Abstract
Background
Although studies suggest that omega-3 fatty acids intake may reduce cardiovascular disease (CVD) mortality risk, few studies have differentiated dietary eicosapentaenoic/docosahexaenoic acid (EPA/DHA) from alpha-linolenic acid (ALA), and epidemiological research in Asian populations is limited.
Methods and Results
The Singapore Chinese Health Study is a population-based cohort that recruited 63,257 Chinese adults aged 45-74 years from 1993 to 1998. Usual diet was measured at recruitment using a validated semi-quantitative food-frequency questionnaire, and mortality information was identified via registry linkage up to 31 December 2011. Cox proportional hazard models were used to calculate hazard ratios (HRs) with adjustment for potential confounders. We documented 4,780 total cardiovascular deaths (including 2,697 coronary heart disease [CHD] deaths and 1,298 stroke deaths) during 890,473 person-years of follow-up. Omega-3 fatty acids intake was monotonically associated with reduced risk of cardiovascular mortality. Compared to the lowest quartile, the HR [95% confidence interval (CI)] was 0.88 (0.81-0.96), 0.88 (0.80-0.97), and 0.83 (0.74-0.92) for the second, third and highest quartile, respectively (P-trend=0.003). Both EPA/DHA and ALA were independently associated with reduced risk of cardiovascular mortality: the HR (95% CI) comparing extreme quartiles was 0.86 (0.77-0.96; P-trend=0.002) and 0.81 (0.73-0.90; P-trend<0.001), respectively. The associations were similar for deaths from coronary heart disease and stroke, and persisted in participants who were free of CVD at baseline.
Conclusions
Higher relative intake of both marine (EPA/DHA) and plant (ALA) omega-3 fatty acids are associated with reduced risk of cardiovascular mortality in a Chinese population.
Keywords: epidemiology, cardiovascular diseases, mortality, nutrition, fatty acids
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide, and the prevalence and incidence has increased dramatically in the past several decades in Asian countries.1, 2 Among the many risk factors, diet plays an important role in the development and prognosis of CVD.3, 4 These is a long-standing interest in the plausible cardiovascular benefits of omega-3 fatty acids given the strong effects on several cardio-metabolic pathways (e.g., lipid profile, blood pressure, resting heart rate, endothelial function, platelet aggregation, inflammation, antiarrhythmic effects) from pre-clinical studies and short-term interventions.5 Although the results on nonfatal cardiovascular events are still conflicting and less well-established, the evidence from large prospective cohort studies and adequately powered clinical trials, however, has supported the notion that omega-3 fatty acids are associated with reduced risk of CVD mortality.5
There are two major types of omega-3 fatty acids from the diet: long-chain omega-3 fatty acids including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), which are mainly from marine-based foods, and alpha-linolenic acid (ALA, 18:3n-3) which is mainly the plant-derived omega-3 fatty acid found in seeds, nuts, and their oils. Mounting evidence has suggested that dietary intake of fish and EPA/DHA is associated with a lower risk of CVD mortality5-8, while it is less clear whether ALA also possesses the cardiovascular benefits, and the results from several prospective studies are mixed.9-14
The majority of the studies have been conducted in North American and European countries, and the studies of the association between omega-3 fatty acids and CVD risk in Asian populations are limited. Three cohort studies in Chinese men and women and one cohort study in Japanese adults have consistently found an inverse association between fish and EPA/DHA intake and risk of cardiovascular death; however, the results are not consistent with regard to coronary heart disease (CHD) or stroke mortality.15-17 Nevertheless, ALA was not examined in those studies. The plant oils have been widely used in Asian cooking, and legumes, soy and grains are major food items in the Asian diet. Thus, the ALA is a major source of omega-3 fatty acids, but no study has specifically examined the relation between ALA intake and risk of cardiovascular mortality in Asian population.
Therefore, to address the question of whether EPA/DHA or ALA has a beneficial association with cardiovascular mortality, we used data from the Singapore Chinese Health Study (SCHS), a population-based prospective cohort of approximately 63,000 Chinese men and women in Singapore. We hypothesized that both EPA/DHA and ALA were associated with a lower risk of cardiovascular death in the Chinese population. We further tested the hypothesis in CVD-free individuals (primary prevention) and people with CVD (secondary prevention) at baseline), as well as the relation with coronary heart disease (CHD) and stroke mortality separately.
METHODS
Study Population
The design of the SCHS has been described previously.18 Briefly, 35,303 Chinese women and 27,954 Chinese men aged 45–74 years of age were enrolled in this population-based cohort study between April 1993 and December 1998. The study participants were restricted to two major dialect groups in Singapore, which are the Hokkiens and the Cantonese who originated from Fujian and Guangdong provinces in Southern China. During the enrolment period, all the study participants were residents of government housing estates, where 86% of the Singapore population resided at the time of recruitment. This study was approved by the Institutional Review Board at the National University of Singapore, and all enrolled subjects were given informed consent.
At recruitment, information on demographics, lifestyle factors (physical activity, tobacco use, and alcohol intake), usual diet, and medical history was obtained through in-person in-interviews using structured questionnaires. We excluded individuals who had self-reported cancer at baseline (n=1,936) or who reported extreme sex-specific energy intakes (<600 or >3000 kcal/d for women and <700 or >3700 kcal/d for men; n=1,023). The current analysis included 60,298 participants.
Assessment of Diet and Covariates
A semi-quantitative food-frequency questionnaire (FFQ) including 165 commonly consumed food items in this population was administered during the baseline interview. The respondents were instructed to select from 8 food-frequency categories (ranged from “never or hardly ever” to “two or more times a day”) and three portion sizes (small, median, large) with the aid of photographs. Relevant to this study, the food frequency questionnaire listed 14 seafood items, including fresh fish (fish ball or cake, deep fried fish, pan or stir fried fish, boiled or steamed fish), fresh shellfish (shrimp or prawn, squid or cuttlefish), dried/salted fish (salted fish, ikan bilis, dried fish, other dried seafoods such as dried shrimp, dried oyster, dried cuttlefish), and canned fish (canned tuna, canned sardine). The FFQ had been validated subsequently using 24-hour recalls and re-administration of the FFQ among a subset of 810 participants from this cohort.18 The validation study by these two methods showed similar distributions with most mean pairs for energy and nutrients within 10% of each other’s values. The correlation coefficient by these two methods for each dietary component ranged between 0.24 and 0.79, which is comparable with previous validation study in diverse populations.19 The dietary intake of each nutrient was derived based on the Singapore Food Composition Database, which has been described in detail previously.18 This database was developed specifically for this cohort study and listed approximately 100 nutritional and non-nutritional values per 100 g of the edible raw and cooked foods. The main dietary sources of marine omega-3 fatty acids (EPA and DHA) were fish and seafood, while the main dietary sources of nonmarine omega-3 (ALA) were grains (21% of omega-3 intake), cooking oils (11%), and legumes and soy (9%). Overall, marine-based omega-3 (EPA/DHA) accounted for approximately 36% of population omega-3 intake, and nonmarine-based omega-3 (ALA) made up 64%. Other known or suspected risk factors for CVD assessed with the baseline questionnaire included age, educational level, smoking status, and physical activity. Body mass index (BMI, in kg/m2) was calculated by body weight in kg divided by square of height in meter. Participants also self-reported their history of medical conditions diagnosed by physicians, including diabetes, hypertension, coronary heart disease and stroke.
Assessment of Mortality
Information on date and cause of death was obtained through linkage analysis with the nationwide registry of birth and death in Singapore. Primary cause of death was used for analysis. Vital status for cohort participants was updated through December 31, 2011. As of December 31, 2011, only 47 subjects from this cohort were known to be lost to follow-up due to migration out of Singapore or for other reasons. This suggests that emigration among participants is negligible in this cohort and that vital statistics in follow-up is virtually complete. Underlying causes of death were coded according to the International Classification of Diseases, Ninth Revision; codes 390-459 were used for cardiovascular deaths, codes 410 414 for CHD deaths, codes 430-438 for stroke deaths.
Statistical Analysis
Person-years for each participant were calculated from the date of recruitment to the death or December 31, 2011, whichever came first. All the nutrients, including omega-3 fatty acids, were adjusted for energy using the residual method.20 Cox proportional hazards were used to examine associations between omega-3 fatty acids intake and CVD mortality risk. In the multivariate model, we adjusted for age (continuous), sex, interview year (1993-1995, 1996-1998), dialect group (Hokkien, Cantonese), cigarette smoking (years of smoking and number of cigarettes per day), alcohol frequency (never or monthly, weekly, daily), level of education (none, primary school, secondary school or more), physical activity level (no, 0.5-3.0 hours/wk, ≥4.0 hours/wk), BMI (<20.0, 20.0-23.9, 24.0-27.9, ≥28.0 kg/m2), history of comorbidities at baseline (diabetes, hypertension, CHD and stroke) and total energy intake (continuous). In the final model, we additionally adjusted for dietary variables, including dietary fiber, protein, and all fat subtypes (i.e., omega-6 fatty acids, monounsaturated fat, and saturated fat), all in quartiles. In the analysis of EPA/DHA or ALA, they were included in the model simultaneously. To examine linear trend, median intake values of each quartile was entered as a continuous variable in the model.
Stratified analysis was made a priori by self-reported history of physician diagnosed CHD and stroke at recruitment. The rationale is to test the hypothesis in primary prevention, i.e. in participants without CVD history) and secondary prevention (patients with CVD). We further stratified the analysis in participants with or without baseline history of diabetes/hypertension in the primary prevention setting to evaluate the association persisted in the high-risk group. All the statistical analyses were conducted using SAS 9.1 (SAS Institute Inc, Cary, NC), with 2-sided P value less than 5% as statistical significance.
RESULTS
Table 1 shows the baseline characteristics according to quartiles of total omega-3 fatty acids intake. Participants in the highest quartile of total omega-3 fatty acids intake were slightly younger, more likely to be women, attained higher level of education, and were less likely to be smokers. They also had a higher prevalence of hypertension, diabetes and coronary heart disease. Positive association with total omega-3 fatty acids was found with dietary fiber and other fatty acids. No significant differences were found for physical activity and BMI. Similar pattern was found for intake of marine and nonmarine-based omega-3 fatty acids (Supplementary Table 1).
TABLE 1.
Participant Characteristics According to Quartiles of Omega-3 Fatty Acid Intake in the Singapore Chinese Health Study
| Characteristics | Quartiles of total omega-3 fatty acids
|
|||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| n | 15181 | 15023 | 15023 | 15072 |
| Age, y | 56.8 ± 8.0 | 56.8 ± 8.1 | 56.4 ± 8.0 | 55.6 ± 7.8 |
| Female sex, n (%) | 5987 (39.4) | 8767 (58.4) | 9472 (63.0) | 9238 (61.3) |
| Cantonese Dialect, n (%) | 7453 (49.1) | 6823 (45.4) | 6673 (44.4) | 6974 (46.3) |
| Education more than secondary school, n (%) | 4025 (26.5) | 3997 (26.6) | 4001 (26.6) | 5157 (34.2) |
| Ever smoker, n (%) | 6304 (41.5) | 4500 (30.0) | 3914 (26.1) | 3670 (24.4) |
| Weekly/daily alcohol drinker, n (%) | 2865 (18.9) | 1562 (10.4) | 1236 (8.2) | 1346 (8.9) |
| Hypertension, n (%) | 3332 (22.0) | 3490 (23.2) | 3675 (24.5) | 3792 (25.2) |
| Diabetes, n (%) | 1052 (6.9) | 1274 (8.5) | 1457 (9.7) | 1570 (10.4) |
| Coronary heart disease, n (%) | 548 (3.6) | 607 (4.0) | 629 (4.2) | 681 (4.5) |
| Stroke, n (%) | 211 (1.4) | 212 (1.4) | 233 (1.6) | 232 (1.5) |
| Weekly moderate activity (%) | ||||
| No | 12037 (79.3) | 11854 (78.9) | 11773 (78.4) | 11297 (75.0) |
| 0.5-3.0 hours/week | 2034 (13.4) | 2006 (13.4) | 2018 (13.4) | 2342 (15.5) |
| ≥4.0 hours/week | 1110 (7.3) | 1162 (7.7) | 1232 (8.2) | 1433 (9.5) |
| Body mass index, kg/m2 | 23.0 ± 3.3 | 23.1 ± 3.3 | 23.2 ± 3.2 | 23.3 ± 3.3 |
| Total energy, kcal/d | 1725 ± 553 | 1426 ± 466 | 1414 ± 465 | 1625 ± 521 |
| Saturated fatty acids,* g/d | 12.8 ± 4.7 | 15.6 ± 3.7 | 16.6 ± 3.7 | 17.1 ± 4.8 |
| Monounsaturated fatty acids,* g/d | 12.1 ± 3.8 | 14.8 ± 2.86 | 15.9 ± 2.9 | 16.6 ± 3.7 |
| Total omega-3 fatty acids,* g/d | 0.59 ± 0.12 | 0.79 ± 0.04 | 0.92 ± 0.04 | 1.26 ± 0.33 |
| Marine omega-3 fatty acids,* g/d | 0.19 ± 0.10 | 0.28 ± 0.09 | 0.35 ± 0.10 | 0.46 ± 0.18 |
| Non-marine omega-3 fatty acid,* g/d | 0.40 ± 0.11 | 0.51 ± 0.09 | 0.56 ± 0.10 | 0.80 ± 0.36 |
| Omega-6 fatty acids,* g/d | 5.8 ± 2.5 | 7.5 ± 2.3 | 8.3 ± 2.5 | 9.9 ± 3.5 |
| Fiber,* g/d | 11.0 ± 4.3 | 12.6 ± 3.7 | 13.0 ± 3.6 | 14.1 ± 4.1 |
The data are expressed as n (%) or mean ± standard deviation.
The nutrients were adjusted for energy using the residual methods.
We documented a total of 4780 total cardiovascular deaths (including 2697 coronary heart disease [CHD] deaths and 1298 stroke deaths) during 890,473 person-years of follow-up. In the multivariate model, omega-3 fatty acid intake was monotonically associated with reduced risk of cardiovascular deaths, even after adjustment for established factors for CVD and other dietary variables. Compared to the lowest quartile, the HR [95% confidence interval (CI)] was 0.88 (0.81-0.96), 0.88 (0.80-0.97), and 0.83 (0.74-0.92) for the second, third and highest quartile, respectively (P-trend=0.003; Table 2). When dietary marine (EPA/DHA) and nonmarine-based (ALA) omega-3 fatty acids were examined independently, both types were associated with reduced risk of cardiovascular mortality: the HR (95% CI) comparing extreme quartiles was 0.86 (0.77-0.96) for EPA/DHA (P-trend=0.004) and 0.81 (0.73-0.90) for ALA (P-trend<0.001). We also tested the joint association between marine-based and nonmarine-based omega-3 fatty acids with cardiovascular mortality (Figure 1 and Supplemental Table 2): compared to those who were in the lowest tertiles of both omega-3 fatty acids, individuals in the highest tertiles had 33% (HR 0.67; 95% CI 0.57-0.78) lower risk. The P value for multiplicative interaction was not significant (P-interaction=0.75).
TABLE 2.
Relative Risk (95% Confidence Interval) of Cardiovascular Mortality According to Quartile of Omega-3 Fatty Acids Intake
| Quartiles Quartile of Omega-3 Fatty Acids Intake
|
P for trend* | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Total omega-3 intake | |||||
| Cases/person-years | 1329/220747 | 1200/220839 | 1196/223184 | 1055/225703 | |
| Multivariate Model 1† | 1.00 | 0.90 (0.83-0.98) | 0.93 (0.86-1.00) | 0.88 (0.81-0.95) | 0.006 |
| Multivariate Model 2‡ | 1.00 | 0.88 (0.81-0.96) | 0.88 (0.80-0.97) | 0.83 (0.74-0.92) | 0.003 |
| Marine omega-3 (EPA + DHA) intake | |||||
| Cases/person-years | 1236/219336 | 1233/220994 | 1188/225056 | 1123/225088 | |
| Multivariate Model 1† | 1.00 | 0.99 (0.92-1.08) | 0.96 (0.89-1.04) | 0.95 (0.88-1.03) | 0.16 |
| Multivariate Model 2‡ | 1.00 | 0.96 (0.89-1.05) | 0.90 (0.82-0.99) | 0.86 (0.77-0.96) | 0.004 |
| Non-marine omega-3 (ALA) intake | |||||
| Cases/person-years | 1342/222500 | 1267/221695 | 1156/221955 | 1015/224323 | |
| Multivariate Model 1† | 1.00 | 0.95 (0.88-1.03) | 0.89 (0.82-0.97) | 0.84 (0.77-0.91) | <0.001 |
| Multivariate Model 2‡ | 1.00 | 0.94 (0.86-1.02) | 0.87 (0.79-0.95) | 0.81 (0.73-0.90) | <0.001 |
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ALA, a-linolenic acid.
Linear trend was tested by assigning to participants the median value in each quartile and assessing this as a continuous variable.
Multivariate model 1 adjusted for age, sex, dialect, year of interview, educational level, body mass index, physical activity, smoking status, alcohol use, baseline history of self-reported diabetes, hypertension, coronary heart disease, stroke, and total energy.
Multivariate model 2 further adjusted for intakes of protein, dietary fiber, monounsaturated fat, saturated fat, omega-6 fatty acids, and alternate omega-3 fatty acids (in the analysis of EPA/DHA or ALA).
Figure 1. Relative Risk (95% Confidence Interval) of Total Cardiovascular Mortality According to Joint Distribution of Marine and Non-marine Omega-3 Fatty Acids Intake*.
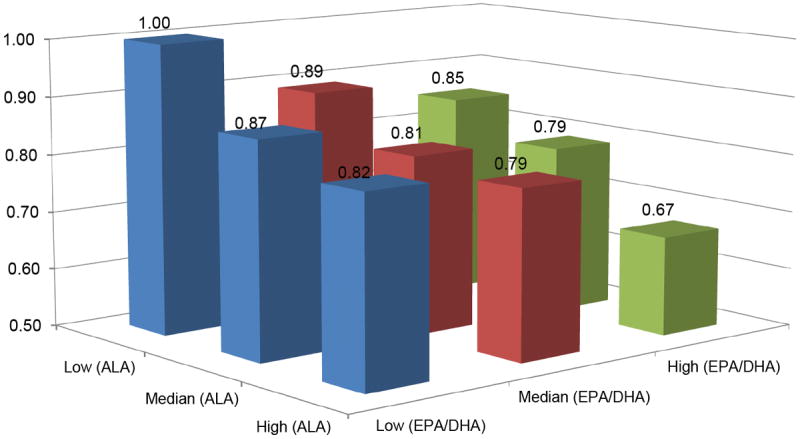
* Multivariate model adjusted for age, sex, dialect, year of interview, educational level, body mass index, physical activity, smoking status, alcohol use, baseline history of self-reported diabetes, hypertension, coronary heart disease, stroke, and intakes of total energy, protein, dietary fiber, monounsaturated fat, saturated fat, and omega-6 fatty acids.
With regard to CHD mortality (Table 3), both marine- and non-marine omega-3 fatty acids alone or in combination were all inversely associated with a lower risk: the HR (95% CI) comparing extreme quartiles was 0.85 (0.73-0.98) for total omega-3 fatty acids (P-trend=0.04), 0.86 (0.74-0.99) for EPA/DHA (P-trend=0.02), and 0.82 (0.71-0.93) for ALA (P-trend=0.001). For stroke mortality, a trend towards inverse associations were found for total, marine and non-marine omega-3 fatty acids, but none of these associations reached statistical significance (Table 3).
TABLE 3.
Relative Risk (95% Confidence Interval) of Ischemic Heart Disease Mortality and Stroke Mortality According to Quartile of Omega-3 Fatty Acids Intake
| Quartiles of fatty acid intake
|
P for trend* | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Coronary Heart Disease Mortality | |||||
| Total omega-3 intake | |||||
| Cases/person-years | 726/220747 | 678/220839 | 675/223184 | 618/225703 | |
| Multivariate Model† | 1.00 | 0.92 (0.81-1.03) | 0.92 (0.80-1.03) | 0.85 (0.73-0.98) | 0.04 |
| Marine omega-3 (EPA + DHA) intake | |||||
| Cases/person-years | 680/219336 | 689/220994 | 700/225056 | 628/225088 | |
| Multivariate Model† | 1.00 | 0.99 (0.88-1.10) | 0.97 (0.85-1.09) | 0.86 (0.74-0.99) | 0.02 |
| Non-marine omega-3 (ALA) intake | |||||
| Cases/person-years | 730/222500 | 733/221695 | 651/221955 | 583/224323 | |
| Multivariate Model† | 1.00 | 1.01 (0.90-1.13) | 0.90 (0.79-1.01) | 0.82 (0.71-0.93) | 0.001 |
| Stroke Mortality | |||||
| Total omega-3 intake | |||||
| Cases/person-years | 373/220747 | 330/220839 | 320/223184 | 275/225703 | |
| Multivariate Model† | 1.00 | 0.86 (0.72-1.01) | 0.84 (0.70-1.01) | 0.82 (0.66-1.01) | 0.10 |
| Marine omega-3 (EPA + DHA) intake | |||||
| Cases/person-years | 345/219336 | 343/220994 | 297/225056 | 313/225088 | |
| Multivariate Model† | 1.00 | 0.96 (0.82-1.12) | 0.82 (0.68-0.98) | 0.91 (0.74-1.12) | 0.28 |
| Non-marine omega-3 (ALA) intake | |||||
| Cases/person-years | 382/222500 | 324/221695 | 316/221955 | 276/224323 | |
| Multivariate Model† | 1.00 | 0.81 (0.68-0.95) | 0.81 (0.68-0.97) | 0.81 (0.67-0.99) | 0.07 |
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ALA, a-linolenic acid.
Linear trend was tested by assigning to participants the median value in each quartile and assessing this as a continuous variable.
Multivariate model adjusted for age, sex, dialect, year of interview, educational level, body mass index, physical activity, smoking status, alcohol use, baseline history of self-reported diabetes, hypertension, coronary heart disease, stroke, intakes of total energy, protein, dietary fiber, saturated fat, monounsaturated fat, omega-6 fatty acids, and alternate omega-3 fatty acids (in the analysis of EPA/DHA or ALA).
When stratified the analysis by self-reported history of physician-diagnosed CHD or stroke at recruitment, significant and similar associations were found among individuals who were free of CVD at baseline (Table 4). Among individuals who were free of CVD at baseline, the HR (95% CI) comparing extreme quartiles was 0.83 (0.73-0.94) for total omega-3 fatty acids (P-trend=0.006), 0.84 (0.74-0.95) for EPA/DHA (P-trend=0.002), and 0.81 (0.72-0.90) for ALA (P-trend<0.001). However, among participants with prior history of CVD, the reduction in risk was attenuated and did not reach statistical significance, although an inverse relationship was still generally present. The P value for interaction was not significant for any of the omega-3 fatty acids (all P-interaction>0.10). A similar pattern was observed for CHD mortality (Supplementary Table 3) and the associations were slightly stronger in people without CVD at baseline. For stroke mortality (Supplementary Table 4), there was a trend towards a lower risk, but the association was not statistically significant for neither EPA/DHA nor ALA. Within the population without a prior history of CVD, the associations were similar between individuals with or without baseline diabetes/hypertension (Table 5), and no significant interaction was found (all P-interaction>0.10).
TABLE 4.
Primary versus Secondary Prevention: Relative Risk (95% Confidence Interval) of Cardiovascular Mortality According to Quartiles of Omega-3 Fatty Acids Intake, Stratified by Self-reported Coronary Heart Disease (CHD) or Stroke at Baseline
| Quartiles of fatty acid intake
|
P for trend* | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Total omega-3 intake | |||||
| No prior history of CHD or stroke | |||||
| Cases/person-years | 1123/212118 | 996/211477 | 974/213135 | 827/214944 | |
| Multivariate Model† | 1.00 | 0.89 (0.81-0.98) | 0.88 (0.79-0.98) | 0.83 (0.73-0.94) | 0.006 |
| Prior history of CHD or stroke | |||||
| Cases/person-years | 206/8630 | 204/9362 | 222/10049 | 228/10759 | |
| Multivariate Model† | 1.00 | 0.84 (0.68-1.04) | 0.90 (0.71-1.14) | 0.85 (0.66-1.10) | 0.35 |
| Marine omega-3 (EPA + DHA) intake | |||||
| No prior history of CHD or stroke | |||||
| Cases/person-years | 1039/210412 | 1021/211538 | 947/215052 | 913/214673 | |
| Multivariate Model† | 1.00 | 0.94 (0.86-1.03) | 0.87 (0.79-0.96) | 0.84 (0.74-0.95) | 0.002 |
| Prior history of CHD or stroke | |||||
| Cases/person-years | 197/8924 | 212/9456 | 241/10004 | 210/10415 | |
| Multivariate Model† | 1.00 | 1.00 (0.81-1.22) | 1.03 (0.83-1.29) | 0.92 (0.71-1.19) | 0.47 |
| Non-marine omega-3 (ALA) intake | |||||
| No prior history of CHD or stroke | |||||
| Cases/person-years | 1130/212813 | 1033/212376 | 966/212984 | 791/213501 | |
| Multivariate Model† | 1.00 | 0.91 (0.83-1.00) | 0.89 (0.80-0.98) | 0.81 (0.72-0.90) | <0.001 |
| Prior history of CHD or stroke | |||||
| Cases/person-years | 212/9687 | 234/9319 | 190/8971 | 224/10822 | |
| Multivariate Model† | 1.00 | 1.08 (0.88-1.32) | 0.85 (0.68-1.06) | 0.87 (0.69-1.10) | 0.14 |
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ALA, a-linolenic acid.
Linear trend was tested by assigning to participants the median value in each quartile and assessing this as a continuous variable.
Multivariate model adjusted for age, sex, dialect, year of interview, educational level, body mass index, physical activity, smoking status, alcohol use, baseline history of self-reported diabetes, hypertension, intakes of total energy, protein, dietary fiber, saturated fat, monounsaturated fat, omega-6 fatty acids, and alternate omega-3 fatty acids (in the analysis of EPA/DHA or ALA).
The P for interaction with baseline history of CHD/stroke was 0.24 for total omega-3 fatty acids, 0.13 for marine omega-3 fatty acids, and 0.91 for non-marine omega-3 fatty acids.
TABLE 5.
Relative Risk (95% Confidence Interval) of Cardiovascular Mortality According to Quartiles of Omega-3 Fatty Acids Intake Among Participants without Coronary Heart Disease or Stroke, Stratified by Self-reported Diabetes or Hypertension at Baseline
| Quartiles of fatty acid intake
|
P for trend* | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Total omega-3 intake | |||||
| No prior history of diabetes and hypertension | |||||
| Cases/person-years | 647/164398 | 530/160521 | 491/158334 | 387/156595 | |
| Multivariate Model† | 1.00 | 0.90 (0.79-1.02) | 0.89 (0.76-1.03) | 0.84 (0.74-1.00) | 0.08 |
| Prior history of diabetes or hypertension | |||||
| Cases/person-years | 476/47720 | 466/50956 | 483/54801 | 440/58349 | |
| Multivariate Model† | 1.00 | 0.89 (0.77-1.02) | 0.85 (0.72-0.99) | 0.80 (0.67-0.96) | 0.03 |
| Marine omega-3 (EPA + DHA) intake | |||||
| No prior history of diabetes and hypertension | |||||
| Cases/person-years | 582/161961 | 523/159177 | 499/160863 | 451/157847 | |
| Multivariate Model† | 1.00 | 0.91 (0.81-1.03) | 0.88 (0.76-1.01) | 0.86 (0.72-1.02) | 0.08 |
| Prior history of diabetes or hypertension | |||||
| Cases/person-years | 457/48451 | 498/52361 | 448/54188 | 462/56825 | |
| Multivariate Model† | 1.00 | 0.96 (0.84-1.10) | 0.81 (0.70-0.94) | 0.81 (0.68-0.96) | 0.005 |
| Non-marine omega-3 (ALA) intake | |||||
| No prior history of diabetes and hypertension | |||||
| Cases/person-years | 631/163532 | 545/159599 | 512/160322 | 367/156395 | |
| Multivariate Model† | 1.00 | 0.97 (0.85-1.10) | 0.99 (0.86-1.14) | 0.84 (0.72-0.99) | 0.04 |
| Prior history of diabetes or hypertension | |||||
| Cases/person-years | 499/49281 | 488/52776 | 454/52662 | 424/57106 | |
| Multivariate Model† | 1.00 | 0.84 (0.73-0.97) | 0.80 (0.69-0.93) | 0.75 (0.64-0.89) | 0.003 |
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ALA, a-linolenic acid.
Linear trend was tested by assigning to participants the median value in each quartile and assessing this as a continuous variable.
Multivariate model adjusted for age, sex, dialect, year of interview, educational level, body mass index, physical activity, smoking status, alcohol use, intakes of total energy, protein, dietary fiber, saturated fat, monounsaturated fat, omega-6 fatty acids, and alternate omega-3 fatty acids (in the analysis of EPA/DHA or ALA).
The P for interaction with baseline history of diabetes/hypertension was 0.21 for total omega-3 fatty acids, 0.41 for marine omega-3 fatty acids, and 0.81 for non-marine omega-3 fatty acids.
DISCUSSION
In this large cohort study of Chinese men and women, intake of omega-3 fatty acids (both marine and nonmarine-based sources) was independently associated with reduced risk of cardiovascular disease mortality. Inverse associations were also found for deaths from CHD and stroke with intake of total omega-3 fatty acids and ALA, although the association between EPA/DHA intake and stroke mortality did not reach statistical significance. The associations were observed after full adjustment for established risk factors for CVD and other dietary and lifestyle confounders for both types of omega-3 fatty acids and the lowest risk was observed in the subjects with high intake for both.
Our results are largely consistent with previous prospective cohort studies on intake of long-chain omega-3 fatty acids and cardiovascular death8, 21. There are several cohort studies in the Asian populations and the results were generally consistent. In a cohort of 18,224 Chinese men in Shanghai, Yuan et al.17 reported that men in the highest quintile of EPA/DHA intake had almost 57% lower risk of mortality from myocardial infarction (n=187 cases), but not stroke (n=480 cases). In a cohort of 57,972 Japanese men and women, Yamagishi et al.16 found that participants in the highest quintile of total omega-3 fatty acids intake had 19% lower risk of cardiovascular death (n=2,045 cases), particularly mortality from heart failure (n=307 cases), but not stroke (n=972 cases). In a recent cohort of 134,296 Chinese men and women in Shanghai, Takata et al.15 observed that women in the highest quintile of EPA/DHA intake had 22% lower risk of cardiovascular mortality (n=1,090 cases), but not in men (n=699 cases). In this study, EPA/DHA intake was not related to hemorrhagic stroke, and the association with ischemic stroke was only evident in the highest quintile but not the other groups.15 Compared to those studies, the current analysis has the largest number of cardiovascular deaths (n=4,780), and we found that EPA/DHA was inversely associated with risk of cardiovascular deaths and mortality from CHD, but not with stroke mortality.
Compared to marine omega-3 fatty acids, studies on the relation between ALA intake and CVD are limited. A recent meta-analysis of 6 prospective cohort studies found that adults in the top tertile had a 20% lower risk of cardiovascular death compared to the bottom tertile,22 although the association was not significant in some of the included studies. This is probably due to the small sample size and limited power (the number of outcomes ranged from 78 to 829).22 Again, our study is the largest so far on the association between ALA intake and cardiovascular deaths (n=4780), and the first report in the Asian population. Our analysis is also one of the few studies that investigated both marine and nonmarine-based omega-3 fatty acids in the same cohort. We found that both types of omega-3 fatty acids were associated with a lower risk, which is consistent with the Cardiovascular Health Study23. One study suggested that ALA intake may particularly reduce CHD risk when marine-based omega-3 fatty acids intake is low24, but in the current analysis, the association appeared to be independent and no significant multiplicative interaction was found, this is consistent with results from the Nurses’ Health Study for the sudden cardiac death.25
The association between EPA/DHA with stroke is less consistent, with modest benefits found in observation cohort studies, but not in clinical trials.26 In our analysis, the association between EPA/DHA with stroke mortality was not significant, which is consistent with studies in Chinese men in Shanghai,17 and Japanese men and women.16 The recent report in a large cohort in Chinese men and women in Shanghai15 found an inverse relation between EPA/DHA with stroke mortality; however, the significant association was only observed in the fifth quintile, but not in others, compared to the first quintile. The observed inverse association between ALA with stroke mortality is in line with a cohort study in The Netherlands where participants in the second to the fifth quintiles had a 35–50% lower risk compared with the reference group.27 A nested case-control study of 96 cases and 96 controls found inverse association between serum concentrations of ALA and risk of stroke in US adults.28 However, null association was also reported in other studies in Western population.29-31 No study has been conducted to investigate the relation of ALA intake and risk of stroke risk in Asian population.
Recently, several clinical trials have been conducted to investigate whether omega-3 fatty acids supplementation reduces risk of cardiovascular events in the secondary prevention setting. A recent meta-analysis of EPA/DHA supplementation found modest but not statistically significant reductions in cardiac death (9% reduction, 13 trials) and sudden death (13% reduction, 7 trials).32 The results were quite mixed: significant reduction in cardiovascular death was found in some early trials,33, 34 but not in several recent trails.35, 36 The recent Alpha Omega Trial35 in 4,837 post-myocardial infarction patients was a 2-by-2 factorial design with 2 g ALA or 400 mg EPA/DHA as the interventions, and no significant effect was found for either EPA/DHA or ALA. In the primary prevention, the recent ORIGIN37 trial randomly assigned 12,536 patients who were at high risk for cardiovascular events (individuals who had impaired fasting glucose, impaired glucose tolerance, or diabetes) to receive 900 mg EPA/DHA intervention or olive oil control. No significant reduction was found for cardiovascular death after 6 years of intervention. However, the use of olive oil in the control group may mask the benefits from omega-3 fatty acids. Furthermore, as the authors acknowledged, participants in the trials were taking more concomitant cardio-protective therapies and may lead to a failure to detect significant benefit with omega-3 fatty acids.37 Indeed, as criticized by Mozaffarian and Wu,5 the statistical power was limited in all the clinical trials. No clinical trial has been done in healthy individuals (those without CVD, diabetes or hypertension) because of low feasibility. Therefore, evidence from both clinical trials and high-quality prospective cohort studies on the topic should be considered as evidence for providing dietary recommendations. The risk reduction among patients with existing CVD events in our study was not significant, although the trend was towards a lower risk and no significant interaction was found. Among individuals without CVD at baseline, our results indicate a similar association in those with or without diabetes/hypertension at baseline, suggesting that omega-3 fatty acids confer cardiovascular benefits in both healthy and high-risk groups.
The strengths of our study include the high response and follow-up rate, detailed collection of data through face-to-face interviews, nearly complete mortality assessment with objectively obtained records on time and cause of death. The dietary intake was assessed by a FFQ that was specifically developed and validated in this population, and has been shown to be internally consistent and reproducible. We are also aware of several limitations. First, the dietary intake was assessed by self-report, and some levels of measurement error were inevitable. However, this would most likely result in non-differential misclassification with respect to disease status and likely underestimation of risk. Second, the self-reported lifestyle-related data and baseline comorbidity status may result in some misclassification and residual confounding. Periodic assessment of such data would have allowed us to examine the potential reported changes in these factors in relation to the outcomes. The residual and unmeasured confounding, as in any observational studies, is still possible. Third, we do not have comprehensive data on incident nonfatal cardiovascular events in the cohorts, and future efforts should be made to validate and confirm the nonfatal cases. Finally, due to the observational study nature, causality should be made with caution.
CONCLUSION
Our results provide supportive evidence that high dietary intake of both marine and nonmarine-based omega-3 fatty acids is associated with reduced risk of cardiovascular death in the Chinese population, particularly for deaths from coronary heart disease and in individuals without cardiovascular disease at baseline. Further studies are still needed to evaluate the association with incident nonfatal cardiovascular events, and confirm our results in other Asian populations.
Supplementary Material
Acknowledgments
We thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study. Finally, we acknowledge the founding, longstanding principal investigator of the Singapore Chinese Health Study, Mimi C. Yu.
Sources of Funding
This work was supported by the Singapore National Medical Research Council (NMRC/EDG/0011/2007), and the United States National Institutes of Health (RO1 CA055069, R35 CA053890, RO1 CA080205, RO1 CA098497, and RO1 CA144034). The funder had no role in the design and conduct of the study; collection; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Disclosures
None.
References
- 1.Ohira T, Iso H. Cardiovascular disease epidemiology in Asia. Circ J. 2013;77:1646–1652. doi: 10.1253/circj.cj-13-0702. [DOI] [PubMed] [Google Scholar]
- 2.Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, Vos T, Wang H, Lopez AD, Murray CJ. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118:230–237. doi: 10.1161/CIRCULATIONAHA.108.771881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR., Jr Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:647–654. doi: 10.3945/ajcn.2009.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 6.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 7.Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–372. doi: 10.1002/clc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Wolk A, Colditz GA, Hennekens CH, Willett WC. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. 1999;69:890–897. doi: 10.1093/ajcn/69.5.890. [DOI] [PubMed] [Google Scholar]
- 10.Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200:177–182. doi: 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]
- 11.Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ. 1996;313:84–90. doi: 10.1136/bmj.313.7049.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, Virtamo J. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol. 1997;145:876–887. doi: 10.1093/oxfordjournals.aje.a009047. [DOI] [PubMed] [Google Scholar]
- 13.Oomen CM, Ocke MC, Feskens EJ, Kok FJ, Kromhout D. alpha-Linolenic acid intake is not beneficially associated with 10-y risk of coronary artery disease incidence: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:457–463. doi: 10.1093/ajcn/74.4.457. [DOI] [PubMed] [Google Scholar]
- 14.Laaksonen DE, Nyyssonen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. 2005;165:193–199. doi: 10.1001/archinte.165.2.193. [DOI] [PubMed] [Google Scholar]
- 15.Takata Y, Zhang X, Li H, Gao YT, Yang G, Gao J, Cai H, Xiang YB, Zheng W, Shu XO. Fish intake and risks of total and cause-specific mortality in 2 population-based cohort studies of 134,296 men and women. Am J Epidemiol. 2013;178:46–57. doi: 10.1093/aje/kws584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A Japan Collaborative Cohort Study for Evaluation of Cancer Risk Study Group. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–816. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 18.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39:187–195. doi: 10.1207/S15327914nc392_5. [DOI] [PubMed] [Google Scholar]
- 19.Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, Henderson BE, Nomura AM, Earle ME, Nagamine FS, Kolonel LN. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151:358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willett W. Nutritional Epidemiology. 3. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 21.Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139:804S–819S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB. Alpha-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96:1262–1273. doi: 10.3945/ajcn.112.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–164. doi: 10.1161/01.CIR.0000152099.87287.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, Willett WC, Hu FB. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112(21):3232–3238. doi: 10.1161/CIRCULATIONAHA.105.572008. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury R, Stevens S, Gorman D, Pan A, Warnakula S, Chowdhury S, Ward H, Johnson L, Crowe F, Hu FB, Franco OH. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. 2012;345:e6698. doi: 10.1136/bmj.e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de GJ, Verschuren WM, Boer JM, Kromhout D, Geleijnse JM. Alpha-linolenic acid intake and 10-year incidence of coronary heart disease and stroke in 20,000 middle-aged men and women in the Netherlands. PLoS ONE. 2011;6:e17967. doi: 10.1371/journal.pone.0017967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon JA, Fong J, Bernert JT, Jr, Browner WS. Serum fatty acids and the risk of stroke. Stroke. 1995;26:778–782. doi: 10.1161/01.str.26.5.778. [DOI] [PubMed] [Google Scholar]
- 29.He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Fish consumption and risk of stroke in men. JAMA. 2002;288:3130–3136. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- 30.Larsson SC, Virtamo J, Wolk A. Dietary fats and dietary cholesterol and risk of stroke in women. Atherosclerosis. 2012;221:282–286. doi: 10.1016/j.atherosclerosis.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 31.Wiberg B, Sundström J, Arnlöv J, Terént A, Vessby B, Zethelius B, Lind L. Metabolic risk factors for stroke and transient ischemic attacks in middle-aged men: a community-based study with long-term follow-up. Stroke. 2006;37:2898–2903. doi: 10.1161/01.STR.0000249056.24657.8b. [DOI] [PubMed] [Google Scholar]
- 32.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 33.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 34.Gissi-HF Investigators. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 35.Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 37.ORIGIN Trial Investigators. Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


