Abstract
Aim
To evaluate the clinical utility of incorporating a novel heavy/light chain immunoassay (HLC) into the existing methods for the assessment of multiple myeloma (MM) patients.
Methods
Convenience sera samples from 90 previously treated IgG and IgA MM patients in different disease stages were analyzed. The study was conducted in Clinical Hospital Center Zagreb between 2011 and 2013. The collected sera were analyzed by standard laboratory techniques (serum protein electrophoresis, quantification of total immunoglobulins, serum immunofixation, serum free light chain [FLC] assay) and HLC assay.
Results
HLC ratios outside the normal range were found in 58 of 90 patients, including 28 out of 61 patients with total immunoglobulin measurements within the normal range and 5 out of 23 patients in complete response. Both elevated HLC isotype level and abnormal HLC ratio correlated with the parameters of tumor burden, including percentage of plasma cells in the bone marrow (P < 0.001 and P = 0.002, respectively) and an abnormal serum FLC ratio (for both P < 0.001). In addition, abnormal HLC isotype level correlated with serum beta-2-microglobulin level (P = 0.038). In terms of prognosis, abnormal HLC isotype level and abnormal HLC ratio were significantly associated with shorter overall survival (P < 0.001 and P = 0.002, respectively). Interestingly, suppression of the uninvolved (polyclonal) isotype pair, but not other non-myeloma immunoglobulin isotypes, was also associated with a shorter overall survival (P = 0.021). In a multivariate analysis, an abnormal HLC ratio and β2-microglobulin level >3.5mg/L were independent risk factors for survival.
Conclusion
The new HLC assay has greater sensitivity in detecting monoclonal protein, correlates with tumor burden markers, and affects patients' outcome.
Hematological malignancy multiple myeloma is characterized by the proliferation of monoclonal plasma cells in the bone marrow, which usually leads to the secretion of monoclonal intact immunoglobulins (Ig), free light chains (FLC), or both, into the serum. Consequently, the measurement of monoclonal proteins is integral to diagnosing multiple myeloma, monitoring of response to treatment, and detecting relapse (1-3). Conventional methods rely principally on serum electrophoresis together with either urine electrophoresis for the detection of FLC M-proteins or nephelometric quantification of total Igs for intact Ig paraprotein measurement. However, these standard techniques often do not have the required sensitivity or do not correlate with the patient’s clinical status (4-7).
The ability to detect monoclonal serum FLC has been substantially increased by the introduction of the serum FLC assay, improving the diagnosis, monitoring, and prognosis of a number of plasma cell dyscrasias (8,9). Still, a number of problems associated with the detection of monoclonal intact Igs persist. Serum Ig concentrations can change for ≥50% with fluctuations in blood volume and/or hematocrit (10). In the case of monoclonal IgGs, the serum concentration can be also influenced by altered metabolism due to recycling of IgG by the neonatal Fc receptors (11-13). Furthermore, monoclonal proteins, in particular IgA paraproteins, may not be accurately quantified by serum protein electrophoresis (SPE) due to co-migration with other serum proteins (14,15). Finally, one of the major limitations of measuring total Igs is the inability to distinguish between the monoclonal component and polyclonal Ig background.
These problems can be addressed by a recently developed heavy/light chain (HLC) immunoassay. Using specific antibodies targeted to junctional epitopes of the heavy and light chains of Ig molecules, HLC immunoassay separately quantifies different light chain types of each Ig class (IgGκ, IgGλ, IgAκ, IgAλ, etc), thus providing accurate quantification of both the involved and uninvolved components of the patient's affected Ig isotype. The ratio of the monoclonal and polyclonal Igs of the same isotype (IgGκ/IgGλ, IgAκ/IgAλ) provides a sensitive measure of monoclonality, while minimizing the influence of changes in blood volume/hematocrit and the effect of IgG recycling (16,17).
Previous studies using this novel assay in specific, controlled patient cohorts found the HLC ratio to be useful for screening, monitoring, and risk stratification of patients with multiple myeloma, but also with other monoclonal gammopathies, such as monoclonal gammopathy of undetermined significance (MGUS) and amyloid light-chain (AL) amyloidosis (18-20). The aim of this study was to evaluate the ability of the new assay – HLC isotype measurements – to detect monoclonal protein (ie, residual disease) in previously treated multiple myeloma patients and to determine whether these patients can benefit from adding the new assay into a routine assessment.
Patients and methods
The study included 90 unselected intact Ig multiple myeloma patients in different clinical stages (symptomatic disease) who had received at least one line of different treatment regiments (the number of patients was limited by availability of serum assays but also by the number of patients who were willing to enter the study). Written informed consent was obtained from each patient before entering the study. Patients were enrolled consecutively as they presented for routine assessment at our hospital between January 2011 and October 2013. At the time of the assessment, all patients already completed treatment regiments. The median follow-up period was 17.4 months (range 0.5-46.1 months; follow up period is defined as time elapsed from the day of the sampling until January 2015 when statistical analysis was performed). Median age was 61 years (range 39-84). A complete clinical and laboratory assessment was performed on convenience samples using standard laboratory techniques (SPE, nephelometric quantification of total immunoglobulins, serum immunofixation [IFE], and serum FLC assay) in addition to the novel HLC assay, complete blood count, and bone marrow analysis. Biochemical parameters – serum albumin, β2-microglobulin, serum creatinine, serum calcium levels, and lactate dehydrogenase were also measured. This study was approved by the Ethics Committee of the University Hospital Center Zagreb and Ethics Committee of the Medical School, University of Zagreb.
Serum HLC and FLC levels were measured using the polyclonal antisera assays Hevylite® and Freelite®, respectively (The Binding Site, Birmingham, UK) and performed on a Dade Behring BNII analyzer (Siemens AG, Munich, Germany) according to the manufacturer's instructions. Normal ranges for HLC isotypes and their ratios were obtained from the manufacturer's instructions. SPE and sIFE were performed on a Hydrasys platform (Sebia, Paris, France). Total Igs were measured using Tina-quant Gen.2 reagents (Roche, Rotkreuz, Switzerland) on a Cobas® 6000 platform (Roche) according to the manufacturer's instructions.
The data were analyzed using the R package (21). Subgroups of patients were compared using the Mann-Whitney test for continuous variables and the χ2 for categorical variables. Overall survival (OS) was estimated with the Kaplan-Meier method and the subgroups of patients were compared using the log-rank test. The association of time to death with relevant variables was evaluated in a multivariate analysis with the use of Cox’s proportional-hazard regression model. P values ≤0.05 were considered significant.
Results
A total of 66/90 (73%) patients had IgG myeloma (47 IgGκ and 19 IgGλ) and 24/90 (27%) had IgA myeloma (13 IgAκ and 11 IgAλ). According to international response criteria (22), at the moment of the study 67/90 patients had achieved a partial response or better (10 partial responses, 34 very good partial responses, and 23 complete responses), 1 patient showed minimal response, 10 patients had stable disease, 7 patients had progressive disease, and 5 patients were at relapse (Table 1).
Table 1.
Study population characteristics at re-evaluation. Values are shown as medians and range
| All | IgG patients | IgA patients | |
|---|---|---|---|
| Number of patients |
90 |
66 |
24 |
| Age (years) |
61 (39-84) |
61 (39-84) |
64 (45-84) |
| Sex (male/female) |
42/48 |
32/34 |
10/14 |
| Total immunoglobulin – standard method (g/L) |
10.48 (1.05-54.32) |
11.48 (2.85-54.32) |
4.76 (1.05-35.49) |
| Serum free light chain ratio |
1.08 (0.0-299.0) |
1.16 (0.0-299.0) |
0.75 (0-25.8) |
| β-2-microglobulin (mg/L) |
2.34 (1.3-11.15) |
2.3 (1.3-11.15) |
2.45 (1.70-9.78) |
| Albumin (g/L) |
41.8 (16.8-69.0) |
42.15 (30.6-69.0) |
39.2 (16.8-44.9) |
| Hemoglobin (g/L) |
125 (86-179) |
125 (86-149) |
125 (101-179) |
| Thrombocytes ( × 109/L) |
187 (43-567) |
190 (43-567) |
184 (99-378) |
| Creatinine (µmol/L) |
90 (47-256) |
90 (47-172) |
77 (51-256) |
| Serum calcium (mmol/L) |
2.34 (1.82-2.86) |
2.33 (1.97-2.79) |
2.43 (1.82-2.86) |
| Lactate dehydrogenase (U/L) |
182 (79-862) |
190 (79-862) |
158 (113-264) |
| Percentage of plasma cells |
6 (0-95) |
6 (0-70) |
7.5 (0-95) |
| International Staging System stage I / II / III (No.) |
53 / 34 / 3 |
36 / 29 / 1 |
17 / 5/ 2 |
| Complete response (No.) |
23 |
15 |
8 |
| Very good partial response (No.) |
34 |
28 |
6 |
| Partial response (No.) |
10 |
8 |
2 |
| MR/SD/PD/relapse (No.) | 1 /10 / 7 / 5 | 1 / 5 / 4 / 5 | 0 / 5 / 3 / 0 |
*MR – minimal response; SD – stable disease; PD – progressive disease.
The ability of the new assay to detect monoclonal immunoglobulin
One of the aims of our study was to determine the ability of HLC isotype measurement to detect monoclonal protein and to compare it with the standard test for Ig measurement. Standard test showed abnormal (elevated) total Ig measurement in 29/90 (32%) patients and Ig measurement within the normal range in 61/90 (68%) of patients. All patients with abnormal total Ig values according to standard test also had abnormal Ig values according to the new assay (abnormal involved HLC isotype value and/or abnormal HLC ratio). However, 28 (46%) of the 61 patients with normal Ig values according to the standard test (28/90 of total, 31%) had an abnormal HLC ratio, signifying the presence of monoclonal protein.
Relationship between HLC ratio and serum FLC ratio
Abnormal HLC ratio in the presence of normal FLC ratio revealed monoclonal protein in additional 4/23 (17%) patients in complete response, 8/34 (24%) patients with very good partial response, and 3/10 (30%) patients with stable disease (Table 2). In all patients with progressive disease, both assays were positive. HLC and FLC assays provided sometimes differing but complementary information, ie, in some patients both assays indicated the presence of monoclonal protein and in others only one of the assays did so. This was perhaps most notable in 8/23 patients who had achieved complete response (Table 2). Nevertheless, 2 of the 5 patients in complete response but with an abnormal HLC ratio suffered a relapse during the follow up period.
Table 2.
Results of heavy/light chain (HLC) and free light chain (FLC) assays in multiple myeloma patients in different disease stages
| Number of patients with: | ||||
|---|---|---|---|---|
| Results |
complete response
(N = 23) |
very good partial response
(N = 34) |
partial response (N = 10) |
stable disease (N = 10) |
| FLC κ/λ ratio*
and
HLC Ig´κ/Ig´λ ratio both normal |
14 |
12 |
0 |
0 |
| FLC κ/λ ratio
and
HLC Ig´κ/Ig´λ ratio both abnormal |
1 |
12 |
10 |
6 |
| FLC κ/λ ratio abnormal and
HLC Ig´κ/Ig´λ ratio normal |
4 |
2 |
0 |
1 |
| FLC κ/λ ratio normal and HLC Ig´κ/Ig´λ ratio abnormal | 4 | 8 | 0 | 3 |
*free κ/λ light chain ratio.
†heavy/light Ig’κ/Ig’λ chain ratio.
Disease variables and involved (monoclonal) HLC measurements
In order to assess whether the new assay truly reflected disease activity and provided good measurement of tumor burden (ie, residual disease), we tested the correlations between HLC measurements (involved HLC isotype values and HLC ratio) and other disease variables. Looking at the entire cohort, abnormal HLC ratio values correlated with the percentage of clonal plasma cells in the bone marrow (P = 0.002) and an abnormal serum FLC ratio (P < 0.001). Involved HLC isotype concentration above normal values correlated with serum beta-2-microglobulin values (P = 0.038), percentage of clonal plasma cells in the bone marrow (P < 0.001), and abnormal serum FLC ratio (P < 0.001).
Analyzing the data according to Ig isotype, abnormal IgGκ/IgGλ HLC ratios (normal range 0.98-2.75) correlated with the percentage of clonal plasma cells in the bone marrow (P = 0.020), abnormal serum FLC ratio (P < 0.001), and hemoglobin concentration (P = 0.032). Monoclonal IgGκ or IgGλ isotype concentrations above the normal upper limit (for IgGκ above 9.78 g/L and for IgGλ above 5.71 g/L) correlated with serum beta-2-microglobulin concentration (P = 0.011), percentage of clonal plasma cells in the bone marrow (P = 0.007), and abnormal serum FLC ratio (P = 0.002).
Abnormal IgAκ/IgAλ ratios (normal range 0.80-2.04) correlated with abnormal serum FLC ratio (normal range 0.26-1.65; P = 0.023) and serum beta-2-microglobulin concentration (P = 0.04), while correlation with the percentage of clonal plasma cells in the bone marrow was not as strong (P = 0.08). Monoclonal IgAκ or IgAλ isotype concentrations higher than normal upper limit (for IgAκ above 2.82 g/L and for IgAλ above 1.98 g/L) correlated with serum beta-2-microglobulin concentration >3.5 mg/L (P = 0.050) and abnormal serum FLC ratio (P = 0.005).
Disease variables and uninvolved (polyclonal) HLC isotype level
Correlations regarding the suppression of the polyclonal HLC isotype pair (ie, suppression of IgGλ in a IgGκ myeloma patient), were also assessed. Suppression was defined as an istoype pair value below normal range. In the entire group, there were significant correlations between isotype pair suppression and abnormal serum FLC ratio (P < 0.001), as well as hemoglobin concentration <100 g/L (P = 0.005). The same was found when looking at IgG group alone (abnormal serum FLC ratio, P < 0.001 and hemoglobin <100 g/L, P < 0.001), while in the IgA group there were no significant correlations between the suppressed polyclonal isotype and other tumor markers, possibly due to the small number of patients.
HLC measurements and overall survival
The aim of this study was to determine not only the ability of the new assay to detect monoclonal protein, ie, residual disease but also the clinical relevance of HLC assay measurement in multiple myeloma patients regardless of the type of treatment and time elapsed from the last course of therapy. For this purpose, Kaplan-Meier analysis was performed for OS of patients from the date of enrolment onwards. Patients were stratified according to HLC ratio values, and OS was significantly shorter in patients with abnormal HLC ratio value (median OS not reached, P < 0.001) (Figure 1A). When patients were stratified according to monoclonal isotype concentrations, OS was significantly shorter in patients with values above normal range (median 31.7 months vs median not reached, P = 0.002) (Figure 1B).
Figure 1.
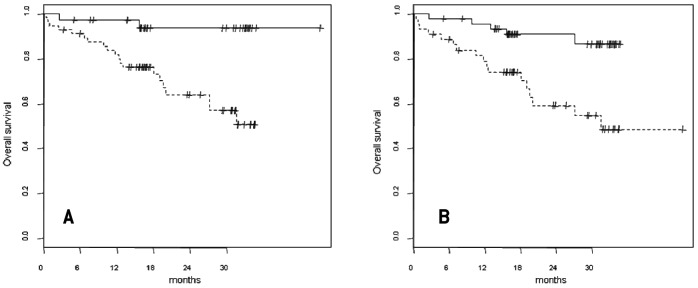
Overall survival in study population. (A) Patients stratified according to heavy/light chain ratio. Those with an abnormal value had significantly shorter overall survival (dotted line) than those with normal values (full line, P = 0.0019); median survival was not reached in either group. (B) Patients stratified according to monoclonal isotype absolute values. Median survival was 31.7 months in patients with monoclonal isotype values above normal (dotted line) and was not reached in patients with monoclonal isotype values with normal range (full line, P = 0.002).
Interestingly, when patients were stratified according to the presence or absence of suppression of the uninvolved polyclonal HLC isotype pair, those with isotype suppression also had significantly shorter OS (median 31 months vs median not reached, P = 0.021, Figure 2).
Figure 2.
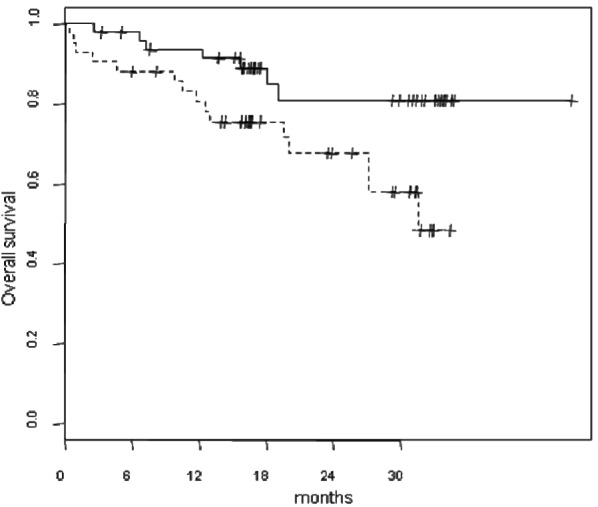
Overall survival in patients stratified according to polyclonal heavy/light chain (HLC) isotype pair suppression. Median overall survival was 31 months in patients showing suppressed polyclonal HLC isotypes (dotted line) and was not reached in patients without suppression (full line, P = 0.021).
The clinical relevance of the HLC assay was even more evident when Kaplan-Meier analysis was performed in patients who achieved very good partial responses (defined as negative SPE and positive IFE) and complete response (both SPE and IFE negative). When this subgroup of patients was stratified according to HLC ratio value, OS was significantly shorter in patients with abnormal HLC ratio values than in very good partial responses/complete response patients with normal HLC ratio (median 27.2 month vs median not reached, P = 0.033) (Figure 3).
Figure 3.
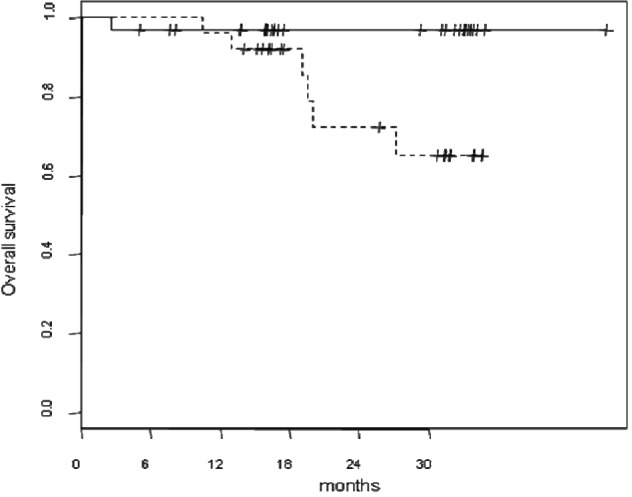
Overall survival in patients who achieved very good partial response/complete response stratified according to heavy/light chain ratio. Patients with an abnormal value had significantly shorter overall survival than those with normal values; median survival was 27.2 months (dotted line) vs not reached (full line, P = 0.002).
Prognostic significance of HLC measurements
In order to assess the prognostic significance of the new assay, Cox multivariate regression analysis was performed for the entire study population and for the group of patients who achieved very good partial response or better. An abnormal HLC ratio as well as high beta-2-microglobulin (>3.5 mg/L) concentration were independent predictors of OS for the entire cohort (risk ratio [RR] 4.7, confidence interval [CI] 0.96-23.47, P = 0.050; RR 4.9, CI 1.59-15.34, P = 0.005, respectively) (Table 3). When assessing the patients with very good partial response or better, the significance of abnormal HLC ratio was not as strong as for the entire group (RR 3.18, CI 0.84-12.04, P = 0.087).
Table 3.
Multivariate analysis showed abnormal heavy/light chain ratio to be an independent risk factor for overall survival
| Risk factor | Relative risk | 95% confidence interval | P |
|---|---|---|---|
|
β2-microglobulin >3.5 mg/L |
4.94 |
1.59-15.34 |
0.005 |
|
Abnormal heavy/light chain ratio* |
4.74 |
0.96-23.47 |
0.050 |
| Abnormal free light chain ratio |
3.29 |
0.93-11.69 |
0.064 |
| Serum albumin <35 g/L |
2.18 |
0.67-7.06 |
0.191 |
| International staging system (stage III) | 0.71 | 0.13-3.90 | 0.699 |
*Variables in bold denote statistically significant results (P ≤ 0.05).
Discussion
Detection of monoclonal immunoglobulin
Using HLC assay, we successfully detected isotype and quantified monoclonal Ig in all multiple myeloma patients. HLC assay had greater sensitivity for monoclonal disease detection than total Ig result. It revealed an abnormal HLC ratio in almost half of the patients with total Ig levels within normal range, signifying the presence of monoclonal protein. Moreover, an abnormal HLC ratio was found in almost 20% of complete response patients, thus detecting the presence of residual disease when other laboratory techniques could not detect it. In support of this, 2 of the 5 patients in complete response but with an abnormal HLC ratio suffered relapse during the follow up period. Similar observations of abnormal HLC ratios in patients in complete response have been reported previously (18,23).
The HLC and serum FLC assays provided differing but complementary information. This was most apparent in patients in complete response: 4 patients had abnormal serum FLC ratio and normal HLC ratio, whereas 4 patients had the opposite results. Knowing the metabolism of FLC and intact Igs, one would expect that the normalization of the FLC ratio will precede or occur simultaneously with the normalization of HLC ratio. However, our data showed that normal HLC ratio values did not necessarily mean normal FLC ratio values or vice versa. Similar observations were reported previously (18) and are consistent with the two assays providing information on two independent markers of myeloma activity – the intact Igs and the FLC (24,25). These data suggest that both assays are required for the optimal follow-up of multiple myeloma patients. In support of this, when response of myeloma patients to treatment was assessed, it was shown that in patients achieving complete response or better, patients with abnormal both HLC and FLC ratio had a significantly shorter 2-year progression free survival (PFS) than those with either normal HLC or FLC ratio (26).
Significance of the uninvolved HLC isotype pair suppression
Our results showing a strong association of suppression of the uninvolved HLC isotype pair with both markers of tumor burden (ie, FLC and hemoglobin) and shorter OS are particularly interesting. An analogous correlation of abnormal HLC ratios with shorter PFS in myeloma patients, particularly with more extreme HLC ratios, has been observed previously (27). Interestingly, this association has been shown to be driven primarily and specifically by suppression of the uninvolved polyclonal HLC isotype pair, while suppression of the non-tumor Igs of different isotypes showed no significant correlation with PFS. Similarly, HLC pair suppression has been shown to be an additional independent risk factor for progression to malignancy in MGUS patients alongside serum monoclonal protein size, heavy chain isotype, and an abnormal serum FLC ratio (20). The reason for the association of the outcome specifically with HLC pair-suppression in myeloma and MGUS remains unexplained. However, several studies in MGUS patients have noted higher levels of HLC pair suppression in IgG patients compared to IgA and IgM patients (20,28). An explanation for this is that due to relatively higher levels of background polyclonal IgG, which may help to prevent invasion of clonal plasma cells (29), suppression of normal IgG plasma cells may be a requirement for progression in IgG MGUS. Conversely, suppression of normal plasma cells may be less of a requirement for IgA MGUS progression and may help to explain the higher risk of progression shown by IgA MGUS patients (28). Interestingly, in multiple myeloma patients, we observed similar results of more frequent HLC pair suppression in IgG myeloma, with 35/66 (53%) IgG patients showing HLC pair suppression compared to 7/24 (29%) IgA patients, although the relative number of IgA patients was much lower. As multiple myeloma has been shown to be preceded consistently by MGUS (30,31), our data suggests that this possible differential requirement for isotype-specific pair suppression between IgG and IgA MGUS is maintained upon progression to multiple myeloma. Therefore, HLC pair suppression may be a useful marker of malignant disease, suggesting that it is useful to monitor the response of multiple myeloma patients to treatment.
HLC measurement values and impact on patients’ outcome
Despite the fact that the data in this study were obtained from a heterogeneous group of multiple myeloma patients, we found a strong correlation between an abnormal HLC ratio and other markers of tumor burden (the percentage of clonal plasma cells in the bone marrow and an abnormal serum FLC ratio). Given the statistical strength of the correlation, one might expect this to impact the patient outcome. Indeed, patients with abnormal level of monoclonal HLC isotype or abnormal HLC ratio had significantly shorter OS than patients with normal monoclonal HLC isotype level or HLC ratio. In the group of patients who achieved very good partial response or better, HLC ratio value maintained its impact on patients' outcome while monoclonal HLC isotype concentrations above normal range did not.
Prognostic significance of the HLC measurements
In order to further assess the clinical relevance of HLC assay in the management of previously treated multiple myeloma patients, we additionally performed multivariate Cox regression analysis for the entire group as well as for patients in very good partial response or better. The analysis for the entire cohort confirmed the prognostic relevance of the HLC ratio along with high β2-microglobulin values. Interestingly, the serum FLC ratio, which has been reported as predictive of outcome in several previous studies (32-35), was not significantly associated with OS in our study, most likely due to the fact that we did not include light chain multiple myeloma patients. The prognostic utility of HLC ratios in multiple myeloma using clinical presentation samples of untreated patients or patients recruited to clinical trials where all individuals received the same chemotherapeutic treatment followed by autologous stem cell transplantation (ASCT) treatment has recently been shown (18,27). However, in our study, patients were unselected and so were at different clinical stages of disease, received different chemotherapeutic regimes, and only a proportion had received ASCT treatment. Even in such a sample, we were able to evaluate the clinical utility of the HLC assay in everyday practice. Therefore, our finding that an abnormal HLC ratio, irrespective of disease stage and treatment regimen, is predictive of a poorer outcome in multiple myeloma is particularly noteworthy.
In conclusion, our results corroborate previous studies and show potential clinical utility of the HLC assay. In our study, the HLC ratio values correlated well with other prognostic tumor burden markers, showed enhanced sensitivity for the detection and quantification of monoclonal proteins, and affected patient outcome using a cohort of unselected patients in a daily practice setting. Interestingly, HLC pair suppression also correlated well with other tumor markers and was prognostic for shorter overall survival. Multivariate analysis confirmed an abnormal HLC ratio as an independent prognostic factor. Although larger studies using more defined groups of patients are required, our data suggest a clear role for HLC measurement in monitoring and response assessment of multiple myeloma patients with potential role in predicting patients’ outcome.
Acknowledgments
We thank The Binding Site Ltd for donating Hevylite® kits and Maritim d.o.o. for all the support and help in communication with The Binding Site Ltd
Funding This study was supported by a grant from the Ministry of Science, Education and Sports of the Republic of Croatia (project number 108-1081872-2061).
Ethical approval received from the Ethics Committee of the University Hospital Center Zagreb and Ethics Committee of the Medical School, University of Zagreb.
Declaration of authorship JB designed and supervised the study and wrote the manuscript. DN designed the study, performed patient follow-up, and reviewed the manuscript. ZP performed statistical analysis and reviewed the data. JL reviewed and revised the manuscript. SP, KD, DB, DSeg, LV, and DM performed the laboratory analysis. DSer, IR, SB-K performed patient follow-up and provided the clinical samples. BL reviewed the manuscript.
Competing interests JL is an employee of The Binding Site Group Ltd, which donated Hevylite® kits. All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Anderson KC, Alsina M, Bensinger W, Biermann JS, Chanan-Khan A, Cohen AD, et al. NCCN Clinical practice guidelines in oncology for multiple myeloma. J Natl Compr Canc Netw. 2011;9:1146–83. doi: 10.6004/jnccn.2009.0061. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San MJ, Chanan-Khan A, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701–5. doi: 10.1182/blood-2010-10-299529. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587–600. doi: 10.1200/JCO.2013.48.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding S, Young P, Di Fazio M, Hughes R. Intact immunoglobulin heavy/light chain paired assays. Biochim Clin. 2013;37:365–9. [Google Scholar]
- 5.Murray DL, Ryu E, Snyder MR, Katzmann JA. Quantitation of serum monoclonal proteins: relationship between agarose gel electrophoresis and immunonephelometry. Clin Chem. 2009;55:1523–9. doi: 10.1373/clinchem.2009.124461. [DOI] [PubMed] [Google Scholar]
- 6.Katzmann JA, Kyle RA, Benson J, Larson DR, Snyder MR, Lust JA, et al. Screening panels for detection of monoclonal gammopathies. Clin Chem. 2009;55:1517–22. doi: 10.1373/clinchem.2009.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzmann JA, Snyder MR, Rajkumar SV, Kyle RA, Therneau TM, Benson JT, et al. Long-term biologic variation of serum protein electrophoresis M-spike, urine M-spike, and monoclonal serum free light chain quantification: implications for monitoring monoclonal gammopathies. Clin Chem. 2011;57:1687–92. doi: 10.1373/clinchem.2011.171314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–24. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 9.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–9. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 10.Alexanian R. Blood volume in monoclonal gammopathy. Blood. 1977;49:301–7. [PubMed] [Google Scholar]
- 11.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology. 1996;89:573–8. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Hayton WL, Robinson JM, Anderson CL. Kinetics of FcRn-mediated recycling of IgG and albumin in human: pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin Immunol. 2007;122:146–55. doi: 10.1016/j.clim.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–8. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 14.Keren DF. Serum protein electrophoresis evaluation of monoclonal gammopathies (M-proteins). J Ligand Chem. 2005;27:218–26. [Google Scholar]
- 15.Katzmann JA, Clark R, Sanders E, Landers JP, Kyle RA. Prospective study of serum protein capillary zone electrophoresis and immunotyping of monoclonal proteins by immunosubtraction. Am J Clin Pathol. 1998;110:503–9. doi: 10.1093/ajcp/110.4.503. [DOI] [PubMed] [Google Scholar]
- 16.Bradwell AR, Harding SJ, Fourrier NJ, Wallis GL, Drayson MT, Carr-Smith HD, et al. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin kappa/lambda ratios. Clin Chem. 2009;55:1646–55. doi: 10.1373/clinchem.2009.123828. [DOI] [PubMed] [Google Scholar]
- 17.Keren DF. Heavy/light-chain analysis of monoclonal gammopathies. Clin Chem. 2009;55:1606–8. doi: 10.1373/clinchem.2009.132753. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig H, Milosavljevic D, Zojer N, Faint JM, Bradwell AR, Hubl W, et al. Immunoglobulin heavy/light chain ratios improve paraprotein detection and monitoring, identify residual disease and correlate with survival in multiple myeloma patients. Leukemia. 2013;27:213–9. doi: 10.1038/leu.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wechalekar A, Faint J, Lachmann H, Hawkins P, Harding S, Bradwell A. Significance of abnormal serum immunoglobulin heavy/light chain ratios (Hevylite) in 294 patients with systemic AL amyloidosis. Haematologica. 2011;96:392. [Google Scholar]
- 20.Katzmann JA, Clark R, Kyle RA, Larson DR, Therneau TM, Melton LJ, III, et al. Suppression of uninvolved immunoglobulins defined by heavy/light-chain pair suppression is a risk factor for progression of MGUS. Leukemia. 2013;27:208–12. doi: 10.1038/leu.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing 2011;ISBN: 3-900051-07-0. Available from: http://www.R-project.org. Accessed: May 11, 2015.
- 22.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakomy D, Lemaire-Ewing S, Denimal D, Bastie JN, Lafon I, Caillot D. Evaluation of the new Hevylite IgA assay for the diagnosis and follow-up of monoclonal gammopathies. Ann Biol Clin (Paris) 2013;71:157–63. doi: 10.1684/abc.2013.0805. [in French] [DOI] [PubMed] [Google Scholar]
- 24.Ayliffe MJ, Behrens J, Stern S, Sumar N. Association of plasma cell subsets in the bone marrow and free light chain concentrations in the serum of monoclonal gammopathy patients. J Clin Pathol. 2012;65:758–61. doi: 10.1136/jclinpath-2012-200669. [DOI] [PubMed] [Google Scholar]
- 25.Ayliffe MJ, Davies FE, de Castro D, Morgan GJ. Demonstration of changes in plasma cell subsets in multiple myeloma. Haematologica. 2007;92:1135–8. doi: 10.3324/haematol.11133. [DOI] [PubMed] [Google Scholar]
- 26.Drayson MT, Berlanga O, Plant T, Newnham NJ, Young P, Harding S. Immunoglobulin heavy/light chain measurements during monitoring provide prognostic information of relapse after therapy in myeloma patients. Blood. 2012;120:3964a. [Google Scholar]
- 27.Bradwell A, Harding S, Fourrier N, Mathiot C, Attal M, Moreau P, et al. Prognostic utility of intact immunoglobulin Ig'kappa/Ig'lambda ratios in multiple myeloma patients. Leukemia. 2013;27:202–7. doi: 10.1038/leu.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espino M, Medina S, Blanchard MJ, Villar LM. Involved/uninvolved immunoglobulin ratio identifies monoclonal gammopathy of undetermined significance patients at high risk of progression to multiple myeloma. Br J Haematol. 2014;164:752–5. doi: 10.1111/bjh.12679. [DOI] [PubMed] [Google Scholar]
- 29.Paiva B, Perez-Andres M, Vidriales MB, Almeida J, de las Heras N, Mateos MV, et al. Competition between clonal plasma cells and normal cells for potentially overlapping bone marrow niches is associated with a progressively altered cellular distribution in MGUS vs myeloma. Leukemia. 2011;25:697–706. doi: 10.1038/leu.2010.320. [DOI] [PubMed] [Google Scholar]
- 30.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–22. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rhee F, Bolejack V, Hollmig K, Pineda-Roman M, Anaissie E, Epstein J, et al. High serum-free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood. 2007;110:827–32. doi: 10.1182/blood-2007-01-067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snozek CL, Katzmann JA, Kyle RA, Dispenzieri A, Larson DR, Therneau TM, et al. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: proposed incorporation into the international staging system. Leukemia. 2008;22:1933–7. doi: 10.1038/leu.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, Sachanas S, Tzenou T, Papadogiannis A, et al. Prognostic value of serum free light chain ratio at diagnosis in multiple myeloma. Br J Haematol. 2007;137:240–3. doi: 10.1111/j.1365-2141.2007.06561.x. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor P, Gertz MA, Dispenzieri A, Lacy MQ, Dingli D, Hayman SR, et al. Importance of Achieving Sustained Stringent Complete Response (sCR) Following Autologous Stem Cell Transplantation in Multiple Myeloma. Blood. 2012;120:1988a. [Google Scholar]


