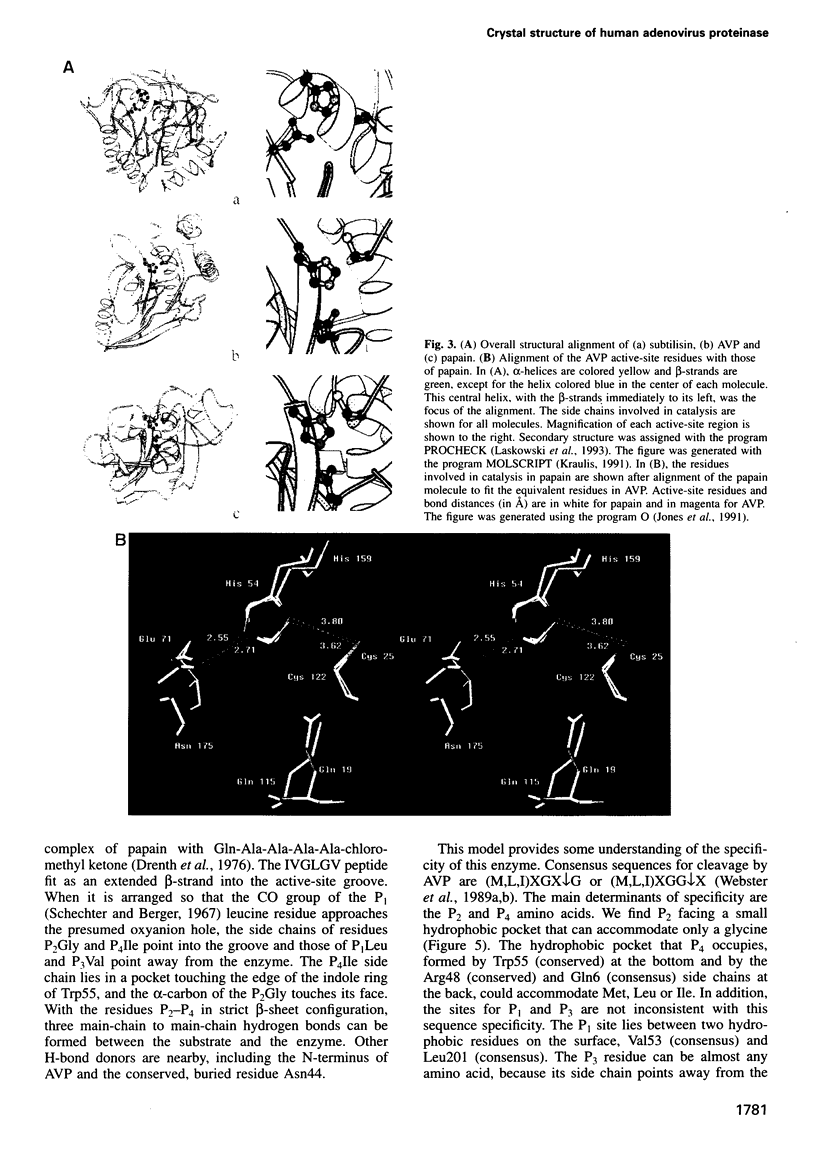
- Drenth J., Kalk K. H., Swen H. M. Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry. 1976 Aug 24;15(17):3731–3738. doi: 10.1021/bi00662a014. [DOI] [PubMed] [Google Scholar]
- Hannan C., Raptis L. H., Déry C. V., Weber J. Biological and structural studies with an adenovirus type 2 temperature-sensitive mutant defective for uncoating. Intervirology. 1983;19(4):213–223. doi: 10.1159/000149363. [DOI] [PubMed] [Google Scholar]
- Holm L., Sander C. The FSSP database of structurally aligned protein fold families. Nucleic Acids Res. 1994 Sep;22(17):3600–3609. [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Mangel W. F., McGrath W. J., Toledo D. L., Anderson C. W. Viral DNA and a viral peptide can act as cofactors of adenovirus virion proteinase activity. Nature. 1993 Jan 21;361(6409):274–275. doi: 10.1038/361274a0. [DOI] [PubMed] [Google Scholar]
- Mangel W. F., Toledo D. L., Brown M. T., Martin J. H., McGrath W. J. Characterization of three components of human adenovirus proteinase activity in vitro. J Biol Chem. 1996 Jan 5;271(1):536–543. doi: 10.1074/jbc.271.1.536. [DOI] [PubMed] [Google Scholar]
- Mirza A., Weber J. Infectivity and uncoating of adenovirus cores. Intervirology. 1980;13(5):307–311. doi: 10.1159/000149139. [DOI] [PubMed] [Google Scholar]
- Ollis D. L., Cheah E., Cygler M., Dijkstra B., Frolow F., Franken S. M., Harel M., Remington S. J., Silman I., Schrag J. The alpha/beta hydrolase fold. Protein Eng. 1992 Apr;5(3):197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- Polgár L. Mercaptide-imidazolium ion-pair: the reactive nucleophile in papain catalysis. FEBS Lett. 1974 Oct 1;47(1):15–18. doi: 10.1016/0014-5793(74)80415-1. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. Families of cysteine peptidases. Methods Enzymol. 1994;244:461–486. doi: 10.1016/0076-6879(94)44034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertus J. D., Kraut J., Alden R. A., Birktoft J. J. Subtilisin; a stereochemical mechanism involving transition-state stabilization. Biochemistry. 1972 Nov 7;11(23):4293–4303. doi: 10.1021/bi00773a016. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Tihanyi K., Bourbonnière M., Houde A., Rancourt C., Weber J. M. Isolation and properties of adenovirus type 2 proteinase. J Biol Chem. 1993 Jan 25;268(3):1780–1785. [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A., Hay R. T., Kemp G. The adenovirus protease is activated by a virus-coded disulphide-linked peptide. Cell. 1993 Jan 15;72(1):97–104. doi: 10.1016/0092-8674(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Webster A., Russell S., Talbot P., Russell W. C., Kemp G. D. Characterization of the adenovirus proteinase: substrate specificity. J Gen Virol. 1989 Dec;70(Pt 12):3225–3234. doi: 10.1099/0022-1317-70-12-3225. [DOI] [PubMed] [Google Scholar]
- Webster A., Russell W. C., Kemp G. D. Characterization of the adenovirus proteinase: development and use of a specific peptide assay. J Gen Virol. 1989 Dec;70(Pt 12):3215–3223. doi: 10.1099/0022-1317-70-12-3215. [DOI] [PubMed] [Google Scholar]
- Yeh-Kai L., Akusjärvi G., Aleström P., Pettersson U., Tremblay M., Weber J. Genetic identification of an endoproteinase encoded by the adenovirus genome. J Mol Biol. 1983 Jun 15;167(1):217–222. doi: 10.1016/s0022-2836(83)80044-8. [DOI] [PubMed] [Google Scholar]