Abstract
Purpose
To establish procedures for functional magnetic resonance imaging in rats without the need for anaesthetic agents.
Materials and Methods
Rats were trained to habituate to restraint in a harness and scanner noise. Under anaesthesia, rats were then prepared with a cranial implant that permitted stabilization of the head during subsequent imaging experiments. The cranial implant included an RF coil that was used to transmit and receive radiofrequency signals during imaging. Further training was then conducted to habituate the animals to head fixation whilst in the MR scanner.
Results
Using this method, we were able to successfully and repeatedly record BOLD fMRI responses to hypercapnia and whisker stimulation in awake rats. Electrical stimulation of the whisker pad produced a ~7% increase in BOLD signal in the corresponding barrel cortex as well as adjacent negative BOLD responses, whilst hypercapnia produced larger increases in BOLD signal amplitude.
Conclusion
This methodology leaves the face and limbs free from obstruction, making possible a range of behavioural or sensory stimulation protocols. Further development of this animal model could enable traditional behavioural neuroscience techniques to be combined with modern functional neuroimaging.
Keywords: Functional magnetic resonance imaging, rat, anaesthesia
INTRODUCTION
Functional magnetic resonance imaging (fMRI) has become established as a major research tool in neuroscience. It has enabled researchers from a wide range of disciplines to address fundamental questions of brain function and dysfunction non-invasively in human subjects. In many circumstances however, there remains a need for comparable animal models in which repeatable non-invasive imaging can be combined with other experimental manipulations (e.g. pharmacological, surgical and behavioural) that would not be permissible or practical in human subjects. Additionally, since accurate interpretation of fMRI data is constrained by our as yet incomplete knowledge of how neuroimaging signals are related to underlying changes in neuronal activity, there remains a pressing for physiological studies in animal models to investigate the mechanisms and parameters of neuronal-fMRI signal coupling.
A major limitation of magnetic resonance imaging is the requirement for almost complete immobilisation of the subject that in animal models usually necessitates the use of anaesthetic agents. However, such agents interfere with normal neuronal, metabolic and haemodynamic function (1-5), in addition to precluding the use of operant and behavioural experimental protocols. In response to these issues, we have developed procedures and apparatus to enable MRI and fMRI studies to be conducted in conscious rats. As a starting point, we took methodology previously described for conducting optical imaging studies in the un-anaesthetized rat (6), as this has proved effective in reducing movement artefacts and minimizing stress in the animals, the central considerations in this work. In this paper we report details of the animal training and surgical procedures together with the design details of the customised animal holding device, the chronically implanted MRI surface coil and design of the associated tuning circuit. Using this methodology we have successfully recorded functional imaging responses to both whisker stimulation and hypercapnia in fully conscious animals over a period of several weeks. This method leaves the face and limbs free from obstruction, making possible a range of behavioural or sensory stimulation protocols. Chronic implantation of the surface coil will also reduce variability in MR signals and images over repeated data acquisition sessions and reduce the time required to optimally install the animal into the imaging system. Development of this animal model could enable traditional neuroscientific techniques to be combined with modern functional neuroimaging in chronic research designs.
MATERIALS AND METHODS
All procedures were carried out with the approval of both the local ethics committee and the UK Home Office under the Animals (Scientific Procedures) Act 1986.
The Restraint Harness And Animal Holder
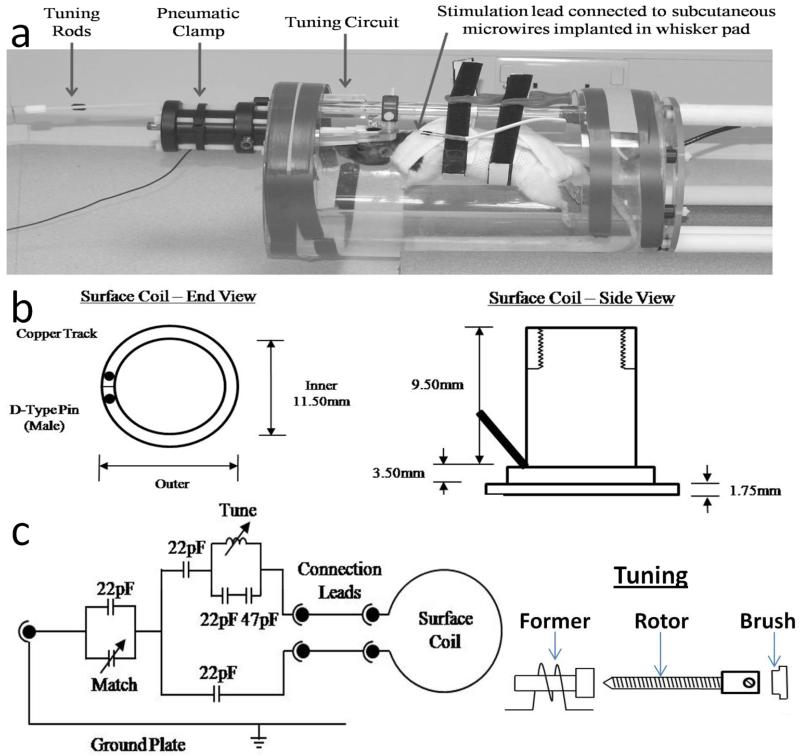
The harness was constructed from non-elastic washable materials, with Velcro strips stitched into the appropriate positions to enable the harness to be suspended from a frame (see Figure 1a). The animal holder was constructed in house by cutting out a semi-cylindrical section from a Perspex tube, 200mm diameter, ~8mm wall thickness. Circular Perspex discs were bonded to the open ends of the cylinder with holes drilled as appropriate to attach the support rod from which the harness was suspended, the implant-restraint clamp, and the tuning circuit (Figure 1).
Figure 1.
(a) Photograph of the customised animal holding device with animal in situ. (b) Schematic diagram of the chronically implanted combined surface coil and head fixation mechanism. (c) The tuning circuit, with capacitor values optimized for our imaging system shown. Matching was achieved with an externally adjustable variable capacitor (Polyflon, USA, 2-25pF). Tuning was achieved via an externally adjustable variable inductor constructed from 1mm copper wire wound twice on a 4mm diameter former, 0.8mm spacing and a 35mm long brass rotor (6BA), as illustrated.
The Cranial Implant, RF Coil And Tuning Circuit
The implant consisted of a small cylinder cut from a solid nylon tube (cylinder diameter 12mm, wall thickness 1mm). The RF coil was constructed by bonding a loop of copper foil to the base of the cranial implant and soldering this to gold pin connectors that protruded from the rear of the implant (Figure 1b). The tuning circuit was constructed in-house, according to the specifications detailed in Figure 1c: pilot work was undertaken to determine the optimum configuration of the circuit (e.g. capacitance, tuning range) based on the design of the implanted RF coil. Once complete, the tuning circuit was protected by encasing it in Perspex, with rods to enable manual tuning and matching protruding from the casing and out of the front of the animal holder.
Animal Training
Female Hooded Lister rats (n=4, 200-350g) were kept in a 12h dark/light cycle at a temperature of 22°C with food and water ad libitum. Animals were habituated to body restraint and head restraint as described previously for the use of optical imaging techniques in un-anaesthetized animals (6). In the present study, it was also necessary to acclimatise animals to the noise of the scanner environment. Briefly, animals were first exposed to gradually increasing periods of restraint in the body harness (2-45 minutes) which were combined with alternate and then concurrent periods of gradually increasing scanner noise amplitude (controlled by simply positioning animals closer to the magnet room and ultimately adjacent to the magnet itself). These training sessions were conducted once or twice daily for approximately two weeks prior to surgery. Surgical procedures were then conducted to implant the head fixation device and surface coil (see below) and after a period of post-operative recovery (approximately 4 days), animals were habituated to head restraint through training sessions conducted approximately twice each day over a period of 1 week, as described in Martin et al., (2002) (6). Animals were given a food reward after each restraint/training session.
Surgical Procedures
Surgical procedures were identical to those described previously (4) for the implantation of a head fixation device and optical imaging chamber in anaesthetized rats. Briefly, anaesthetized, homeothermically maintained animals were first implanted with subcutaneous stimulating microwires in the whisker pad which were attached to connectors fixed to the skull using dental cement (7). Using a dental drill, 2-4 small holes were made into the exposed skull into which nylon retaining skull screws was inserted. A layer of cyanoacrylate were then applied, engulfing the nylon screws and the implant was positioned on the skull surface and affixed using additional cyanoacrylate and a layer of dental cement. Care was taken to ensure there were no bubbles present in either the cyanoacrylate or dental cement, and that the set cement contained no sharp edges that could irritate the skin. All wounds were closed and between 0.5 and 1.0ml saline was injected sub-cutaneously to replace lost body fluids in addition to an analgesic (Rimadyl, 0.05ml, s.c.). Animals were prepared in pairs and subsequently caged together: we found this promoted recovery and ease of handling, with no problems such as animals removing each others’ stitches.
MRI Data Acquisition And Analysis
Once trained, animals were placed into the harness and animal holding apparatus and connections were made to the implanted RF coil and stimulating wires. The animal holder was then positioned in the magnet at a precise, predetermined location (established during the training sessions) which placed the RF coil in the magnet isocentre. The magnet used for all experiments was a 7 Tesla Bruker BioSpecAVANCE (310mm bore, MRI system B/C 70/30). The surface coil was tuned and matched to 300MHz using the MultlinkTM 1H preamplifier with a built-in tune/match display. The coils were connected to an active decoupling coil control unit operated from the console using a DC/pulse-in to ensure accurate transmit/receive times. For anatomical localization, high spatial resolution gradient echo scans were performed (256*256 pixels, FOV = 30mm, slice thickness = 1mm, TR = 1000ms, TE = 15ms, flip angle=90°, 2 averages). A transverse slice was used to identify the position of bregma from which a coronal scan 2mm posterior was taken to image the whisker barrel cortex: this position also used for all subsequent functional scans. Using this coronal section an oblique (tangential) slice was also identified through the somatosensory cortex to provide functional images. Functional data were acquired from the tangential and coronal slices using a single shot MBEST Gradient Echo - Echo Planar Imaging (GE-EPI) sequence (raw data matrix = 64*64, data sampling interval = 5μs, FOV = 30mm, slice thickness = 2mm, TR = 1000, TE = 12ms, flip angle 90o, 10 dummy scans). Read-out direction was left-right for both slice orientations. Standard phase correction (8) was used to minimise Nyquist ghosting. Imaging data were converted to thresholded z-score activation maps (see Figure 2) which enabled selection of regions of interest for extraction of time-series data (see Figures 2-5). MRI data acquisition sessions typically lasted less than 1 hour from initial installation of the animal into the holding apparatus to removal. Animals were imaged a maximum of twice each day and imaging was conducted on consecutive days.
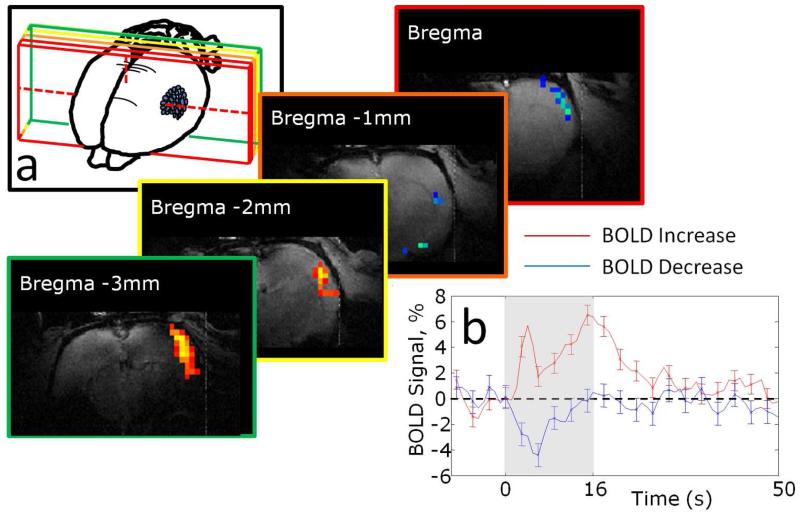
Figure 2.
Example BOLD fMRI activation map following whisker stimulation in awake rats. (a) Example activation maps showing positive and negative BOLD signal changes overlying somatosensory (barrel) cortex with positions of slices relative to bregma indicated. (b) Time series extracted from the positive and negative BOLD response regions shown in (a).
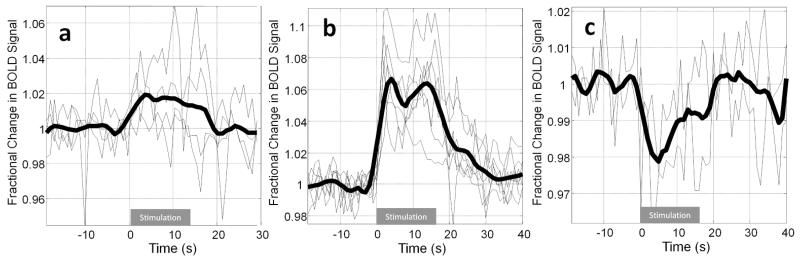
Figure 3.
Group BOLD fMRI response time series: (a) Mean responses recorded from somatosensory cortex to 10 repeats of a 5Hz, 16s stimulation of the contralateral whisker pad. Mean responses shown for 4 experimental sessions using 3 animals. Grand mean (smoothed) shown as emboldened line. (b) As (a) but with a stimulation frequency of 40Hz, 7 sessions from 3 animals. (c) Negative BOLD responses recorded from two animals following 40Hz stimulation from cortical regions adjacent to the positive BOLD response.
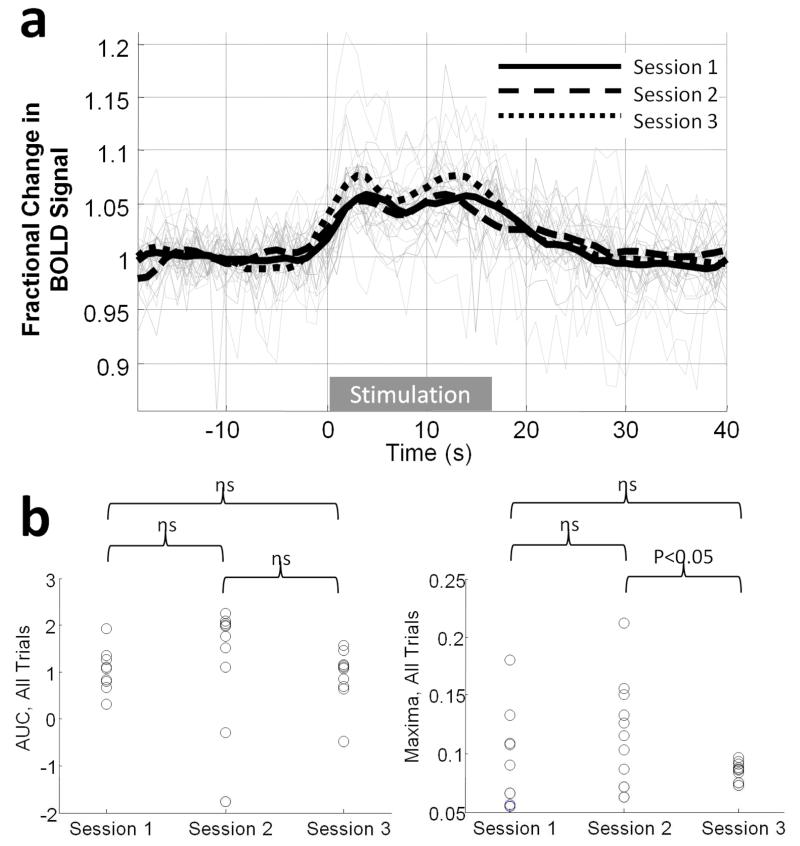
Figure 4.
Response consistency over individual 40Hz stimulus trials and over experimental sessions within a single animal. (a) All responses from three, 10-trial experimental sessions conducted on different days (grey lines) with session-averages overlayed (black lines). (b) Scatter plot of response magnitude for each trial over the three sessions in terms of either area under the curve (left) or maxima (right).
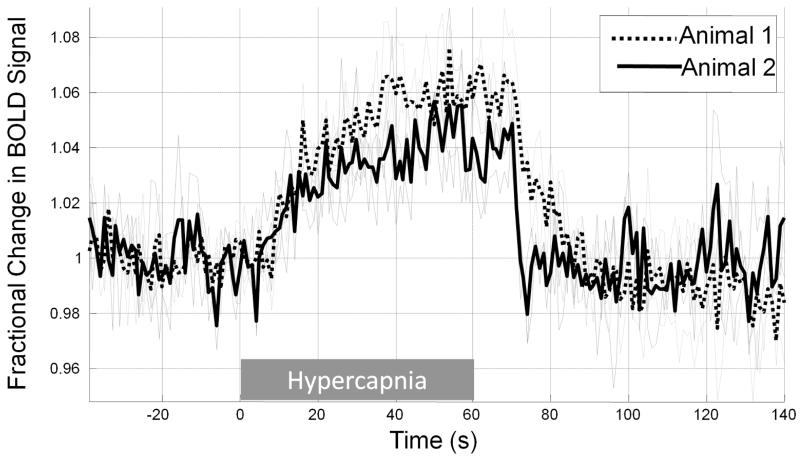
Figure 5.
Cortical BOLD response to hypercapnic challenge recorded from two animals. Data were acquired for 3, 60s epochs of ~5% CO2 in each animal. Responses to each epoch are shown in grey, with the mean response for each animal overlayed in black. Responses are displayed as fractional change in BOLD signal intensity with respect to a 30s pre-hypercapnia baseline.
Stimulation And Hypercapnia
The left whisker pad was electrically stimulated using a constant current stimulator device. In all cases the square wave stimulation pulses had a width of 0.3ms and the stimulation duration was 16s. Stimulation intensity was fixed at 0.4mA which has been shown to provide robust responses without any evidence of being noxious (3). Stimulation frequency was either 5Hz or 40Hz. Each experimental session consisted of 30 stimulation trials, with an inter-trial-interval of 40s, giving a total acquisition time of 1200s. All stimulus control was performed using a CED1401 (Cambridge Electronic Design Ltd, UK) which was stimulus locked to echo acquisition.
To elevate CO2 concentrations in inspired air to induce hypercapnia, 5% CO2 was added to a gentle air flow directed from a tube positioned 3cm from the front of the nose and mouth (4). Data were recorded from up to five trials, each trial consisting of 1 minute normal air mix, 1 minute of 5% CO2, 1 minute normal air, in addition to 1 minute of normal air at the beginning and end of each of these sessions.
RESULTS
Two widely used experimental paradigms for neuroimaging, somatosensory stimulation and hypercapnic challenge were employed to determine the effectiveness of the method described herein for obtaining functional brain imaging data from awake rats. Whisker stimulation elicited positive BOLD signal changes in somatosensory cortex (Figure 2 & 3), with an average (peak) magnitude of approximately 2% or 6% for the 5Hz and 40Hz stimulation conditions respectively (Figure 3a, b). In some animals negative BOLD signal changes in response to the 40Hz stimulation condition were also apparent in regions surrounding the sites of positive BOLD activation (Figure 3c). Figure 4a shows time-series from all 30 trials (40Hz stimulation) for a single animal, acquired in epochs of 10 trials over three separate experimental sessions (conducted on separate days): the mean response amplitude over these three experimental sessions shows a high level of consistency in terms of both response maxima and area under the curve (Figure 4b, c). A one-minute hypercapnic challenge (5% CO2 concentration) elicited widespread positive BOLD signal increases which peaked at approximately 5% and were sustained until the CO2 concentration was reduced to normal (air) levels whereupon BOLD signals returned rapidly to baseline (Figure 5).
The success rate of the techniques (determined as the proportion of trained animals from which) was high, with BOLD fMRI responses obtained under somatosensory stimulation conditions from all animals. Failure rates due to motion was low since a criterion for successful pre-imaging training was low levels of movement: less than 10% of imaging sessions were aborted or the data discounted due to high levels of motion. In one of our first two animals (trained and surgically prepared in parallel) implant removal occurred after over 10-days of successful head restraint habituation and/or experimental data collection, whereupon experiments were terminated and this animal euthanized. Additional skull screws and cyanoacrylate to more firmly bond the implant to the skull appeared to prevent this from re-occuring in other animals.
DISCUSSION
A small number of other laboratories have described alternative methodologies for conducting MRI studies in conscious rats (9-16). We distinguish our approach from these other solutions on the basis of 4 features of our method: (1) no use of anaesthetic or sedative agents to place the animal in the imaging apparatus; (2) animals were fully habituated to the imaging environment prior to the conduct of any experiments; (3) the restraint apparatus left whiskers and limbs free from obstruction in order to enable a wide range of sensory stimulation and the possibility of behavioural tasks being incorporated into the experimental paradigm; (4) chronic implantation of the (transmit/receive) radiofrequency (RF) coil to improve signal consistency across imaging sessions and reduce the time taken to setup for functional scanning.
An important consideration in this work is that of restraint-stress and we are mindful of the possibility of substituting the experimental confounds of anaesthetic agents with the (neuro)physiological effects of stress. As has been reported in previous work using optical imaging and electrophysiological techniques with the same animal training and restraint regimen as used in the present work, indications of stress were minimal and animals appeared to habituate well to the apparatus. Vocalisations and resistance to handling rapidly abated after initial training sessions and there were no indications of stress-related chromodacryorrhoea secretion (17) or abnormal behaviour associated with acute stress or that might precede development of learned helplessness. In work conducted in other laboratories using restrained rats for fMRI, physiological and corticosterone measurements indicate significant reductions in stress after 5-8 days of training (King et al., 2005). We did not measure physiological parameters in the current study in order to minimise the procedures the animals were exposed to and partly in recognition of the fact that un-anesthetized animals are better able to maintain homestasis (thus there is less cause for concern about an ‘un-physiological’ preparation than when using anaesthetized animals). We do however recognise that non-invasive monitoring (e.g. non-invasive pulse oximetry and blood pressure measurements) would be a useful addition to future work using this method.
There is a strong scientific case to develop methodology to enable functional neuroimaging experiments to be conducted in un-anaesthetized rats. Whilst neuroimaging studies in awake non-human primates continues to be important for research in some fields (18), we believe that by avoiding the confounding effects of anaesthesia using methods such as that reported here, rodent models could potentially be used in a wider range of neuroimaging research paradigms, including those found in behavioural neuroscience. Additionally, the likely volume of experimental studies that will be required in this area in order to keep pace with burgeoning applications of neuroimaging technology to study normal function, disease and drug effects, provides a strong motivation for the use of rodent models as the lowest vertebrate group with suitably comparative neurophysiology.
In conclusion, we report a novel methodological approach for acquiring BOLD fMRI data in awake rats. This methodology leaves the face and limbs free from obstruction, making possible a range of behavioural or sensory stimulation protocols. The observation of negative, as well as positive BOLD signal changes following somatosensory stimulation in the awake rat concurs with recent findings from neurovascular coupling studies using anaesthetized animals (19) and suggests the model reported herein may be wellEsuited to further investigation of negative BOLD signals. Further development of this un-anaesthetized animal model could enable traditional behavioural neuroscience techniques to be combined with modern functional neuroimaging.
ACKNOWLEDGEMENTS
This work was supported by the Royal Society (CM - University Research Fellowship) and the Medical Research Council (Research Grant). The authors would like to thank the technical staff of the laboratory (Department of Psychology, University of Sheffield): M. Simkins, M. Benn, N. Kennerley, M. Port, and A. Ham.
Grant Support: Royal Society University Research Fellowship (CM); MRC Grant G1002194 (JB).
REFERENCES
- 1.Chin CL, Tovcimak AE, Hradil VP, et al. Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI. British journal of pharmacology. 2008;153(2):367–379. doi: 10.1038/sj.bjp.0707506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Li R, Yang Z, Hudetz AG, Li SJ. Differential effect of isoflurane, medetomidine, and urethane on BOLD responses to acute levo-tetrahydropalmatine in the rat. Magn Reson Med. 2011 doi: 10.1002/mrm.23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. NeuroImage. 2006;32(1):33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, Jones M, Martindale J, Mayhew J. Haemodynamic and neural responses to hypercapnia in the awake rat. Eur J Neurosci. 2006;24(9):2601–2610. doi: 10.1111/j.1460-9568.2006.05135.x. [DOI] [PubMed] [Google Scholar]
- 5.Masamoto K, Fukuda M, Vazquez A, Kim SG. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci. 2009;30(2):242–250. doi: 10.1111/j.1460-9568.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin C, Berwick J, Johnston D, et al. Optical imaging spectroscopy in the unanaesthetised rat. Journal of neuroscience methods. 2002;120(1):25–34. doi: 10.1016/s0165-0270(02)00185-1. [DOI] [PubMed] [Google Scholar]
- 7.Devilbiss DM, Waterhouse BD. Determination and quantification of pharmacological, physiological, or behavioral manipulations on ensembles of simultaneously recorded neurons in functionally related neural circuits. Journal of neuroscience methods. 2002;121(2):181–198. doi: 10.1016/s0165-0270(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 8.Bruder H, Fischer H, Reinfelder HE, Schmitt F. Image reconstruction for echo planar imaging with nonequidistant k-space sampling. Magn Reson Med. 1992;23(2):311–323. doi: 10.1002/mrm.1910230211. [DOI] [PubMed] [Google Scholar]
- 9.Becerra L, Pendse G, Chang PC, Bishop J, Borsook D. Robust reproducible resting state networks in the awake rodent brain. PLoS One. 2011;6(10):e25701. doi: 10.1371/journal.pone.0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res. 2007;1135(1):186–194. doi: 10.1016/j.brainres.2006.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology. 2005;30(5):936–943. doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Imaging brain activity in conscious animals using functional MRI. Journal of neuroscience methods. 1998;82(1):75–83. doi: 10.1016/s0165-0270(98)00037-5. [DOI] [PubMed] [Google Scholar]
- 13.Liang Z, King J, Zhang N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci. 2011;31(10):3776–3783. doi: 10.1523/JNEUROSCI.4557-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peeters RR, Tindemans I, De Schutter E, Van der Linden A. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn Reson Imaging. 2001;19(6):821–826. doi: 10.1016/s0730-725x(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 15.Sicard K, Shen Q, Brevard ME, et al. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23(4):472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N, Rane P, Huang W, et al. Mapping resting-state brain networks in conscious animals. Journal of neuroscience methods. 2010;189(2):186–196. doi: 10.1016/j.jneumeth.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason G, Wilson D, Hampton C, Würbel H. Non-invasively assessing disturbance and stress in laboratory rats by scoring chromodacryorrhoea. Alternatives to Laboratory Animals. 2004;32(Suppl 1A) doi: 10.1177/026119290403201s25. [DOI] [PubMed] [Google Scholar]
- 18.Ku SP, Tolias AS, Logothetis NK, Goense J. fMRI of the Face-Processing Network in the Ventral Temporal Lobe of Awake and Anesthetized Macaques. Neuron. 2011;70(2):352–362. doi: 10.1016/j.neuron.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Boorman L, Kennerley AJ, Johnston D, et al. Negative Blood Oxygen Level Dependence in the Rat: A Model for Investigating the Role of Suppression in Neurovascular Coupling. Journal of Neuroscience. 2010;30(12):4285–4294. doi: 10.1523/JNEUROSCI.6063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]