Abstract
Context
The characterization of the urinary metabolome may yield biomarkers indicative of pancreatitis.
Objectives
We establish a non-invasive technique to compare urinary metabolic profiles in patients with acute and chronic pancreatitis to healthy controls.
Methods
Urine was obtained from healthy controls (HC, n=5), in patients with mild acute pancreatitis (AP, n=5), and outpatients with chronic pancreatitis (CP, n=5). Proton nuclear magnetic resonance spectra were obtained for each sample. Metabolites were identified and quantified in each spectrum; resulting concentrations were normalized to account for differences in dilution among samples. Kruskal-Wallis test, post-hoc Mann-Whitney U tests, and principal component analysis were performed to identify metabolites that discriminate healthy controls, acute pancreatitis, and chronic pancreatitis.
Results
Sixty metabolites were identified and quantified; five were found to differ significantly (P<0.05) among the three groups. Of these, citrate and adenosine remained significant after validation by random permutation. Principal component analysis demonstrated that healthy control urine samples can be differentiated from patients with chronic pancreatitis or acute pancreatitis; chronic pancreatitis patients could not be distinguished from acute pancreatitis patients.
Conclusions
This metabolomic investigation demonstrates that this non-invasive technique offers insight into the metabolic states of pancreatitis. Although the identified metabolites cannot conclusively be defined as biomarkers of disease, future studies will validate our findings in larger patient cohorts.
Keywords: Metabolomics; Nuclear Magnetic Resonance, Biomolecular; Pancreatitis, Acute Necrotizing; Pancreatitis, Chronic; Urine
Introduction
Diseases of the pancreas affect greater than 1 million persons in the United States annually, resulting in nearly $3 billion in direct and indirect medical costs. The most prevalent disorder of the pancreas is pancreatitis. This disease is characterized by inflammation of the exocrine portion of the pancreas that secretes digestive enzymes into the duodenum. Pancreatitis can take two forms, acute and chronic. Acute pancreatitis begins abruptly and either resolves or worsens within several days. In contrast, chronic pancreatitis is a chronic inflammatory disorder with a protracted disease course developing and progressing over several years.
Acute pancreatitis is the most common cause of hospitalization for pancreatic disorders in the United States and is associated with significant resource utilization, morbidity and mortality. Recent national survey data indicate increasing frequency of hospitalizations (4.6 of every 1,000 hospital admissions, greater than 200,000 admissions in the U.S. annually) and costs ($2 billion in direct annual costs) associated with acute pancreatitis in the United States [1, 2]. Currently, diagnoses of acute pancreatitis are made by standard laboratory testing and radiologic imaging [3]. Severe cases may be associated with complications such as organ failure, necrosis, and death; approximately 2% of total acute pancreatitis attacks are fatal.
Although the exact pathogenesis of chronic pancreatitis remains to be determined, a variety of etiologic risk factors have been outlined, including repeated bouts of acute pancreatitis [4]. Objective features of chronic pancreatitis, detectable by current diagnostic radiologic, endoscopic and biochemical studies, are associated with moderate to advanced stage disease. However, at these stages, pathological structural and functional changes are irreversible and only symptomatic treatment is possible [5, 6]. Furthermore, the advanced stages of chronic pancreatitis are associated with diabetes, severe pain, malabsorption of nutrition, and the development of pancreatic cancer. The prevalence of chronic pancreatitis in industrialized countries, although frequently misdiagnosed [7] or neglected [8], is approximately 30 per 100,000 inhabitants [9, 10]. No specific therapy is available to arrest the acute inflammatory response seen in acute pancreatitis or to retard the progression of chronic pancreatitis.
Metabolomics, the systematic, high-throughput profiling of the host of metabolites in an organism, can be used to characterize an organism's metabolic response to a pathophysiologic state [11]. Nuclear magnetic resonance (NMR) spectroscopy-based approaches are well-suited for high-throughput studies, offering the ability to profile a wide range of metabolites in a single spectrum with minimal sample preparation [12, 13]. Such studies are commonly used to identify biomarkers or sets of biomarkers of disease [14, 15, 16].
The characterization of the metabolic response in pancreatitis is a potentially useful strategy to determine the effects of pancreatic injury in both chronic and acute pancreatitis. Improved understanding of the metabolic consequences of these diseases may yield non-invasive diagnostic biomarkers and therapeutic targets. To that end, we performed a pilot study to determine if differences could be detected among NMR-based urinary metabolic profiles of patients with chronic pancreatitis, acute pancreatitis, and healthy controls.
Methods
Study Population
The study population comprised adult patients referred to the Center for Pancreatic Disease at Brigham and Women's Hospital in Boston, MA, USA for evaluation of abdominal pain, and healthy adult volunteers with no pancreatic disease or risk factors for pancreatic disease. Three cohorts were studied: inpatients with mild acute pancreatitis (AP, n=5), outpatients with chronic pancreatitis (CP, n=5), and healthy controls (HC, n=5).
Urine Collection
Clean-catch urine was collected for all subjects. Second void of the day urine was collected using the clean-catch method for acute and chronic pancreatitis patients. Healthy control and chronic pancreatitis collections were done in the outpatient setting. Acute pancreatitis samples were collected within 48 hours of admission. All urine was promptly aliquoted and stored at -80°C until analysis.
Urine Sample Processing
Urine samples were thawed at the time of preparation for NMR analysis. One mL of thawed urine was mixed with 0.5 mL of 0.2 M sodium phosphate buffer prepared with D2O to control pH. The mixture was placed on ice for 10 minutes and then centrifuged at 7,000 g for 10 minutes. A total of 500 μL of the supernatant was withdrawn and combined with 50 μL of the internal standard 3-(trimethylsilyl) propionic acid (TSP, Sigma-Aldrich, St. Louis, MO, USA) to a concentration of 1 mM [17]. The internal standard and buffer were prepared with D2O to provide a lock for the NMR signal. The pH of the final solution was recorded and the mixture was transferred to separate 5 mm NMR tubes (Wilmad, LabGlass, Vineland, NJ, USA).
Nuclear Magnetic Resonance (NMR) Spectroscopy
Proton NMR spectra were collected with a Bruker Avance spectrometer with autosampler and 5 mm triple resonance 1H/13C/15N TXI CryoProbe with Zgradient, running TopSpin v. 2.16 (Bruker BioSpin, Fremont, CA, USA) at 700.13 MHz. A 1D NOESY (nuclear Overhauser effect spectroscopy) pulse sequence was used. The 90° pulse width was calibrated for each sample, and was generally 12-13 μs. The relaxation time was defined by each sample's 90° pulse width. The relaxation delay was 2 s, the acquisition time was 3 s, the spectral width was 10 kHz, the total number of data points collected was 63,000, and the number of transients collected was 128, for a total experiment time of 11 minutes and 17 seconds. During the relaxation period, the water resonance was presaturated. All spectra were collected at a temperature of 298 °K. Line broadening at 0.5 Hz was applied before fast Fourier transform (FFT); autophasing and auto-baseline correction were applied by TopSpin.
Chenomx software (Edmonton, AB, Canada) [18] was used to identify and quantify a portion of the metabolites present in each urine sample. Fine manual phasing and baseline corrections and the software's Reference Deconvolution algorithm was applied to each spectrum before targeted profiling of the metabolites was performed. Sixty metabolites were fit in each urine sample in this study, resulting in a profile containing the concentration of each identified metabolite in millimoles per liter (mM). The metabolomic profiles containing the urine concentrations were then normalized using the probabilistic quotient method [19, 20] to correct for differences in dilution among samples.
Ethics
Informed consent was given by all patients and healthy controls enrolled in this study. This protocol was approved by the Institutional Review Board at Brigham and Women's Hospital (BWH; IRB #2007-P-002480/1). The study protocol conforms to the ethical guidelines of the “World Medical Association (WMA) Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended by the 59th WMA General Assembly, Seoul, South Korea, October 2008.
Statistics
Data are reported as mean and range or mean ± standard deviation (SD). Significant urinary metabolites according to the three groups (acute pancreatitis, chronic pancreatitis, healthy control) were identified by Kruskal-Wallis test. Differences between metabolite concentrations were assessed with post-hoc Wilcoxon rank-sum tests with Bonferroni correction to accommodate the small sample size. These results were then validated with random permutations. All statistical tests were done with R software (R Foundation for Statistical Computing, http://cran.r-proiect.org/) [21]. R software was also used to generate a principal component analysis model with respect to patient group, and to create boxplots of profiled metabolites (Supplementary Figure).
To check for consistency, absolute metabolite concentrations were normalized to creatinine and compared to values reported in the Human Metabolome Database (HMDB; http://www.hmdb.ca) [22]. Values obtained herein were compared to those also obtained by NMR as reported in HMDB entries (S. Bouatra et al., 2012, The Human Urine Metabolome; manuscript in preparation: http://www.hmdb.ca).
Results
Patient characteristics are listed in Table 1. All of the patients with chronic pancreatitis had alcohol consumption as a related factor and half of the patients with acute pancreatitis had alcohol consumption as a related factor. Peak HCO3- concentrations were obtained from i.v. secretin-stimulated endoscopic pancreatic function tests (ePFT) [23]. Accepted normal values are greater than 75 mEq/L [24]. The observed low peak HCO3- concentration values and Cambridge III imaging scores for chronic pancreatitis patients were consistent with advanced disease.
Table 1.
Demographics for acute pancreatitis (AP) patients, chronic pancreatitis (CP) patients, and healthy controls (HC). Observed low peak HCO3-, concentration values and Cambridge III imaging scores for chronic pancreatitis patients were consistent with advanced disease.
| ID | Gender | Race | Age (years) | MANNHEIM CP diagnosis | Atlanta AP severity | Ranson criteria | Imaging | Etiology | TIGAR-O CP classification |
|---|---|---|---|---|---|---|---|---|---|
| Acute pancreatitis | |||||||||
| AP1 | Female | White | 58 | N/A | Mild | 2 | Normal MRI | Gallstone | N/A |
| AP2 | Male | Black | 42 | N/A | Mild | 2 | Balthazar C | Alcohol | N/A |
| AP3 | Male | White | 44 | N/A | Mild | 1 | Balthazar C | Obstructive | N/A |
| AP4 | Male | Not known | 59 | N/A | Mild | 2 | Diffuse enlargement on abdominal US | Alcohol | N/A |
| AP5 | Female | Black | 63 | N/A | Mild | 2 | Balthazar A | Gallstone | N/A |
| Chronic pancrtatitis | |||||||||
| CP1 | Male | White | 66 | Definite | N/A | N/A | Cambridge III | Alcohol, smoking | Toxic-metabolic |
| CP2 | Female | White | 52 | Definite | N/A | N/A | Cambridge III | Alcohol, smoking, RAP | Toxic-metabolic |
| CP3 | Male | White | 77 | Definite | N/A | N/A | Cambridge III | Alcohol, smoking | Toxic-metabolic |
| CP4 | Male | White | 42 | Definite | N/A | N/A | Cambridge III | Alcohol, hyperlipidemia | Toxic-metabolic |
| CP5 | Female | White | 62 | Definite | N/A | N/A | Cambridge III | Alcohol, smoking | Toxic-metabolic |
| Healthy controls | |||||||||
| HC1 | Male | White | 24 | N/A | N/A | N/A | N/A | N/A | N/A |
| HC2 | Male | Asian | 32 | N/A | N/A | N/A | N/A | N/A | N/A |
| HC3 | Female | White | 34 | N/A | N/A | N/A | N/A | N/A | N/A |
| HC4 | Female | White | 62 | N/A | N/A | N/A | N/A | N/A | N/A |
| HC5 | Female | White | 33 | N/A | N/A | N/A | N/A | N/A | N/A |
|
| |||||||||
| RAP: recurrent acute pancreatitis | |||||||||
| ID | Peak HCo3- concentration (mEq/L) | Smoking status | Smoking amount (packs/year) | Alcohol consumption | Alcohol amount | ||||
|---|---|---|---|---|---|---|---|---|---|
| Acute pancreatitis | |||||||||
| AP1 | N/A | Current | 20 | Not known | Not known | ||||
| AP2 | N/A | Never | 0 | Current | Moderate | ||||
| AP3 | N/A | Past | 17 | Past | Excessive | ||||
| AP4 | N/A | Current | >30 | Current | Excessive | ||||
| AP5 | N/A | Current | 67.5 | Not known | Not known | ||||
| Chronic pancreatitis | |||||||||
| CP1 | 39 | Current | 96 | Current | Increased | ||||
| CP2 | 38 | Current | 10 | Past | Excessive | ||||
| CP3 | 36 | Past | 25 | Past | Excessive | ||||
| CP4 | 40 | Never | 0 | Current | Increased | ||||
| CP5 | 37 | Past | >40 | Past | Excessive | ||||
| Healthy controls | |||||||||
| HC1 | N/A | N/A | N/A | N/A | N/A | ||||
| HC2 | N/A | N/A | N/A | N/A | N/A | ||||
| HC3 | N/A | N/A | N/A | N/A | N/A | ||||
| HC4 | N/A | N/A | N/A | N/A | N/A | ||||
| HC5 | N/A | N/A | N/A | N/A | N/A | ||||
Nuclear Magnetic Resonance (NMR) Spectroscopy Identified Metabolites in the Urine of all Three Cohorts
Sixty metabolites were identified and quantified in the urine. Urea was subsequently omitted from the data set because its protons are in exchange with water and the signal is compromised by water suppression. Boxplots of the remaining 59 metabolites and their averages and standard deviations are reported in the Supplementary Table and Figure. Concentrations of a subset of metabolites identified here are compared with those reported in the Human Metabolome Database in Table 2 and demonstrate that the results of this study are comparable to known concentration ranges.
Table 2.
Comparison of select metabolite concentrations obtained from the HMDB (Human Metabolome Database) and from these experiments. For the sake of comparison, absolute metabolite concentrations were normalized to creatinine concentrations. Values are reported as mean (range) with units of μmol/mmol creatinine.
| Common urinary metabolites | HMBD healthy referencea | Acute pancreatitis | Chronic pancreatitis | Healthy controls |
|---|---|---|---|---|
| Adenosine | 1.4 (0.9-2.3) | 3.6 (1.3-5.3) | 4.4 (3.1-6.4) | 1.6 (0.31-3.9) |
| Alanine | 21.8 (7.1-43.1) | 20 (10-41) | 15 (10-29) | 19 (12-33) |
| Choline | 3.5 (1.4-6.1) | 11 (3.6-19) | 7.9 (2.5-18) | 3.1 (1.3-4.9) |
| Citrate | 203 (49-600) | 120 (6.6-260) | 82 (10-250) | 240 (130-340) |
| Formate | 26.8 (6.0-120.9) | 33 (8.1-63) | 12 (2.2-24) | 27 (15-49) |
| Hippurate | 198 (26-718) | 170 (39-320) | 120 (14-250) | 120 (59-205) |
| Lactate | 11.6 (3.5-29.3) | 15 (13-22) | 12 (7.7-16) | 17 (11-28) |
| Quinolinate | 5.2 (1.0-17.5) | 6.4 (4.5-9.1) | 5.2 (2.0-12) | 7.7 (2.1-17) |
| Trigonelline | 31.1 (5.5-109.3) | 16 (1.8-40) | 27 (0.60-81) | 29 (3.2-73) |
| Valine | 5.5 (2.7-9.8) | 4.1 (2.1-6.1) | 3.4 (1.7-6.3) | 3.8 (2.9-5.0) |
HMDB healthy reference values were obtained via NMR as reported by S. Bouatra et al. 2012 (The Human Urine Metabolome; manuscript in preparation: http://www.hmdb.ca)
Of the 59 metabolites analyzed in this study, five were found to differ significantly among the three groups by Kruskal-Wallis test: acetone, adenosine, citrate, ribose, and 3-indoxylsulfate. These five metabolites were then subjected to pairwise Wilcoxon rank-sum tests with Bonferroni correction in a post-hoc evaluation (Table 3). Validation by random permutation confirmed that of these, only citrate and adenosine were significantly different among the groups. Citrate and adenosine concentrations in each of the groups are shown in Figure 1. Urinary citrate concentrations in healthy controls were greater than those in chronic pancreatitis patients (P=0.019, validation by random permutation). Conversely, urinary adenosine concentrations were lower in healthy controls than in chronic pancreatitis patients (P=0.026, validation by random permutation).
Table 3.
Significant urinary metabolites identified by Kruskal-Wallis test according to group (AP: acute pancreatitis, CP: chronic pancreatitis, HC: healthy control). Wilcoxon rank-sum tests with Bonferroni correction are then applied to test these metabolites; those with significant P values (P<0.05) are bolded. After the significant Wilcoxon rank-sum test results are validated by random permutation, only citrate and adenosine results are significant (P=0.019 and P=0.026, respectively).
| Significant metabolitesa | Exact P valueb | Comparison by group |
|---|---|---|
| Acetone | 0.095 | AP > CP |
| Acetone | 0.024 | AP > HC |
| Adenosine | 0.048 | CP > HC |
| Citrate | 0.095 | AP < HC |
| Citrate | 0.048 | CP < HC |
| Ribose | 0.048 | AP > HC |
| Ribose | 0.095 | CP > HC |
| 3-Indoxylsulfate | 0.095 | AP < HC |
Identified by Kruskal-Wallis test
Wilcoxon rank-sum test with Bonferroni correction
Figure 1.
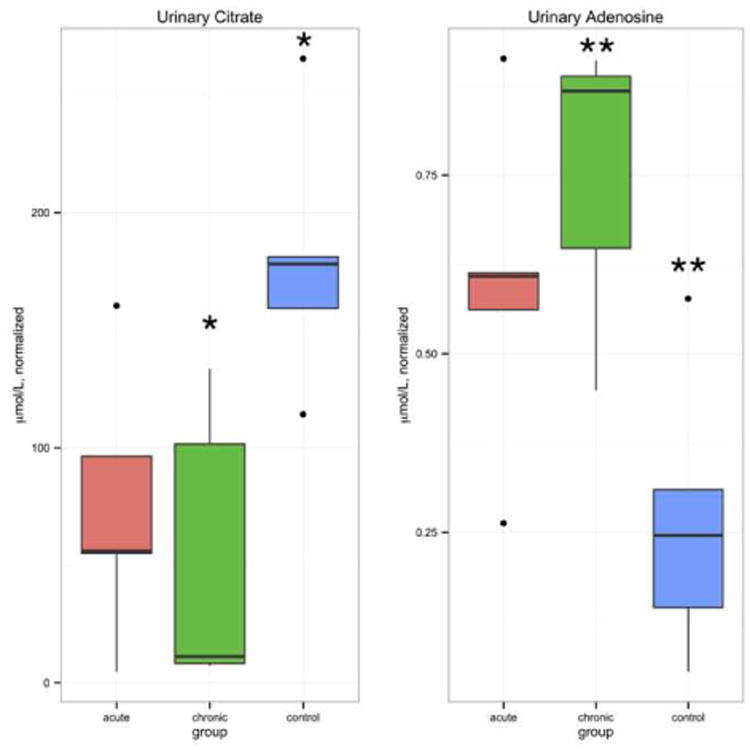
Boxplots depict concentrations of two significant metabolites identified after Wilcoxon rank-sum tests and validation by random permutation. Data are reported in μmol/L (normalized). Citrate (left) concentration was significantly lower in chronic pancreatitis (CP) patients compared to healthy controls (* P=0.019). Adenosine (right) concentration was significantly higher in chronic pancreatitis patients compared to healthy controls (** P=0.026). The box covers the first (Q1) and third (Q3) quartile of the data. The line in the box represents the median value. The whiskers extend to ±1.5 interquartile range (IQR), where IQR=Q3-Q1. Outliers are shown as points.
Principal Component Analysis Revealed Separation Between Healthy Controls and Pancreatitis Patients
Principal component analysis (PCA) was used to model the metabolic profiles according to group (chronic pancreatitis, acute pancreatitis, or healthy control). The first three components collectively explain 41% of the variance in the data (PC1=16%, PC2=13%, PC3=12%). Examination of the three-dimensional scores plot (Figure 2) showed that the control urine samples in green were generally separated from the urine samples obtained from pancreatitis patients. PCA demonstrated no separation between chronic pancreatitis and acute pancreatitis urine samples. The loadings plot (Figure 3) demonstrated the contribution of each of the metabolites to the model; citrate and adenosine are highlighted in blue. Though there is little pattern in the distribution of the metabolites, it is clear that citrate and adenosine lie far from each other in the loadings.
Figure 2.
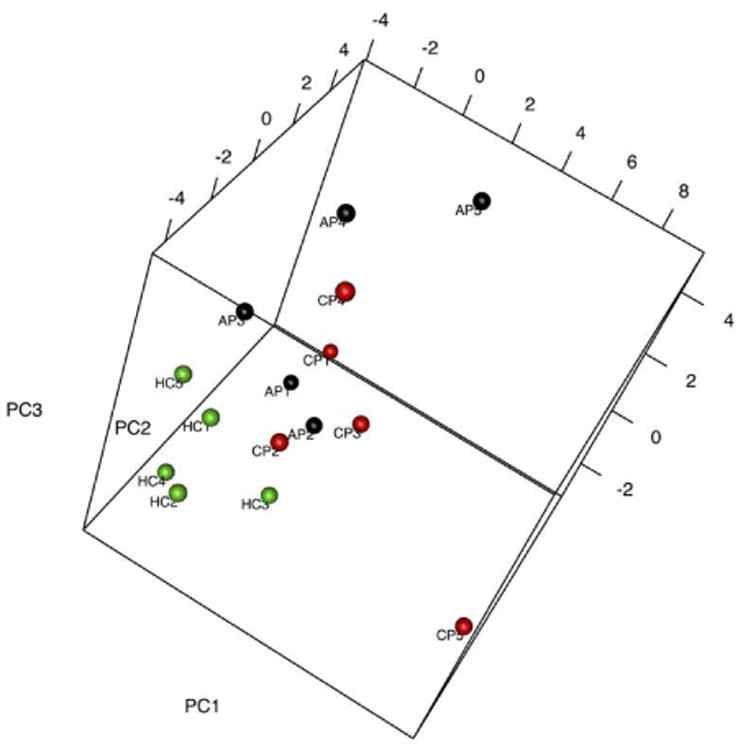
Principal component (PC) scores plot of urinary metabolic profiles shows acute pancreatitis (AP: black), chronic pancreatitis (CP: red), and healthy control (HC: green) urine samples in three dimensions. Separation of the healthy control urine samples from acute pancreatitis and chronic pancreatitis samples is observed. No separation in acute pancreatitis and chronic pancreatitis urine samples is observed.
Figure 3.
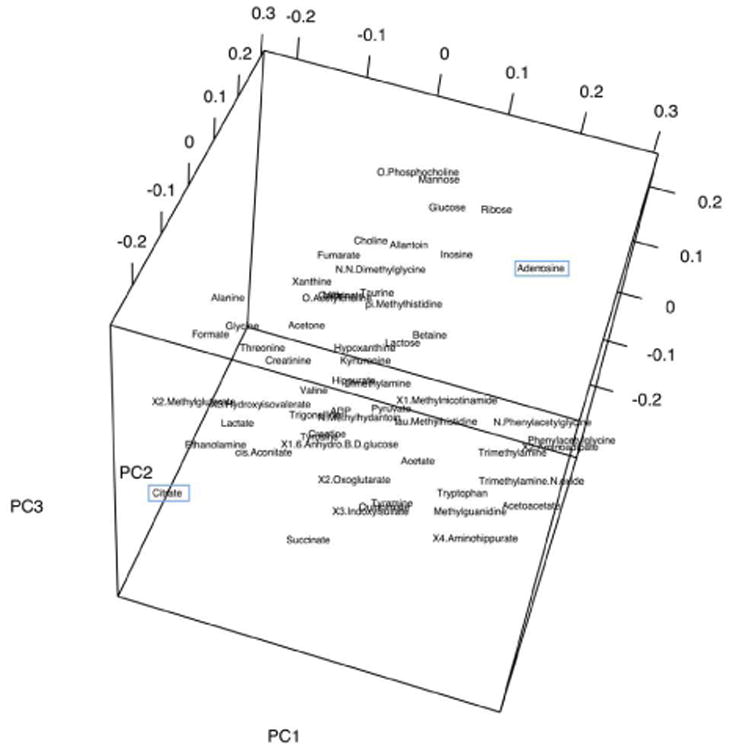
Principal component (PC) loadings plot of urinary metabolic profiles shows the influence of the metabolites on the scores. It can be seen that citrate and adenosine (boxed) are well-separated in the model.
Discussion
This pilot study demonstrates that the urinary metabolome can be used to differentiate patients with acute or chronic pancreatitis from healthy controls. Although only two potential biomarkers were identified, establishing the utility of this methodology is important for future metabolomic studies of pancreatic disease.
Of five identified metabolites that differed significantly among groups by Kruskal-Wallis test, four remained significant after evaluation by Wilcoxon rank-sum tests with Bonferroni correction (acetone, adenosine, citrate, ribose). Of these metabolites, only adenosine and citrate survived validation by random permutation. These two metabolites could reasonably be expected to differ among pancreatitis patients and healthy controls. Citrate is known to be a significant component of pancreatic secretions [25]. Given that alcohol use or abuse has been associated with a decrease in citrate in pancreatic secretions [26], it is notable that most patients in the chronic pancreatitis and acute pancreatitis groups reported some form of regular alcohol consumption (Table 1). The decreased urinary citrate in the pancreatitis cohorts compared to healthy subjects may be associated with decreased citrate in pancreatic secretions due to alcohol metabolism. This weakens citrate's candidacy as a biomarker of pancreatitis considerably, as the observed differences are potentially confounded by alcohol consumption. Still, future studies controlling for alcohol consumption may support citrate as a urinary biomarker of pancreatitis. Other etiologies of low urinary citrate include metabolic acidosis, potassium depletion, and acetazolamide [27, 28]. These conditions do not appear to be relevant in the population studied here.
Adenosine may prove to be a better marker of pancreas disease since it possesses known anti-inflammatory properties [29]. However, adenosine as a marker of inflammation is unlikely to be specific to pancreatitis [30], thus limiting its utility as a biomarker if not validated in further studies. While urinary adenosine concentrations may vary with changes in extracellular adenosine [31], variation in urinary adenosine concentration is known to be low in humans, and is unaffected by sodium content or changes in fluid homeostasis [32]. This implies that renal handling of adenosine does not further confound our results.
As suggested above, this study has several limitations, which may be attributed to small sample size. We attempted to minimize the effects of sample size on the statistical analysis of the data by using Kruskal-Wallis global test, followed by Wilcoxon rank-sum tests with Bonferroni correction and validation by random permutation. We also chose to use PCA, an unsupervised multivariate analysis technique, instead of a supervised technique. Supervised analyses such as partial least squares discriminant analysis (PLS-DA) are common in large metabolomics studies, but are inappropriate for small studies such as this. Additionally, we have not accounted for all confounding variables. As discussed, citrate's relationship to alcohol consumption compromises its usefulness as a biomarker of pancreatitis. Other factors such as age, differences in liver function, and dietary status likely exist among the patients providing samples. Furthermore, the possibility of differences among patient populations, etiology, and disease severity inducing variations in metabolomes presents an additional challenge. A larger study of the urine, serum, and pancreas fluid metabolomes with well-defined patient enrollment criteria, controlling for age, disease severity, and etiology of pancreatitis will account for confounding factors. This would result in a higher proportion of variance explained in the PCA model. A larger study with better-defined cohorts would also allow for partial least squares discriminant analysis modeling. We are currently performing a larger metabolomics study of ERCP-induced pancreatitis that will address many of these issues.
Our study demonstrates that metabolomics techniques are capable of distinguishing urine samples obtained from pancreatitis patients and from those obtained from healthy controls. This is a novel, non-invasive technique that can provide insight into the metabolic states of patients with chronic and acute pancreatitis. The significant increase in urinary adenosine and decrease in urinary citrate among pancreatitis patients compared to healthy controls may have reflected the patients' inflammatory state and alcohol consumption, and as such, validation studies will have to be performed. Although we cannot definitively conclude that these metabolites are biomarkers of pancreatitis, further analyses with larger cohorts will refine our results and define their diagnostic utility. The methodology described here presents a definitive strategy upon which further analyses with larger cohorts can be tested.
Supplementary Material
Supplementary Figure. Boxplots of absolute (non-normalized) concentrations of 59 profiled urinary metabolites. The box covers the first (Q1) and third (Q3) quartile of the data. The line in the box represents the median value. The whiskers extend to ±1.5 interquartile range (IQR), where IQR=Q3-Q1. Outliers are shown as points. Concentration units are expressed in μmol/L.
Supplementary Table. Absolute (non-normalized) concentrations of 59 metabolites profiled by NMR in each urine sample for acute pancreatitis patients, chronic pancreatitis patients, and healthy controls. Values are reported as mean±SD with units of μmol/L. Boxplots of each metabolite (non-normalized concentrations) are shown the Supplementary Figure.
Acknowledgments
Financial support None
Abbreviations
- AP
acute pancreatitis
- CP
chronic pancreatitis
- HC
healthy controls
- HMDB
Human Metabolome Database
- NMR
nuclear magnetic resonance
- PCA
principal component, analysis
Footnotes
Conflicts of interest None
References
- 1.Fagenholz PJ, Castillo CFD, Harris NS, Pelletier AJ, Camargo CA. Increasing United States hospital admissions for acute pancreatitis, 1988–2003. Ann Epidemiol. 2007;17(7):491–497. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Fagenholz PJ, Fernández-del Castillo C, Harris NS, Pelletier AJ, Camargo CA. National study of united states emergency department visits for acute pancreatitis, 1993–2003. BMC emergency medicine. 2007;7(1):1. doi: 10.1186/1471-227X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappell MS. Acute pancreatitis: Etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am. 2008;92(4):889–923. doi: 10.1016/j.mcna.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Etemad B, Whitcomb DC. Chronic pancreatitis: Diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 5.Warshaw AL, Banks PA, Fernāndez-del Castillo C. AGA technical review: Treatment of pain in chronic pancreatitis. Gastroenterology. 1998;115(3):765–776. doi: 10.1016/s0016-5085(98)70157-x. [DOI] [PubMed] [Google Scholar]
- 6.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: Challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132(4):1557–1573. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Flasar MH, Goldberg E. Acute abdominal pain. Med Clin North Am. 2006;90(3):481–504. doi: 10.1016/j.mcna.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Lankisch P. The problem of diagnosing chronic pancreatitis. Dig Liver Dis. 2003;35(3):131–134. doi: 10.1016/s1590-8658(03)00019-7. [DOI] [PubMed] [Google Scholar]
- 9.Otsuki M. Chronic pancreatitis in japan: Epidemiology, prognosis, diagnostic criteria, and future problems. J Gastroenterol. 2003;38(4):315–326. doi: 10.1007/s005350300058. [DOI] [PubMed] [Google Scholar]
- 10.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: The North American pancreatitis study 2 (NAPS2) Pancreatology. 2008;8(4-5):520–531. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiehn O. Metabolomics–the link between genotypes and phenotypes. Plant Mol Biol. 2002;48(1):155–171. [PubMed] [Google Scholar]
- 12.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 13.Madsen R, Lundstedt T, Trygg J. Chemometrics in metabolomics--A review in human disease diagnosis. Anal Chim Acta. 2010;659(1-2):23–33. doi: 10.1016/j.aca.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Sreekumar A, Poisson LM, Rajendiran TM, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 15.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: A review. Clin Cancer Res. 2009;15(2):431–40. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carraro S, Rezzi S, Reniero F, et al. Metabolomics applied to exhaled breath condensate in childhood asthma. Am J Respir Crit Care Med. 2007;175(10):986–90. doi: 10.1164/rccm.200606-769OC. [DOI] [PubMed] [Google Scholar]
- 17.Mortishire-Smith RJ, Skiles GL, Lawrence JW, et al. Use of metabonomics to identify impaired fatty acid metabolism as the mechanism of a drug-induced toxicity. Chem Res Toxicol. 2004;17(2):165–173. doi: 10.1021/tx034123j. [DOI] [PubMed] [Google Scholar]
- 18.Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM. Targeted profiling: Quantitative analysis of 1H NMR metabolomics data. Anal Chem. 2006;78(13):4430–4442. doi: 10.1021/ac060209g. [DOI] [PubMed] [Google Scholar]
- 19.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. application in 1H NMR metabonomics. Anal Chem. 2006;78(13):4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 20.Lusczek ER, Nelson T, Lexcen D, Witowski NE, Mulier KE, Beilman G. Urine metabolomics in hemorrhagic shock: Normalization of urine in the face of changing intravascular fluid volume and perturbations in metabolism. J Bioanal Biomed. 2011;3:038–048. [Google Scholar]
- 21.R Development Core Team. R Foundation for Statistical Computing; Vienna Austria: 2010. R: A Language and Environment for Statistical Computing. http://cran.r-project.org/ [Google Scholar]
- 22.Wishart DS, Knox C, Guo AC, et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009;37(suppl 1):D603–D610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu B, Conwell DL. The endoscopic pancreatic function test. Am J Gastroenterol. 2009;104(10):2381–2383. doi: 10.1038/ajg.2008.181. [DOI] [PubMed] [Google Scholar]
- 24.Stevens T, Conwell DL, Zuccaro G, Lewis SA, Love TE. The efficiency of endoscopic pancreatic function testing is optimized using duodenal aspirates at 30 and 45 minutes after intravenous secretin. Am J Gastroenterol. 2007;102(2):297–301. doi: 10.1111/j.1572-0241.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- 25.Boustière C, Sarles H, Lohse J, Durbec J, Sahel J. Citrate and calcium secretion in the pure human pancreatic juice of alcoholic and nonalcoholic men and of chronic pancreatitis patients. Digestion. 1985;32(1):1–9. doi: 10.1159/000199209. [DOI] [PubMed] [Google Scholar]
- 26.Sarles H, Bernard J, Johnson C. Pathogenesis and epidemiology of chronic pancreatitis. Annu Rev Med. 1989;40(1):453–468. doi: 10.1146/annurev.me.40.020189.002321. [DOI] [PubMed] [Google Scholar]
- 27.Simpson D. Citrate excretion: A window on renal metabolism. American Journal of Physiology-Renal Physiology. 1983;244(3):F223–F234. doi: 10.1152/ajprenal.1983.244.3.F223. [DOI] [PubMed] [Google Scholar]
- 28.Hamm LL. Renal handling of citrate. Kidney Int. 1990;38(4):728–735. doi: 10.1038/ki.1990.265. [DOI] [PubMed] [Google Scholar]
- 29.Noji T, Nan-ya K, Mizutani M, et al. KF24345, an adenosine uptake inhibitor, ameliorates the severity and mortality of lethal acute pancreatitis via endogenous adenosine in mice. Eur J Pharmacol. 2002;454(1):85–93. doi: 10.1016/s0014-2999(02)02476-7. [DOI] [PubMed] [Google Scholar]
- 30.Bours M, Swennen E, Di Virgilio F, Cronstein B, Dagnelie P. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Thompson CI, Sparks HV, Spielman WS. Renal handling and production of plasma and urinary adenosine. American Journal of Physiology-Renal Physiology. 1985;248(4):F545–F551. doi: 10.1152/ajprenal.1985.248.4.F545. [DOI] [PubMed] [Google Scholar]
- 32.Heyne N, Benöhr P, Mühlbauer B, Delabar U, Risler T, Osswald H. Regulation of renal adenosine excretion in humans—role of sodium and fluid homeostasis. Nephrology Dialysis Transplantation. 2004;19(11):2737–2741. doi: 10.1093/ndt/gfh492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Boxplots of absolute (non-normalized) concentrations of 59 profiled urinary metabolites. The box covers the first (Q1) and third (Q3) quartile of the data. The line in the box represents the median value. The whiskers extend to ±1.5 interquartile range (IQR), where IQR=Q3-Q1. Outliers are shown as points. Concentration units are expressed in μmol/L.
Supplementary Table. Absolute (non-normalized) concentrations of 59 metabolites profiled by NMR in each urine sample for acute pancreatitis patients, chronic pancreatitis patients, and healthy controls. Values are reported as mean±SD with units of μmol/L. Boxplots of each metabolite (non-normalized concentrations) are shown the Supplementary Figure.


