Abstract
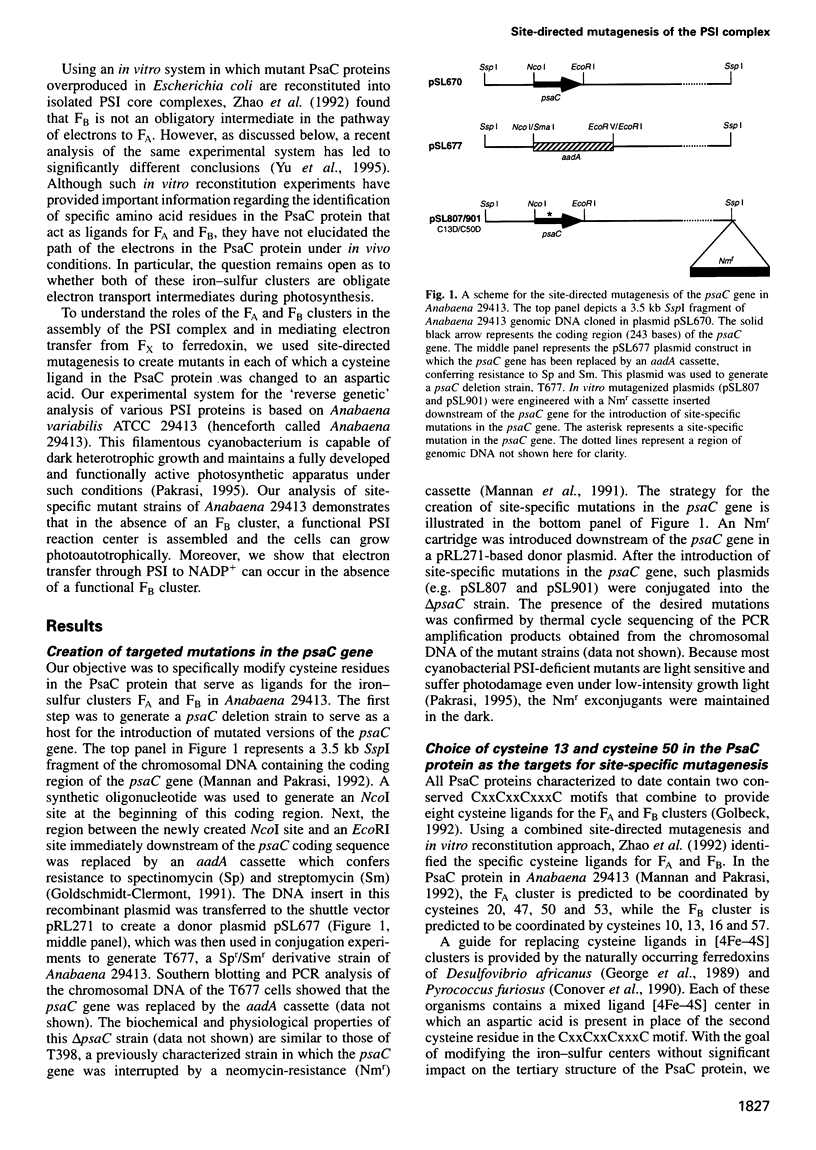
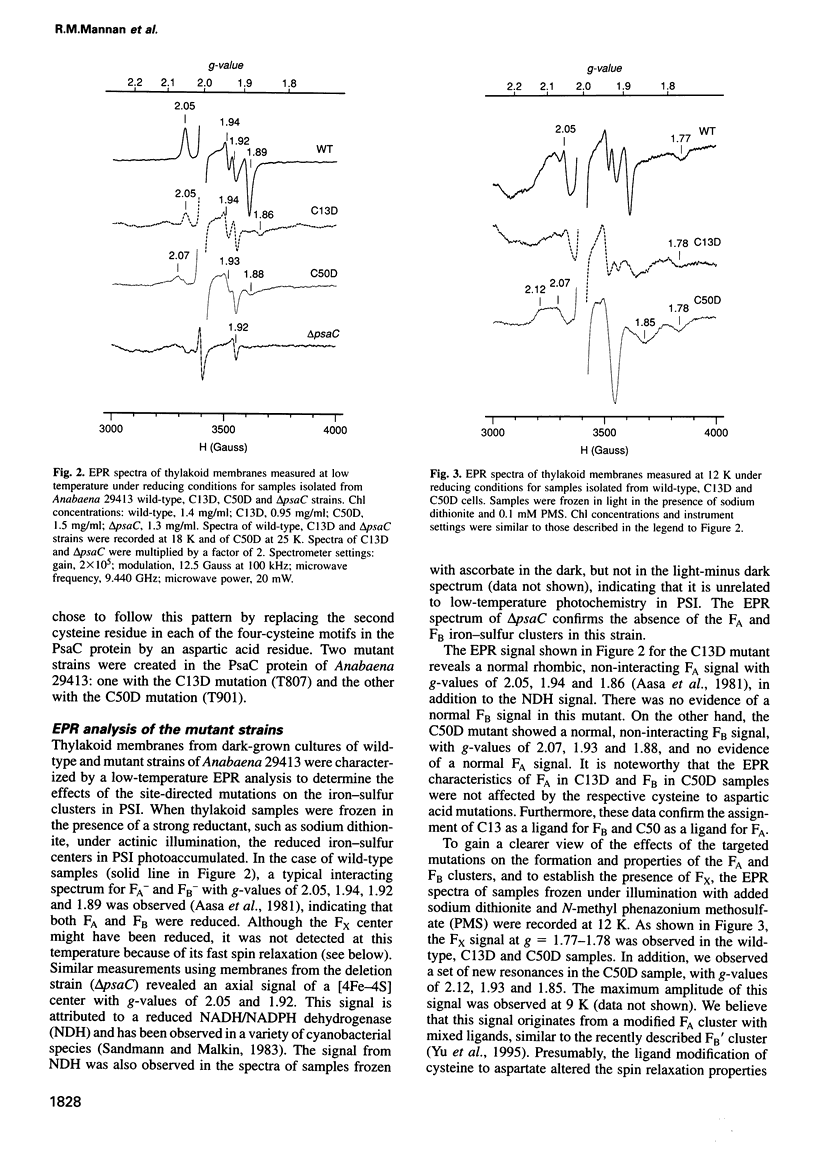
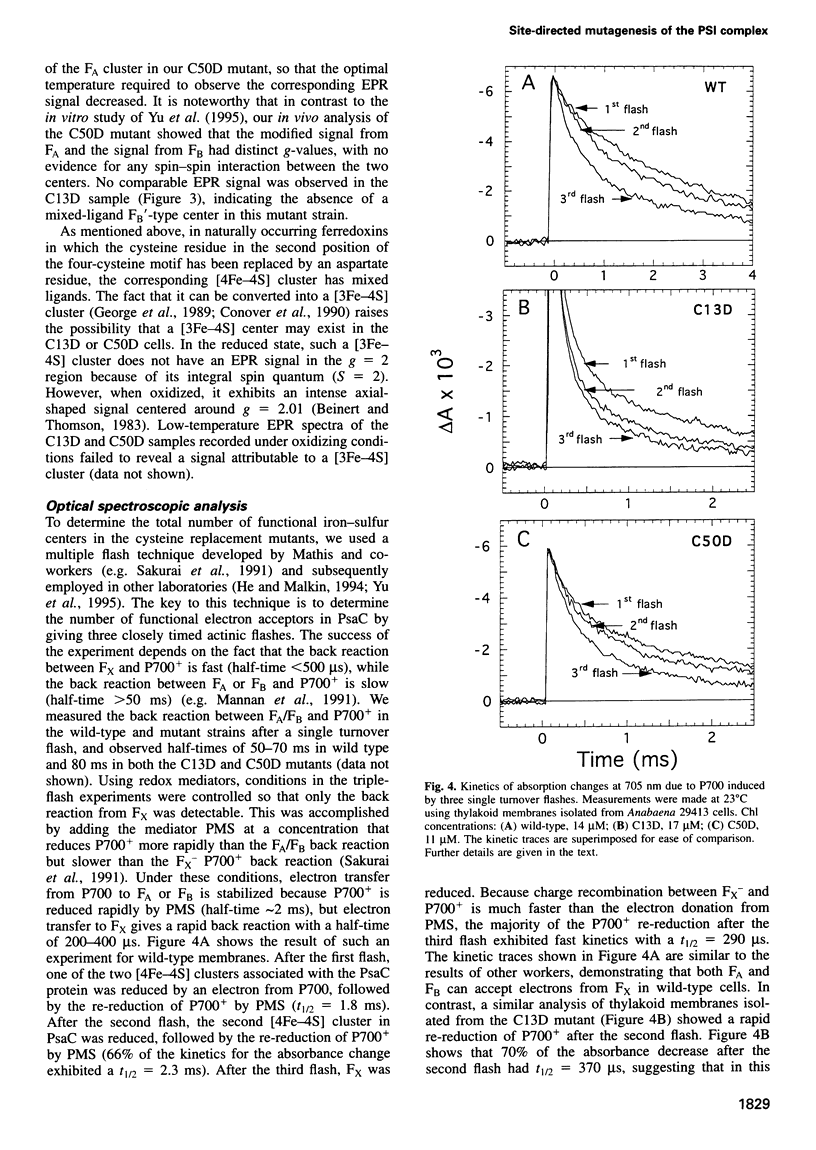
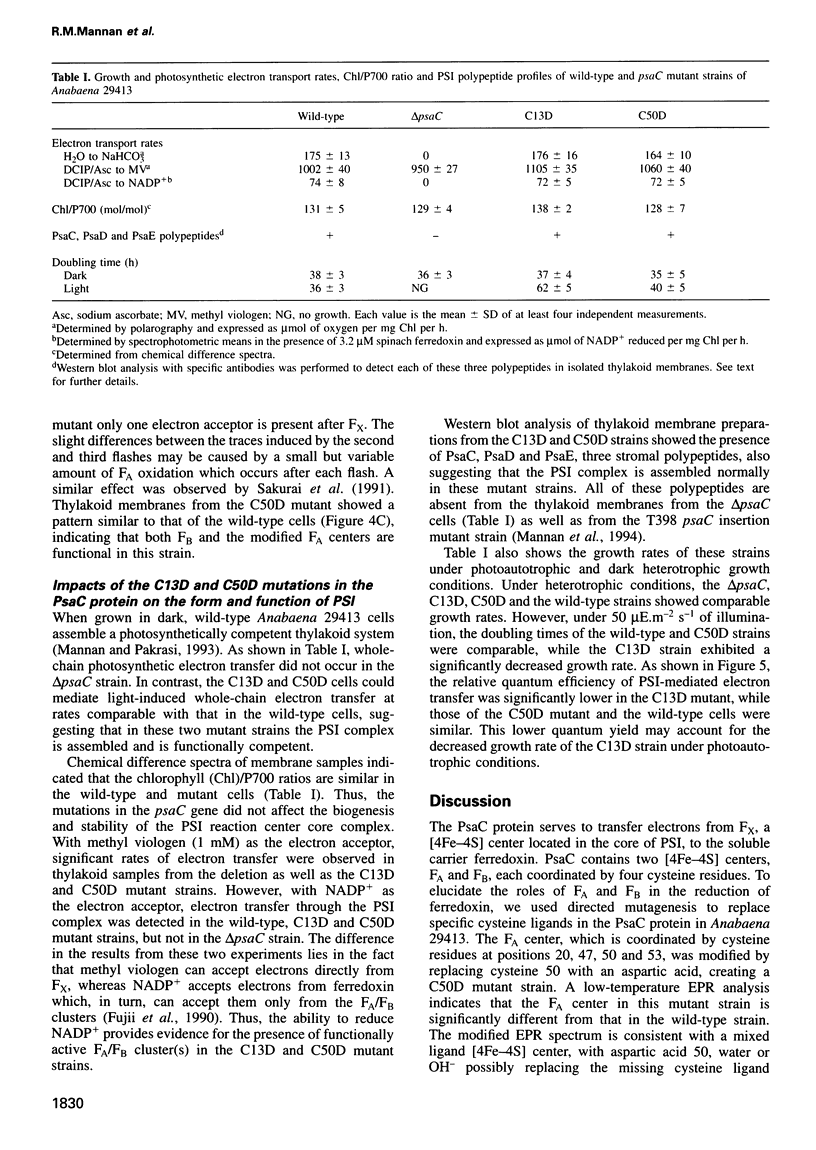
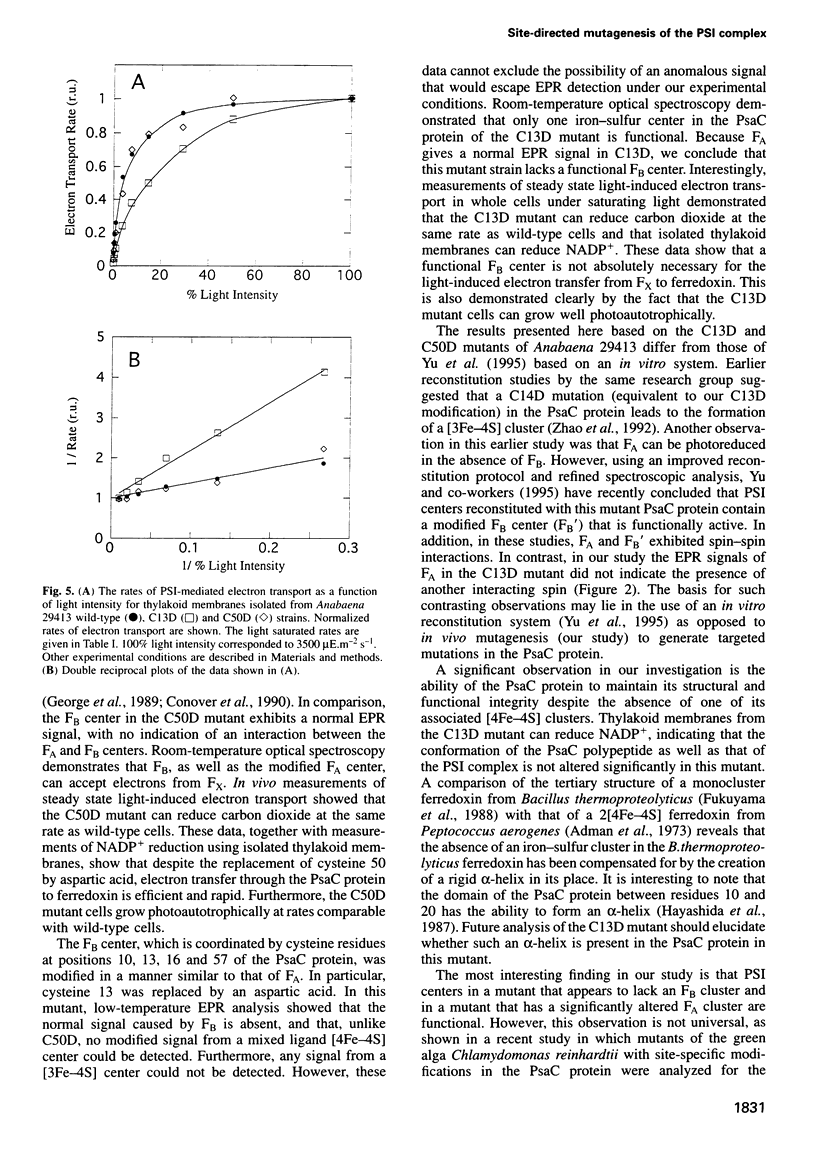
The PsaC protein of the Photosystem I (PSI) complex in thylakoid membranes coordinates two [4Fe-4S] clusters, FA and FB. Although it is known that PsaC participates in electron transfer to ferredoxin, the pathway of electrons through this protein is unknown. To elucidate the roles of FA and FB, we created two site-directed mutant strains of the cyanobacterium Anabaena variabilis ATCC 29413. In one mutant, cysteine 13, a ligand for FB was replaced by an aspartic acid (C13D); in the other mutant, cysteine 50, a ligand for FA was modified similarly (C50D). Low-temperature electron paramagnetic resonance studies demonstrated that the C50D mutant has a normal FB center and a modified FA center. In contrast, the C13D strain has normal FA, but failed to reveal any signal from FB. Room-temperature optical studies showed that C13D has only one functional electron acceptor in PsaC, whereas two such acceptors are functional in the C50D and wild-type strains. Although both mutants grow under photoautotrophic conditions, the rate of PSI-mediated electron transfer in C13D under low light levels is about half that of C50D or wild type. These data show that (i) FB is not essential for the assembly of the PsaC protein in PSI and (ii) FB is not absolutely required for electron transfer from the PSI reaction center to ferredoxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- Beinert H., Thomson A. J. Three-iron clusters in iron-sulfur proteins. Arch Biochem Biophys. 1983 Apr 15;222(2):333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- Conover R. C., Kowal A. T., Fu W. G., Park J. B., Aono S., Adams M. W., Johnson M. K. Spectroscopic characterization of the novel iron-sulfur cluster in Pyrococcus furiosus ferredoxin. J Biol Chem. 1990 May 25;265(15):8533–8541. [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Lord A. V. Evidence for the role of a bound ferredoxin as the primary electron acceptor of photosystem I in spinach chloroplasts. Biochim Biophys Acta. 1972 Jun 23;267(3):530–537. doi: 10.1016/0005-2728(72)90181-8. [DOI] [PubMed] [Google Scholar]
- Fukuyama K., Nagahara Y., Tsukihara T., Katsube Y., Hase T., Matsubara H. Tertiary structure of Bacillus thermoproteolyticus [4Fe-4S] ferredoxin. Evolutionary implications for bacterial ferredoxins. J Mol Biol. 1988 Jan 5;199(1):183–193. doi: 10.1016/0022-2836(88)90388-9. [DOI] [PubMed] [Google Scholar]
- George S. J., Armstrong F. A., Hatchikian E. C., Thomson A. J. Electrochemical and spectroscopic characterization of the conversion of the 7Fe into the 8Fe form of ferredoxin III from Desulfovibrio africanus. Identification of a [4Fe-4S] cluster with one non-cysteine ligand. Biochem J. 1989 Nov 15;264(1):275–284. doi: 10.1042/bj2640275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker of site-directed transformation of chlamydomonas. Nucleic Acids Res. 1991 Aug 11;19(15):4083–4089. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida N., Matsubayashi T., Shinozaki K., Sugiura M., Inoue K., Hiyama T. The gene for the 9 kd polypeptide, a possible apoprotein for the iron-sulfur centers A and B of the photosystem I complex, in tobacco chloroplast DNA. Curr Genet. 1987;12(4):247–250. doi: 10.1007/BF00435285. [DOI] [PubMed] [Google Scholar]
- Heathcote P., Williams-Smith D. L., Sihra C. K., Evans M. C. The role of the membrane-bound iron-sulphur centres A and B in the photosystem I reaction centre of spinach chloroplasts. Biochim Biophys Acta. 1978 Aug 8;503(2):333–342. doi: 10.1016/0005-2728(78)90192-5. [DOI] [PubMed] [Google Scholar]
- Ke B., Hansen R. E., Beinert H. Oxidation-reduction potentials of bound iron-sulfur proteins of photosystem I. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2941–2945. doi: 10.1073/pnas.70.10.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B. R., Blakesley R. W., Berg D. E. Linear amplification DNA sequencing directly from single phage plaques and bacterial colonies. Nucleic Acids Res. 1991 Mar 11;19(5):1153–1153. doi: 10.1093/nar/19.5.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldener I., Lockau W., Cai Y. P., Wolk C. P. Calcium-dependent protease of the cyanobacterium Anabaena: molecular cloning and expression of the gene in Escherichia coli, sequencing and site-directed mutagenesis. Mol Gen Genet. 1991 Jan;225(1):113–120. doi: 10.1007/BF00282649. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan R. M., Pakrasi H. B. Dark heterotrophic growth conditions result in an increase in the content of photosystem II units in the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Plant Physiol. 1993 Nov;103(3):971–977. doi: 10.1104/pp.103.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan R. M., Pakrasi H. B. Molecular Analysis of the psaC Gene Encoding the F(A)/F(B) Apoprotein of Photosystem I in the Filamentous Cyanobacterium Anabaena variabilis ATCC 29413. Plant Physiol. 1992 Feb;98(2):798–800. doi: 10.1104/pp.98.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan R. M., Pakrasi H. B., Sonoike K. The PsaC protein is necessary for the stable association of the PsaD, PsaE, and PsaL proteins in the photosystem I complex: analysis of a cyanobacterial mutant strain. Arch Biochem Biophys. 1994 Nov 15;315(1):68–73. doi: 10.1006/abbi.1994.1472. [DOI] [PubMed] [Google Scholar]
- Mannan R. M., Whitmarsh J., Nyman P., Pakrasi H. B. Directed mutagenesis of an iron-sulfur protein of the photosystem I complex in the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10168–10172. doi: 10.1073/pnas.88.22.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., De Ciechi P., Whitmarsh J. Site directed mutagenesis of the heme axial ligands of cytochrome b559 affects the stability of the photosystem II complex. EMBO J. 1991 Jul;10(7):1619–1627. doi: 10.1002/j.1460-2075.1991.tb07684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B. Genetic analysis of the form and function of photosystem I and photosystem II. Annu Rev Genet. 1995;29:755–776. doi: 10.1146/annurev.ge.29.120195.003543. [DOI] [PubMed] [Google Scholar]
- Sétif P., Ikegami I., Biggins J. Light-induced charge separation in Photosystem I at low temperature is not influenced by vitamin K-1. Biochim Biophys Acta. 1987 Nov 19;894(2):146–156. doi: 10.1016/0005-2728(87)90184-8. [DOI] [PubMed] [Google Scholar]
- Yu L., Bryant D. A., Golbeck J. H. Evidence for a mixed-ligand [4Fe-4S] cluster in the C14D mutant of PsaC. Altered reduction potentials and EPR spectral properties of the FA and FB clusters on rebinding to the P700-FX core. Biochemistry. 1995 Jun 20;34(24):7861–7868. doi: 10.1021/bi00024a010. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li N., Warren P. V., Golbeck J. H., Bryant D. A. Site-directed conversion of a cysteine to aspartate leads to the assembly of a [3Fe-4S] cluster in PsaC of photosystem I. The photoreduction of FA is independent of FB. Biochemistry. 1992 Jun 9;31(22):5093–5099. doi: 10.1021/bi00137a001. [DOI] [PubMed] [Google Scholar]



