Abstract
OBJECTIVE
Irreversible electroporation is a nonthermal ablative tool that uses direct electrical pulses to create irreversible membrane pores and cell death. The ablation zone is surrounded by a zone of reversibly increased permeability; either zone can cause cardiac arrhythmias. Our purpose was to establish a safety profile for the use of irreversible electroporation close to the heart.
MATERIALS and METHODS
The effect of unsynchronized and synchronized (with the R wave on ECG) irreversible electroporation in swine lung and myocardium was studied in 11 pigs. Twelve lead ECG recordings were analyzed by an electrophysiologist for the presence of arrhythmia. Ventricular arrhythmias were categorized as major events. Minor events included all other dysrhythmias or ECG changes. Cardiac and lung tissue was submitted for histopathologic analysis. Electrical field modeling was performed to predict the distance from the applicators over which cells show electroporation-induced increased permeability.
RESULTS
At less than or equal to 1.7 cm from the heart, fatal (major) events occurred with all unsynchronized irreversible electroporation. No major and three minor events were seen with synchronized irreversible electroporation. At more than 1.7 cm from the heart, two minor events occurred with only unsynchronized irreversible electroporation. Electrical field modeling correlates well with the clinical results, revealing increased cell membrane permeability up to 1.7 cm away from the applicators. Complete lung ablation without intervening live cells was seen. No myocardial injury was seen.
CONCLUSION
Unsynchronized irreversible electroporation close to the heart can cause fatal ventricular arrhythmias. Synchronizing irreversible electroporation pulse delivery with absolute refractory period avoids significant cardiac arrhythmias.
Keywords: ablation, cardiac arrhythmia, irreversible electroporation
Electroporation is permeabilization of the cell membrane resulting from the application of an electric field across the cell. Electroporation can be of two types: reversible when the permeabilization is temporary and does not lead to cell death and irreversible when it leads to cell death. It is thought that the permeabilization is caused by electric field–induced elevated cell transmembrane potential and the consequent formation of nanoscale defects in the cell membrane (pores)—hence, the name “electroporation” [1]. Reversible electroporation of cells in tissue is used for two clinical applications: genetic treatment of diseases in which genes are introduced into the temporarily permeabilized tissue cells and electrochemotherapy for treatment of cancer in which various drugs, such as bleomycin, are introduced into temporarily permeabilized tumor cells [2]. Irreversible electroporation of cells in tissue is currently under investigation for its potential role in tumor ablation [3]. Irreversible electroporation is a potential alternative to the thermal-based ablation techniques that are limited by the heat-sink effect and thermal injuries to vital structures [4–7].
In current clinical applications, irreversible electroporation is produced through a series of electric pulses that are locally deposited via an applicator (i.e., electrode). The electric fields form around the electrodes in such a way that their magnitude decreases from the electrodes outward into the tissue. The fields are of such a nature that immediately near the electrodes there is a region in which they induce irreversible electroporation [8]. This irreversible electroporation–inducing region is surrounded by another region of lower electric fields that can induce reversible electroporation. In both regions of reversible and irreversible electroporation, the increased cell membrane permeability opens a path for ion transport, which can induce cardiac arrhythmias and defibrillation [9–11]. Mali et al. [12] investigated the likelihood of developing cardiac arrhythmias resulting from the increase in cell permeability caused by electrical pulses applied for electrochemotherapy and found no dysrhythmias on the ECG. However, the electroporation pulses were applied on the extremities of subjects at a large distance from the myocardium, and the pulse parameters used (eight pulses at 1,000 V/cm, each lasting 100 microseconds) were different from the ones used for irreversible electroporation. Hence, there is no direct evidence of the safety of irreversible electroporation pulse application close to the heart. It is known that external electrical stimuli delivered during the absolute refractory period of the heart are incapable of inducing an action potential [13]. Hence, synchronization of irreversible electroporation pulse delivery with the absolute refractory period of the cardiac cycle should reduce the risk of developing arrhythmias.
Our study evaluates the risk of developing a significant arrhythmia when irreversible electroporation is performed close to the heart and whether synchronization of irreversible electroporation pulse delivery with the absolute refractory period of the cardiac cycle mitigates this risk. We performed electric field modeling to predict empirical lesion size and to predict the distance from the applicators over which cells show increased permeability.
Materials and Methods
Cardiac Synchronization
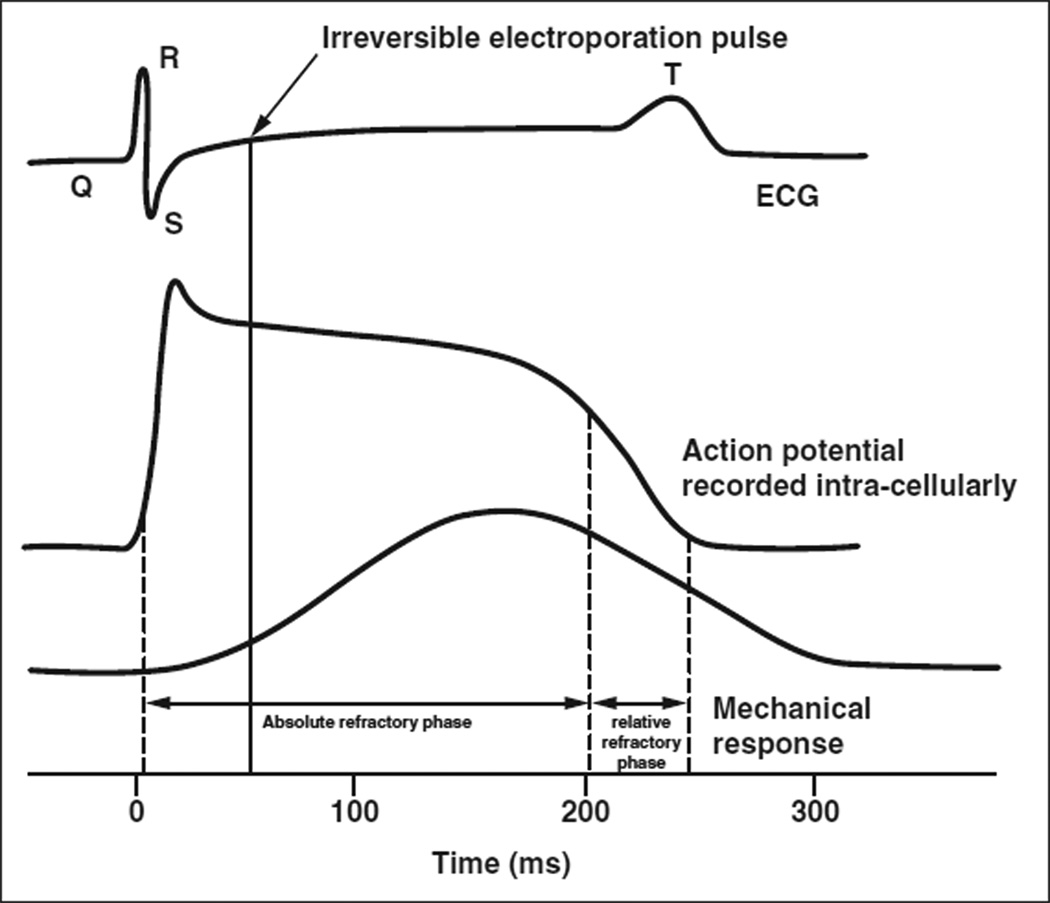
“Cardiac synchronization” refers to the synchronization of irreversible electroporation pulse delivery with the cardiac rhythm. A preprogrammed commercial ECG trigger monitor (Accusync 42/72, Accusync Medical Research Corporation) analyzes the animal’s ECG rhythm to detect the R wave. An irreversible electroporation generator (NanoKnife, AngioDynamics) then delivers a pulse 50 milliseconds after each R wave, in the absolute refractory period of the cardiac cycle (Fig. 1). Synchronization of pulses with the QRS complex increases the total treatment time (114 seconds vs 46 seconds with unsynchronized irreversible electroporation) but is identical to unsynchronized pulses in all other parameters.
Fig. 1.
Action potential, contractile response, and ECG. At 50 milliseconds after R wave, cardiac muscle is absolutely refractory. Synchronized irreversible electroporation pulse, which lasts for 70 microseconds, is denoted by continuous vertical line. Adapted from Ganong [13].
Electric Field Modeling
Electric field modeling was performed to assess the maximal region of increased cell membrane permeability resulting from electric field–induced irreversible and reversible electroporation due to the application of typical clinical irreversible electroporation pulses to the lung. This region of increased permeability should correspond to the distance from the electrode applicators over which cardiac myocytes could become electroporated and reveal a potential for conduction abnormalities due to increased cell membrane permeability. The electric field is calculated from the equation.
∇ × (σ∇ϕ) = 0 ∇ × (σ∇ϕ) = 0
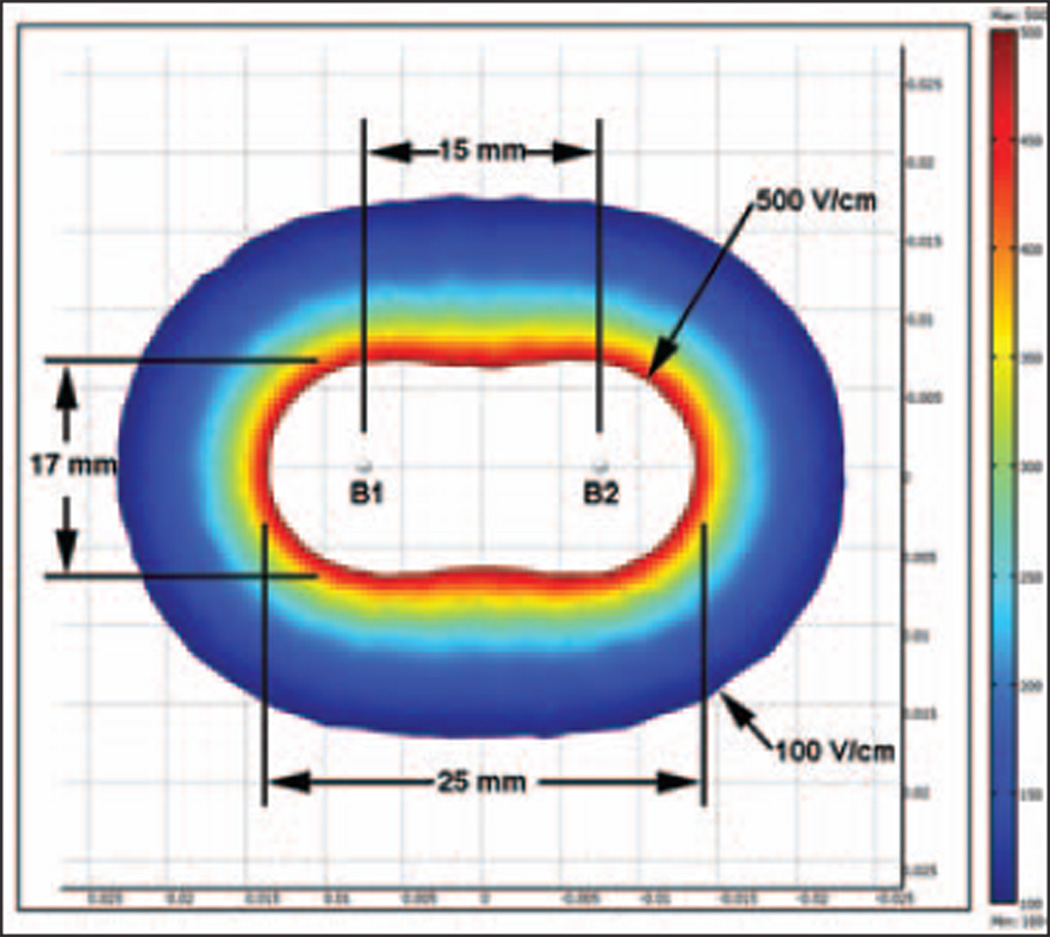
The domain of analysis was taken to be a homogeneous cylinder, 10 cm in diameter and height. The dimensions of the cylinder were selected so that they were large relative to the applicators, and the calculations would produce an upper limit for the distance from the electrodes over which irreversible electroporation in the lung could cause electroporation of heart cells. The applicators were modeled as two parallel stainless steel rods (1 mm in diameter and 20 mm long) 15 mm apart from each other. The applicators were oriented along the center of the cylinder to effectively eliminate any effects the cylinder edges might have on the electric field calculations. Table 1 shows the electric tissue parameters considered [14–18]. The boundary conditions were an electric potential difference of 2,500 V between the electrodes and electrically insulating outer boundary of the domain. The finite element method was used to calculate the electric field associated with an electroporation pulse [8] with MultiPhysics software (COMSOL). Ablation parameters (two probes, 15 mm between probes, 20 mm applicators, and 2,500 V) were selected on the basis of previous work on swine liver [19]. In this model, electric field strength of 100 V/cm was considered as bounding the outer extent of the zone of reversible electroporation, and electric field strength greater than 500 V/cm was considered as the zone of cell death due to irreversible electroporation [8]. These values of 100 and 500 V/cm are also consistent with the size and shape of the irreversible electroporation ablation zone obtained with previously performed in vivo swine liver irreversible electroporation (Fig. 2).
TABLE 1.
Electric Field Modeling Parameters
| Region, Parameter | Value |
|---|---|
| Region A (homogeneous universe) | |
| Volume | 776.4 cm3 cylinder with a diameter of 10 cm and height of 10 cm |
| Conductivity (S/m) | 0.3 |
| Region B1/B2 (applicators) | |
| Diameter (mm) | 1 |
| Exposed length (mm) | 20 |
| Distance between B1 and B2 (mm) | 15 |
| Potential between B1 and B2 (V) | 2,500 |
| Conductivity (S/m) | 4.032 e6 |
Fig. 2.
Electrical field modeling shows extent of zone of reversibly increased permeability (arrows). Ablation zone size with two applicators (B1 and B2) 15 mm apart and 2-cm active applicator is superimposed.
Animal Model
Female swine (n = 11) weighing 35–50 kg were studied for 33 lung and four myocardial ablations. Of the 11 swine, four were followed for 3 weeks (long-term study group) and seven were sacrificed within 24 hours of the ablation (short-term study group). The protocol was approved by our Institutional Animal Care and Use Committee. Sedation was achieved with IV tiletamine hydrochloride and zolazepam hydrochloride (6 mg/kg; Telazol, Fort Dodge Animal Health). General anesthesia was maintained by inhaled isofluorane (2%; Aerrane, Baxter Healthcare) after endotracheal intubation. Pancuronium bromide (0.1 mg/kg; Pancurox, Hospira), a neuromuscular blockade agent, was administered before the procedure by the veterinary staff and was titrated to ensure muscle paralysis. Adequate neuromuscular blockade was verified by observation after a test pulse delivered through the applicators placed in the lungs or myocardium. An external biphasic defibrillator was immediately available for treatment of ventricular arrhythmias. Cardiac rhythm was recorded for each procedure and at 1 and 3 weeks in the survival animals with a 12-lead ECG (using a Marquette Mac 5000 Premium Resting ECG machine, GE Healthcare). These ECG rhythms were archived for independent analysis by an electrophysiologist. Ventricular arrhythmias (tachycardia and fibrillation) were categorized as major events. Transient supraventricular arrhythmias, ST-T segment elevations, and transient T wave changes were classified as minor events.
Each animal underwent multiple (two or more) lung ablations in the same session, unless precluded by development of a fatal arrhythmia. Myocardial ablations were created immediately before a planned sacrifice; an ECG was recorded every 5 minutes for up to 45 minutes after ablation. Of the 11 animals, four animals underwent a total of eight ablations that were followed for 3 weeks (long-term study group) after the initial lung ablations (Tables 2 and 3). The remaining seven animals were sacrificed within 24 hours of the procedure (short-term study group). An overdose of IV pentobarbital sodium (100 mg/kg; Sleepaway, Fort Dodge Animal Health) was used to sacrifice all animals, including those that developed a fatal arrhythmia. A necropsy was then conducted and representative histologic samples were collected.
TABLE 2.
Summary of ECG Results of Unsynchronized Irreversible Electroporation in Swine Lungs
| Shortest Distance From Heart, Actual Distance |
Voltage Used (V) |
Result |
|---|---|---|
| ≤ 1.7 cm | ||
| Within myocardium | 1,500 | Ventricular fibrillation |
| 8 mm | 2,100 | Ventricular fibrillation |
| < 10 mm | 2,500 | Ventricular fibrillation |
| < 10 mm | 1,700 | Transient ventricular tachycardia |
| 10 mm | 2,500 | Ventricular fibrillation |
| 15 mm | 2,000 | Ventricular fibrillation |
| 17 mm | 1,600 | Ventricular fibrillation |
| > 1.7 cm | ||
| 2 cm | 2,100 | Transient supraventicular tachycardia; ventricular rate 120 beats per minute |
| 2.6 cma | 1,700 | Transient supraventicular tachycardia, right bundle branch block, and large T wave |
| 3.2 cm | 2,500 | No change from baseline ECG |
| 3.2 cma | 2,500 | No change from baseline ECG |
| 3.5 cma | 2,500 | No change from baseline ECG |
| 3.9 cm | 2,600 | No change from baseline ECG |
| 4 cm | 2,600 | No change from baseline ECG |
| 6 cm | 2,000 | No change from baseline ECG |
| 6.7 cm | 2,500 | No change from baseline ECG |
| 7 cma | 1,700 | No change from baseline ECG |
| 7.7 cma | 2,500 | No change from baseline ECG |
Long-term study animal. No arrhythmias were detected at 1 and 3 weeks of follow-up.
TABLE 3.
Summary of ECG Results of Synchronized Irreversible Electroporation in Swine Lungs
| Shortest Distance from Heart, Actual Distance |
Voltage Used (V) | Result |
|---|---|---|
| ≤ 1.7 cm | ||
| Within myocardium | 1,400 | ST segment elevation |
| Within myocardium | 1,500 | ST segment elevation |
| Within myocardium | 1,500 (only 10 pulses delivered) | ST segment elevation |
| Within myocardium | 2,500 | ST segment elevation |
| Touching pericardiuma | 2,100 | No change from baseline ECG |
| Touching pericardiuma | 2,100 | Self-limited supraventicular tachycardia (150 beats per minute); transient ST segment elevation |
| 5 mm | 1,700 | No change from baseline ECG |
| 8 mm | 2,100 | Self-limited T wave inversion |
| < 10 mm | 1,700 | No change from baseline ECG |
| < 10 mm | 2,500 | Self-limited T wave inversion |
| 15 mm | 2,000 | No change from baseline ECG |
| 17 mm | 2,000 | No change from baseline ECG |
| > 1.7 cm | ||
| 2 cma | 2,500 | No change from baseline ECG |
| 2 cm | 1,700 | No change from baseline ECG |
| 3.2 cm | 2,500 | No change from baseline ECG |
| 3.5 cm | 2,500 | No change from baseline ECG |
| 4 cm | 2,500 | No change from baseline ECG |
| 6 cm | 2,000 | No change from baseline ECG |
| 6.7 cm | 2,500 | No change from baseline ECG |
Long-term study animal. No arrhythmias were detected at 1 and 3 weeks of follow-up.
Synchronized and Unsynchronized Irreversible Electroporation in Swine Lung and Myocardium
After sterile preparation of the operating field, under CT guidance, multiple paired applicator sets (NanoKnife [part 20400101], Angiodynamics) were placed 9–15 mm apart in the swine lung (2 cm of applicator exposed) and left ventricular myocardium (0.5 cm exposed). A spacer (NanoKnife [part 20400301], Angiodynamics) was used to ensure consistent distance in between the two applicators. After placement, the shortest distance between the heart border and the active part of the applicator was calculated from 3D CT images reconstructed using a workstation (Advantage 4.4, GE Healthcare). Between 1,667 and 1,700 V/cm were applied through the applicators to ensure irreversible electroporation. This voltage was secured by altering the applied voltage depending on the distance between the two applicators; for example, for a distance of 9 mm, 1,500 V were applied, and for a distance of 15 mm, 2,500 V were applied. The electrical pulses were synchronized or unsynchronized with the ECG depending on study design. Each irreversible electroporation treatment consisted of nine groups of 10 pulses (total, 90 pulses; each pulse 70 microseconds long) with a 3-second pause between each group of pulses to permit recharging of the generator. Each pulse was separated by 250 milliseconds in the unsynchronized mode and by one heartbeat in the synchronized mode. Control lesions were created in the pig liver and kidney in unsynchronized mode.
Each animal was imaged at the end of the procedure with an unenhanced and contrast-enhanced CT scan (300 mg/mL iohexol; Omnipaque, GE Healthcare). Pneumothorax was treated by percutaneous insertion of an 8-French multipurpose catheter (Cook Medical) using standard Seldinger technique. This was attached to a portable chest drainage system (Pneumostat chest drain valve, Atrium Medical). In long-term study animals, the chest tube was removed once there was no air leak and a chest radiograph revealed a fully inflated lung. Long-term study animals (n = 4) underwent repeat CT scans and ECG at 1 and 3 weeks.
Histopathologic Analysis
Gross and microscopic (H and E stain) analysis of lung and cardiac tissue was performed. Phosphotungstic acid–hematoxylin stain was used for the cardiac specimens to evaluate for contraction band necrosis. Masson trichrome stain was used on the long-term specimens to confirm fibrosis.
Statistical Analysis
Fisher’s exact test was used to check for statistically significant associations between the presence or absence of ECG synchronizations and complications, stratifying for the shortest distance from the myocardium (≤ 1.7 vs > 1.7 cm). A two-sided p value of 0.05 or less was considered statistically significant. All p value calculations were performed using SAS software (version 9.2, SAS Institute).
Results
Thirty-three lung and four myocardial irreversible electroporation ablations were created. Of these, three synchronized and five unsynchronized lung ablations were followed for 3 weeks. The results of lung irreversible electroporation at varying distances from the heart in the unsynchronized mode (n = 18) are shown in Table 2, and those for the synchronized mode (n = 19) are shown in Table 3. The ST-T segment elevations seen with myocardial ablations trended toward the baseline but did not normalize. A significant pneumothorax developed in only two animals (both from the long-term study group) after irreversible electroporation was performed. A chest tube was placed at the end of the irreversible electroporation ablation. The chest tube was removed on days 2 and 4, respectively, after the procedure, as soon as closure of the pleural puncture was confirmed.
Major Events at Less Than or Equal to 1.7 cm From the Heart
A major event was observed in all seven unsynchronized ablations, but in none of 12 synchronized ablations. This difference is statistically significant with p < 0.001.
Major Events at More Than 1.7 cm From the Heart
No major events were observed with the 11 unsynchronized or seven synchronized ablations.
Minor Plus Major Events at Less Than or Equal to 1.7 cm From the Heart
Minor and major events occurred in all seven unsynchronized and in three of 12 synchronized ablations.
Minor or Major Events at More Than 1.7 cm From the Heart
Minor or major events occurred in two of 11 unsynchronized and zero of seven synchronized ablations. The incidence of complications between the synchronized and unsynchronized groups is significantly different (p < 0.001) when the shortest distance from the myocardium is less than or equal to 1.7 cm but not significantly different (p = 0.497) when the shortest distance is more than 1.7 cm.
Electric Field Modeling
From mathematic models, it was determined that a field strength of greater than or equal to 100 V/cm (threshold for reversible electroporation) extends to a radius of 1.7 cm outward from each unipolar applicator (Fig. 2). This corresponds to an area of reversibly and irreversibly increased permeability. The typical lesion that develops after irreversible electroporation depends on the exposed applicator length and the voltage applied. On the basis of our ex vivo experiments, for 2-cm exposed applicators using 2,500 V, an ellipsoidal lesion of 30 × 25 × 17 mm is formed. Figure 2 depicts the ablation zone as all cells within field strength greater than 500 V/cm (threshold for irreversible electroporation).
Histopathologic Analysis
Evaluation of myocardium specimens from short-term lung study animals showed no gross or microscopic evidence of acute myocardial injury (contraction band necrosis on phosphotungstic acid–hematoxylin stain). Myocardial specimens from long-term lung study animals revealed pericardial and epicardial fibrosis but no myocardial changes. There was a sharp demarcation between the epicardial fibrosis and the myocardium. This finding was confirmed with Masson trichrome stain. With intracardiac applicators, the myocardium showed complete acute necrosis characterized by cytoplasmic hypereosinophilia, contraction bands, and pyknosis with a mild neutrophilic infiltrate. The necrosis was sharply demarcated from surrounding normal myocardium. The treated portion of lung parenchyma showed complete ablation up to the edge of bronchi and arterioles without intervening live cells. Thermal injury was seen within 0.5 mm at the applicator tissue interface but not elsewhere in the ablation zone.
Discussion
Conventional thermal ablation techniques are effectively used in the locoregional treatment of tumors. However, several limitations have surfaced, the most important being incomplete tumor ablation with subsequent tumor recurrence secondary to the heat-sink effect and possible thermal injury to vital structures such as the heart and hila [4–7]. Irreversible electroporation, being a nonthermal technique [20, 21], has generated interest as an alternative ablative technique, and previous literature has shown that irreversible electroporation can be effectively used in tumor ablation [22]. However, the reversibly and irreversibly increased permeability caused by irreversible electroporation electrical pulses can potentially lead to cardiac arrhythmias when used in proximity to the heart. To safely use irreversible electroporation as an ablative tool near the heart, it is imperative to understand the potential risk of arrhythmia associated with its use, the need for synchronization to mitigate such a risk, and the distance at which synchronization is critical. Our study showed that ventricular arrhythmias can be avoided by synchronizing the irreversible electroporation pulse with the cardiac rhythm.
Rhythmic cardiac contraction is governed by the autonomous patterned discharge of electrical impulses (action potential) by the sinoatrial node [23], which is brought about by the opening and closing of specialized proteins in the lipid bilayer that alter ion flux [13]. External electrical stimuli can cause formation of nonspecific ion channels [24] to permit flux of charged particles [25], leading to a localized depolarization [26]. Electrical stimuli that exceed the threshold excitation potential can cause localized depolarization to build into an action potential [27]. Therefore, irreversible electroporation energy could trigger a premature action potential in a cardiac myocyte and lead to cardiac arrhythmias, especially ventricular fibrillation.
To avoid arrhythmias, the electrical energy must be applied in a fashion so as not to cause a critical increase in cell permeability. Previous studies have shown that, for electrical energy to be dysrhythmogenic, it must stimulate the myocardium during its “vulnerable period.” This denotes the period of the cardiac cycle where electrical stimuli of sufficient strength can lead to changes in membrane conductivity with subsequent ion flux and cellular depolarization. The vulnerable period of the ventricular myocardium is denoted by almost the entire T wave as seen on an ECG [12] (Fig. 1). The remaining part of the QRS complex is refractory to all electrical stimuli. Therefore, if irreversible electroporation pulse delivery is adjusted to fall during the absolute refractory period (before the vulnerable period of the myocardium), arrhythmogenic potential is minimized [12, 13, 28]. This is called synchronization. In our study, no lasting dysrhythmia was seen when the irreversible electroporation pulse (lasting 70 microseconds, terminating before vulnerable period) was delivered 50 milliseconds after the R wave (Fig. 1), even with the irreversible electroporation applicators within the myocardium.
As mentioned previously, when an irreversible electroporation pulse is applied between two applicators inserted into tissue, the electrical energy conducted outward from the applicator leads to a permanent (cell death) or a transient increase in cell permeability as a function of conducted voltage strength and duration [22]. Therefore, during irreversible electroporation, two zones are formed: a zone of cell death close to the applicator (tissue voltage, > 500 V/cm) surrounded by a zone of tissue with reversibly increased cell membrane permeability (tissue voltage, 100–500 V/cm) (Fig. 2). Although the latter increase in permeability is reversible, cells can take several seconds to return to a baseline potential difference [26]. If either of these zones was to include the myocardium, they could cause a premature action potential and arrhythmia (Fig. 3). More important, if an operator fails to realize that cells beyond the ablation zone also show increased permeability, he or she may not realize the potential danger of developing an arrhythmia when using unsynchronized irreversible electroporation near the heart. Our results indicate that all cells within a radius of 1.7 cm from each applicator show a transient or permanent increase in cell membrane permeability (i.e., irreversible electroporation pulse delivery within 1.7 cm of the myocardium can cause an arrhythmia). Statistically, therefore, to perform an irreversible electroporation ablation without developing a ventricular arrhythmia, the distance from the heart either needs to be greater than 1.7 cm or irreversible electroporation should be performed in the synchronized mode. Thus, with the protocol used in this study, synchronization appears to be essential within 1.7 cm of the heart (Fig. 3). This correlates well with our results showing that no ventricular arrhythmias developed with unsynchronized irreversible electroporation at more than 1.7 cm from the heart, but at less than or equal to 1.7 cm, fatal ventricular arrhythmias resulted. This observation is also consistent with the safety of irreversible electroporation in the liver, kidney, and peripheral lung (i.e., far away from the heart).
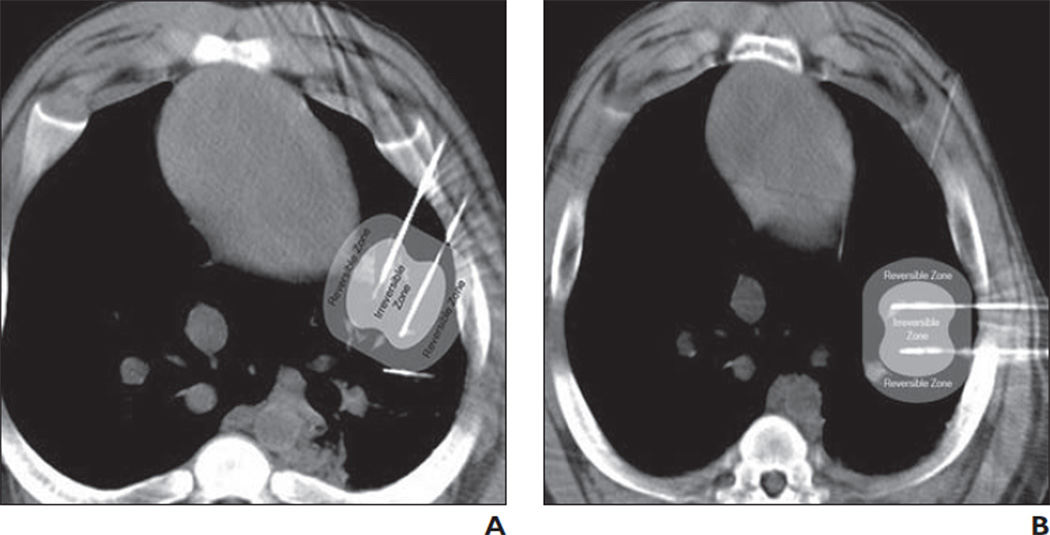
Fig. 3.
Field model zones superimposed on intraprocedure CT scans.
A, Zone of increased permeability (darker gray zone) overlaps myocardium. Lighter gray zone represents ablation zone. Synchronization would be necessary to avoid cardiac arrhythmia.
B, Zone of increased permeability (darker gray zone) is away from myocardium. Lighter gray zone represents ablation zone. Synchronization is not essential.
Our pilot study suggests that synchronized irreversible electroporation can be applied to tissue close to the heart without significant cardiac effects. We conclude that this promising technology should be further investigated to uncover its potential benefits.
This pilot study was performed in normal porcine lung tissue with unipolar applicators. Our sample size was relatively small; larger studies would be required to definitely show safety. Results for bipolar applicators may differ. Pig hearts are known to be hypersensitive to arrhythmias. Further work in animal tumor models would shed light on possible differences in tumor response to irreversible electroporation and in the conduction of electrical pulses by lung tumors versus normal lung tissue [14–18].
Applicator distance from the heart as measured on an axial CT image may not represent the true 3D distance, which is why 3D reconstruction on the workstation was used in this study to make measurements. However, the CT images used for measurement calculation were static images and did not reflect the distance changes that occur with cardiac systole and diastole. Thus, synchronization may be necessary at distances greater than 1.7 cm from the heart (as measured on an axial CT scan).
Unipolar applicators were used in this experiment (i.e., two applicators were necessary to create an ablation zone). Although the literature is divided on the correlation between the number of pleural passes and the incidence of pneumothorax [29, 30], the use of two separate applicators may increase the risk of pneumothorax. Therefore, the use of unipolar applicators could increase the risk of developing a pneumothorax and could affect accurate placement of the applicators within tumor nodules. In our study, only two of the four long-term study animals required placement of a chest tube for pneumothorax; in each of these cases, a significant air leak did not develop until after applicator placement. Similarly, in the seven animals sacrificed within 24 hours of the study, no significant pneumothorax interfering with applicator placement was seen. Bipolar applicators (requiring a single applicator) are now available and can be used for ablation similar to conventional ablation techniques such as radiofrequency or microwave ablation.
Besides the potential for cardiac dysrhythmia, operators must acknowledge that the technology requires use of general anesthesia with a neuromuscular blocker to prevent muscle spasm.
In conclusion, irreversible electroporation pulses delivered in synchrony with the R wave of the ECG permit ablation in proximity to the heart without significant arrhythmia.
Acknowledgments
This work was supported by a research grant from Angiodynamics. S. B. Solomon and B. Rubinsky are scientific advisors to Angiodynamics. G. W. Single and W. C. Hamilton, Jr. are employees of Angiodynamics.
Footnotes
Presented at the 2009 annual meeting of the Radiological Society of North America.
References
- 1.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mir LM. Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochemistry. 2001;53:1–10. doi: 10.1016/s0302-4598(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 3.Rubinsky B. Irreversible electroporation in medicine. Technol Cancer Res Treat. 2007;6:255–260. doi: 10.1177/153303460700600401. [DOI] [PubMed] [Google Scholar]
- 4.Jungraithmayr W, Szarzynski M, Neeff H, et al. Significance of total vascular exclusion for hepatic cryotherapy: an experimental study. J Surg Res. 2004;116:32–41. doi: 10.1016/s0022-4804(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 5.Steinke K, Haghighi KS, Wulf S, Morris DL. Effect of vessel diameter on the creation of ovine lung radiofrequency lesions in vivo: preliminary results. J Surg Res. 2005;124:85–91. doi: 10.1016/j.jss.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Weber SM, Lee FT, Jr, Chinn DO, Warner T, Chosy SG, Mahvi DM. Perivascular and intralesional tissue necrosis after hepatic cryoablation: results in a porcine model. Surgery. 1997;122:742–747. doi: 10.1016/s0039-6060(97)90082-9. [DOI] [PubMed] [Google Scholar]
- 7.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT., Jr Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 8.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khadra A, Nikolski V, Efimov IR. The role of electroporation in defibrillation. Circ Res. 2000;87:797–804. doi: 10.1161/01.res.87.9.797. [DOI] [PubMed] [Google Scholar]
- 10.Nikolski VP, Efimov IR. Electroporation of the heart. Europace. 2005;7(Suppl 2):S146–S154. doi: 10.1016/j.eupc.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Tovar O, Tung L. Electroporation of cardiac cell membranes with monophasic or biphasic rectangular pulses. Pacing Clin Electrophysiol. 1991;14:1887–1892. doi: 10.1111/j.1540-8159.1991.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 12.Mali B, Jarm T, Corovic S, et al. The effect of electroporation pulses on functioning of the heart. Med Biol Eng Comput. 2008;46:745–757. doi: 10.1007/s11517-008-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganong W. Review of medical physiology. 21st ed. New York, NY: Lange Medical Books/McGraw-Hill; 2003. pp. 80–81. [Google Scholar]
- 14.Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues. I. Literature survey. Phys Med Biol. 1996;41:2231–2249. doi: 10.1088/0031-9155/41/11/001. [DOI] [PubMed] [Google Scholar]
- 15.Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues. III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol. 1996;41:2271–2293. doi: 10.1088/0031-9155/41/11/003. [DOI] [PubMed] [Google Scholar]
- 16.Jossinet J. Variability of impedivity in normal and pathological breast tissue. Med Biol Eng Comput. 1996;34:346–350. doi: 10.1007/BF02520002. [DOI] [PubMed] [Google Scholar]
- 17.Jossinet J, Schmitt M. A review of parameters for the bioelectrical characterization of breast tissue. Ann N Y Acad Sci. 1999;873:30–41. doi: 10.1111/j.1749-6632.1999.tb09446.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith SR, Foster KR, Wolf GL. Dielectric properties of VX-2 carcinoma versus normal liver tissue. IEEE Trans Biomed Eng. 1986;33:522–524. doi: 10.1109/TBME.1986.325740. [DOI] [PubMed] [Google Scholar]
- 19.Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007;6:287–294. doi: 10.1177/153303460700600404. [DOI] [PubMed] [Google Scholar]
- 20.Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6:307–312. doi: 10.1177/153303460700600407. [DOI] [PubMed] [Google Scholar]
- 21.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat. 2007;6:37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 22.Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005;4:699–705. doi: 10.1177/153303460500400615. [DOI] [PubMed] [Google Scholar]
- 23.Braunwald E, Fauci AS, Kasper DL, Hauser S, Longo DL, Jameson JL, editors. Harrison’s principles of internal medicine. 15th ed. New York, NY: McGraw-Hill; 2001. [Google Scholar]
- 24.Tovar O, Tung L. Electroporation and recovery of cardiac cell membrane with rectangular voltage pulses. Am J Physiol. 1992;263:H1128–H1136. doi: 10.1152/ajpheart.1992.263.4.H1128. [DOI] [PubMed] [Google Scholar]
- 25.Lodish H, Berk A, Matsudaira P, et al. Molecular cell biology. 5th ed. New York, NY: W.H. Freeman & Company; 2004. [Google Scholar]
- 26.DeBruin KA, Krassowska W. Modeling electroporation in a single cell. I. Effects of field strength and rest potential. Biophys J. 1999;77:1213–1224. doi: 10.1016/S0006-3495(99)76973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng DK, Tung L, Sobie EA. Nonuniform responses of transmembrane potential during electric field stimulation of single cardiac cells. Am J Physiol. 1999;277:H351–H362. doi: 10.1152/ajpheart.1999.277.1.H351. [DOI] [PubMed] [Google Scholar]
- 28.Guyton AC, Hall JE. Textbook of medical physiology. Philadelphia, PA: Elsevier Saunders; 2006. [Google Scholar]
- 29.Cox JE, Chiles C, McManus CM, Aquino SL, Choplin RH. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology. 1999;212:165–168. doi: 10.1148/radiology.212.1.r99jl33165. [DOI] [PubMed] [Google Scholar]
- 30.Moore EH. Technical aspects of needle aspiration lung biopsy: a personal perspective. Radiology. 1998;208:303–318. doi: 10.1148/radiology.208.2.9680552. [DOI] [PubMed] [Google Scholar]