Abstract
Background/Aims
Assessment of bone turnover for management of renal osteodystrophy is part of routine care in chronic kidney disease Stage 5 (CKD-5) patients. Measurement of intact parathyroid hormone (iPTH) is the most commonly used surrogate marker for bone turnover in these patients. The current study was conducted to evaluate the predictive value of the five most commonly used iPTH assays for bone turnover.
Methods
In a cross-sectional study, 84 CKD-5 patients underwent bone biopsy and blood drawings for determination of iPTH and total serum alkaline phosphatase (AP).
Results
Histologically, patients presented with a broad range of bone turnover abnormalities as determined by activation frequency and bone formation rate/bone surface. Results of the five iPTH assays in each patient correlated but were significantly different. There were also significant differences between iPTH measurements at the same bone turnover level. Using Kidney Disease Outcome Quality Initiative recommended iPTH ranges, all assays showed comparably poor diagnostic performance. At 80% specificity, cut-off values of the 5 iPTH assays for low bone turnover varied from 165 to 550 pg/ml and for high bone turnover from 404 to 1,003 pg/ml. Sensitivities at these cutoffs remained below acceptable standards. Addition of AP measurements to iPTH did not improve diagnostic accuracy.
Conclusions
Precise assessment of bone turnover will require utilization of established and novel bone markers reflecting effects of bone turnover rather than measuring only iPTH or other effectors.
Keywords: renal osteodystrophy, bone turnover, parathyroid hormone, PTH assays, alkaline phosphatase, end-stage renal disease, K/DOQI
Introduction
Renal osteodystrophy (ROD) develops early in the process of loss of kidney function, [Malluche et al. 1976] and virtually all patients with chronic kidney disease Stage 5 (CKD-5) requiring renal replacement therapy are affected by this disorder [Malluche et al. 1990]. Abnormalities of bone turnover are major components of ROD [Malluche et al. 2006, Moe et al. 2006].
While bone biopsy is considered the gold standard for diagnosis of the turnover component of ROD [Malluche et al. 1994, Martin et al. 2004], this procedure is invasive, time consuming, often not readily available, and requires specialized processing of bone samples [Hruska et al. 1995, Malluche et al. 1986, 1994]. Parathyroid hormone (PTH) is known to affect bone turnover; therefore, clinicians often use measurements of serum PTH levels for the noninvasive assessment of bone turnover abnormalities (but not mineralization or bone volume) in CKD-5 patients. This practice, however, carries several limitations.
The first generation of PTH assays was introduced with the development of a radioimmunoassay for human PTH [Berson et al. 1968]. Early PTH assays used polyclonal antisera raised against intact PTH purified from animal parathyroid glands, while subsequent assays employed antibodies against human PTH [Fischer et al. 1974, Manning et al. 1980]. Initial experiments with these single-antibody first generation assays showed, however, considerable variance in sensitivity and specificity.
Subsequent studies demonstrated the heterogeneity of PTH peptides present in the circulation; secondary to the short half-life and the complex metabolism of PTH, there is a large variety of lower molecular weight PTH fragments [Mayer et al. 1979, Segre et al. 1977]. Some of these fragments demonstrate increased half-lives, especially in the setting of decreased glomerular filtration rate [Donadio et al. 2007, Herberth et al. 2006, Martin et al. 1979]. Based on these studies, immunometric “sandwich” assays utilizing two antibodies were developed in the 1980s as 2nd generation assays [Brossard et al. 2002, Brown et al. 1987, Nussbaum et al. 1988]. Since the first antibody is usually directed towards the C-terminal region and the second antibody is directed towards the N-terminal 34 amino acids, it was suggested that these assays measure only “intact” PTH 1-84 [Nussbaum et al. 1988]. It was, however, subsequently shown that these 2nd generation “intact” PTH assays do not measure PTH 1-84 exclusively [Brossard et al. 1996].
In today’s clinical practice, intact PTH (iPTH) levels in most CKD-5 patients in the US are measured by one of five different commercially available 2nd generation assays, and nephrologists rely on this information to noninvasively assess bone turnover and guide therapeutic decisions. The Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend 150 – 300 pg/ml as the desirable iPTH concentration in CKD-5 patients when using the 2nd generation iPTH Nichols® Allegro IRMA assay [Martin et al. 2004]. This assay, however, is no longer commercially available, and recent studies report significant variability between the currently available iPTH kits and the Nichols® Allegro assay, as well as between the iPTH assays themselves [Joly et al. 2008, Souberbielle et al. 2006]. In addition, there is increasing evidence showing that the predictability of bone turnover is limited with the exclusive use of iPTH assays [Qi et al. 1995, Wang et al. 1995]. The current study was conducted to evaluate the performance of the five most frequently used iPTH assays and the addition of total serum alkaline phosphatase to iPTH results for assessment of histologically determined bone turnover in CKD-5 patients.
Materials and methods
Study design
Patients agreeing to participate in the Renal Bone Disease Registry of the University of Kentucky were enrolled in this cross-sectional study. Participation in the registry consisted of a blood draw after overnight fast at the time of bone biopsy and agreement to have the bone biopsy sample evaluated by histomorphometry. The protocol was approved by the Institutional Review Board of the University of Kentucky. The study has been conducted in adherence with the Declaration of Helsinki, and all patients provided informed consent.
Patients
Patients were recruited from the years 2000 to 2006 from four US dialysis centers. Etiologies for development of CKD-5 were: diabetic nephropathy (n = 30), hypertension (n = 25), glomerulonephritis (n = 11), reflux nephropathy (n = 6), cystic kidney disease (n = 5), amyloidosis (n = 1), undetermined (n = 6). All patients were receiving stable dialysis treatment with standard dialysate calcium (2.5 mEq/l).
Inclusion criteria
18 years or older; dialysis vintage ≥ 3 months; mental competence; willingness to participate in the study; naïve to vitamin D or on a steady dose of vitamin D analogs for at least 6 months.
Exclusion criteria
Hematocrit ≤ 30%; parathyroidectomy or failed kidney transplant during the past 6 months; pregnancy; uncontrolled systemic illnesses or organ diseases that may affect bone (except Type 1 or 2 diabetes mellitus); treatment during the last 6 months with drugs known to affect bone metabolism (except treatment with vitamin D analogs as outlined in the inclusion criteria); chronic alcoholism and/or drug addiction.
Laboratory analysis
Blood was drawn at the time of the bone biopsy after an overnight fast. PTH was determined in plasma by the following five assays:
N-tact(r) PTH SP from DiaSorin® (Stillwater, MN, USA) (Diasorin) (normal range 3 – 54 pg/ml; intra- and interassay coefficients of variation (CV) < 4% and < 5%).
Roche Elecsys PTH ICMA from Roche Diagnostics (Indianapolis, IN, USA) (Elecsys) (normal range 15 – 65 pg/ml; intra-and interassay CV < 6% and < 9%).
Advia Centaur intact PTH IRMA from Bayer Health Care (Tarrytown, NY, USA) (Centaur) (normal range 14 – 72 pg/ml; intra-and interassay CV < 6% and < 6%).
DPC IMMULITE® PTH IRMA from Diagnostics Products Corporation (Los Angeles, CA, USA) (Immulite) (normal range 16 – 87 pg/ml; intra- and interassay CV < 7% and < 9%).
Total PTH(tm), (Scantibodies Laboratory Inc., Santee, CA, USA) (Scantibodies) (normal range 14 – 66 pg/ml; intra- and interassay CV < 5% and < 7%). This assay uses comparable antibodies that were used for the Nichols® Allegro assay and thus results are equivalent.
Total alkaline phosphatase was determined by a colorimetric assay using the Unicel D×C800 system (Beckman Coulter Inc., Fullerton, CA, USA) (normal range 37 – 110 U/l; intra- and interassay CV 3.5% and 3.3%).
Bone biopsy and mineralized bone histology
Anterior iliac crest bone biopsies were done after tetracycline labeling under local anesthesia and conscious sedation. The labeling schedule consisted of a 2-day oral administration of tetracycline hydrochloride (250 mg bid) followed by a drug-free interval of 10 days and subsequent oral administration of demeclocycline hydrochloride (300 mg bid) for 4 days. Bone biopsies were performed 3 – 4 days after completing the second label by using the one-step electrical drill technique (Straumann Medical, Waldenburg, Switzerland). Bone samples were processed undecalcified and sections were stained with the modified Masson-Goldner trichrome stain, the aurin tricarboxylic acid stain, and solochrome azurin for assessment of stainable aluminum in bone [Malluche and Faugere 1986]. Unstained sections were prepared for phase contrast and fluorescent light microscopy. Histomorphometric analysis was done at standardized sites in cancellous bone using the semi-automatic method (Osteoplan II, Kontron, Munich, Germany) at 200× magnification. Activation frequency (Ac.f.) and bone formation rate/bone surface (BFR/BS) were calculated for assessment of bone turnover. These histomorphometric parameters were calculated according to the guidelines set by the Nomenclature Committee of the American Society of Bone and Mineral Research.
Statistical analysis
Data are presented as mean ± SD (standard deviation of mean) or frequency counts and percentages unless otherwise indicated.
The classification of “low”, “normal”, and “high” bone turnover was based on our normative database [Malluche and Faugere 1986, Malluche et al. 1982, 2008]. The low bone turnover outcome group was defined as Ac.f. < 0.49 year−1 and BFR/BS < 1.8 mm3/cm2/yr. The normal bone turnover outcome group was defined as Ac.f. 0.49 – 0.72 year−1 and BFR/BS 1.8 – 3.8 mm3/cm2/yr. The high bone turnover outcome group was defined as Ac.f. > 0.72 year−1 and BFR/BS > 3.8 mm3/cm2/yr. Differences in patient characteristics between the bone turnover groups were evaluated by using Kruskal-Wallis tests for continuous variables and χ2-tests for frequency counts. Differences between mean iPTH and total alkaline phosphatase levels between the different bone turnover groups were analyzed by using nonparametric Wilcoxon-Mann-Whitney two-sample rank-sum tests, and comparisons of “low”, “normal”, and high bone turnover were performed using Kruskal-Wallis tests.
Receiver operating characteristic (ROC) analysis was employed to evaluate possible roles of independent variables for the prediction of the low and high bone turnover outcomes. Empirical sensitivities and specificities were calculated using the K/DOQI iPTH thresholds of < 150 pg/ml, 150 – 300 pg/ml and > 300 pg/ml. In addition, iPTH thresholds that achieve empirical specificities of at least 80% were calculated, and the corresponding sensitivities were estimated. Sensitivities and specificities were then plotted across the range of different iPTH values. Logistic regression analysis was used to evaluate whether classification accuracy in terms of the area under the curve (AUC) increases when total serum alkaline phosphatase measurements were added to iPTH results.
All calculations were carried out by the statistical program packages SPSS (SPSS 15.0 for Windows, SPSS Inc., Chicago, IL, USA), SAS version 9.1 (SAS Institute Inc., Cary, NC, USA), and the R statistical package (R Foundation for Statistical Computing, Vienna, Austria).
Results
Eighty-four patients were enrolled in the study (Table 1). Overall median age (min-max) was 52.0 years (21.0 – 80.0) (low bone turnover group 58.0 years (25.0 – 74.0); normal bone turnover group 43.5 years (32.0 – 69.0); high bone turnover group 49.5 years (21.0 – 80.0)). Overall median dialysis duration (min-max) was 28.5 months (3.0 – 192.0) (low bone turnover group 24.0 months (6.0 – 77.0); normal bone turnover group 24.5 months (5.0 – 58.0); high bone turnover group 30.0 (3.0 – 192.0)). There were no statistically significant differences between patients with low, normal and high bone turnover with respect to gender, race, age, presence of diabetes mellitus, vitamin D therapy or dialysis duration. Qualitative histologic assessment yielded the following diagnoses according to the old nomenclature: adynamic bone disease (n = 19); mixed uremic osteodystrophy (n = 15); and hyperparathyroid bone disease (n = 50). Since PTH is measured to assess the turnover component of renal osteodystrophy, further analysis was performed based on the quantitative histomorphometric evaluation of bone turnover employing the outcome measures activation frequency (Ac.f.) and bone formation rate/bone surface (BFR/BS).
Table 1.
Characteristics of the study population stratified according to low, normal and high bone turnover. Continuous variables are summarized as mean ± SD and categorical variables are summarized as counts (%). Vitamin D therapy reflects treatment with vitamin D analogues.
| Total | Low turnover | Normal turnover | High turnover | * p | |
|---|---|---|---|---|---|
| N | 84 | 19 | 15 | 50 | |
| Age (years) | 51.0 ± 13.7 | 55.8 ± 15.6 | 48.0 ± 11.7 | 50.1 ± 13.2 | 0.12 |
| Gender (%) | 0.63 | ||||
| Women | 37 (44.0) | 7 (36.8) | 8 (53.3) | 22 (44.0) | |
| Men | 47 (56.0) | 12 (63.2) | 7 (46.7) | 28 (56.0) | |
| Race (%) | 0.36 | ||||
| AA | 44 (52.4) | 8 (42.1) | 10 (66.7) | 26 (52.0) | |
| Caucasian | 40 (47.6) | 11 (57.9) | 5 (33.3) | 24 (48.0) | |
| Diabetes mellitus (%) | 0.38 | ||||
| yes | 30 (35.7) | 9 (47.4) | 6 (40.0) | 15 (30.0) | |
| no | 54 (64.3) | 10 (52.6) | 9 (60.0) | 35 (70.0) | |
| Vitamin D Therapy (%) | 0.52 | ||||
| yes | 45 (53.6) | 10 (52.6) | 10 (66.7) | 25 (50.0) | |
| no | 39 (46.4) | 9 (47.4) | 5 (33.3) | 25 (50.0) | |
| Duration of Dialysis (months) | 34.9 ± 29.5 | 30.5 ± 19.8 | 24.7 ± 16.5 | 40.3 ± 35.1 | 0.24 |
| PTH assay results | |||||
| Diasorin (pg/ml) | 376 ± 327 | 181 ± 246 | 362 ± 338 | 455 ± 324 | < 0.01 |
| Elecsys (pg/ml) | 734 ± 582 | 342 ± 423 | 608 ± 504 | 920 ± 579 | < 0.01 |
| Centaur (pg/ml) | 836 ± 657 | 409 ± 565 | 810 ± 660 | 1009 ± 622 | < 0.01 |
| Immulite (pg/ml) | 937 ± 675 | 554 ± 645 | 651 ± 434 | 1152 ± 658 | < 0.01 |
| Scantibodies (pg/ml) | 615 ± 517 | 335 ± 417 | 539 ± 562 | 755 ± 496 | < 0.01 |
| Alkaline phosphatase (U/l) | 181 ± 156 | 144 ± 116 | 139 ± 139 | 208 ± 171 | 0.01 |
From Kruskal-Wallis or χ2 analysis
Based on our normative database, patients were divided into the bone turnover categories “low”, “normal”, and high (see Material and Methods). Mean Ac.f. and BFR/BS in the low bone turnover group were 0.30 ± 0.12 yr−1 and 1.13 ± 0.40 mm3/cm2/yr; in the normal bone turnover group 0.58 ± 0.07 yr−1 and 2.89 ± 0.65 mm3/cm2/yr; and in the high bone turnover group 1.47 ± 0.73 yr−1 and 7.62 ± 3.11 mm3/cm2/yr, respectively. There were no patients with discrepancies between Ac.f. and BFR/BS that would have put them into different categories. None of the patients had stainable aluminum in bone.
Measurements of the five iPTH assays correlated (r = 0.78 – 0.97; p < 0.01) but all values were significantly different (p < 0.05). There were also significant differences in mean iPTH values when patients with low bone turnover were compared to patients with high bone turnover. Areas under ROC curves for the five iPTH assays for the prediction of low and high bone turnover were similar ranging between 0.76 and 0.81 and between 0.73 and 0.77, respectively (Figure 1A,B).
Figure 1.
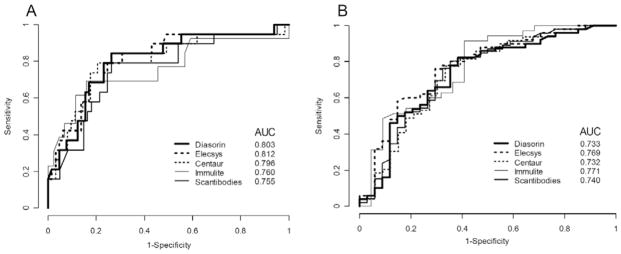
Receiver operating characteristic analysis of five different iPTH assays for predicting the outcome low bone turnover (A) and high bone turnover (B). AUC = Area under the curve.
When using K/DOQI-recommended iPTH target ranges (< 150 pg/ml; 150 – 300 pg/ml; > 300 pg/ml), different assays had comparable sensitivities and specificities for discriminating between different bone turnover categories (Table 2). For the diagnosis of low bone turnover using an iPTH threshold of less than 150 pg/ml, all assays displayed good specificities for ruling out patients with normal or high bone turnover but showed poor diagnostic performance for identifying patients with low bone turnover (sensitivities 0.15 – 0.68). Using the iPTH range of 150 – 300 pg/ml, all assays had acceptable sensitivities for identification of patients with normal bone turnover but were not useful for correctly ruling out low or high bone turnover (specificities 0.13 – 0.33). For the diagnosis of high bone turnover using the iPTH cut-off > 300 pg/ml, all but one (Diasorin) assays performed well for identification of patients in this bone turnover category (sensitivities 0.64 – 0.94), but all five assays had poor to moderate ability to rule out patients with normal or low bone turnover (specificities 0.45 – 0.74).
Table 2.
Sensitivities and specificities of five total iPTH assays for the prediction of bone turnover using Kidney Disease Outcome Quality Initiative (K/DOQI) recommended ranges.
| Assay | Low vs. normal or high < 150 pg/ml | Normal vs. low or high 150 – 300 pg/ml | High vs. normal or low > 300 pg/ml | |||
|---|---|---|---|---|---|---|
| Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | |
| Diasorin | 0.68 | 0.83 | 0.80 | 0.33 | 0.64 | 0.74 |
| Elecsys | 0.42 | 0.94 | 0.84 | 0.27 | 0.84 | 0.56 |
| Centaur | 0.32 | 0.95 | 0.79 | 0.14 | 0.86 | 0.55 |
| Immulite | 0.15 | 1.00 | 0.85 | 0.33 | 0.94 | 0.45 |
| Scantibodies | 0.37 | 0.85 | 0.82 | 0.13 | 0.80 | 0.62 |
Cut-off values for iPTH yielding at least 80% specificity showed remarkable differences between the assays for ruling out low bone turnover (lowest value 165 pg/ml (Diasorin); highest value 550 pg/ml (Immulite)), or high bone turnover (lowest value 404 pg/ml (Diasorin); highest value 1003 pg/ml (Centaur)) (Table 3). By keeping a specificity level of at least 80%, all assays performed equally poorly for diagnosing low bone turnover (sensitivities 0.58 – 0.74), or high bone turnover (sensitivities 0.49 – 0.60). Figure 2A provides information on the specific cut-off values for each iPTH assay in case a different specificity level than 80% is desired for ruling out low bone turnover. Figure 2B then gives information on the sensitivity at these specific cut-off values for each iPTH assay for diagnosing low bone turnover. Changes in the cut-off values and sensitivities for different speficities and for each iPTH assay are shown in Figure 3A and B for high bone turnover.
Table 3.
Cut-off thresholds (pg/ml) that achieve at least 80% specificity for predicting bone turnover abnormalities with corresponding sensitivities (Sens.), positive predictive values (PPV), and negative predictive values (NPV).
| Assay | Low | High | ||||||
|---|---|---|---|---|---|---|---|---|
| Cut-off | Sens. | PPV | NPV | Cut-off | Sens. | PPV | NPV | |
| Diasorin | 165 | 0.68 | 0.69 | 0.79 | 404 | 0.52 | 0.61 | 0.76 |
| Elecsys | 291 | 0.68 | 0.69 | 0.79 | 759 | 0.60 | 0.65 | 0.79 |
| Centaur | 355 | 0.74 | 0.72 | 0.82 | 1003 | 0.49 | 0.59 | 0.75 |
| Immulite | 550 | 0.69 | 0.72 | 0.80 | 979 | 0.51 | 0.61 | 0.77 |
| Scantibodies | 223 | 0.58 | 0.66 | 0.74 | 713 | 0.52 | 0.61 | 0.76 |
Figure 2.
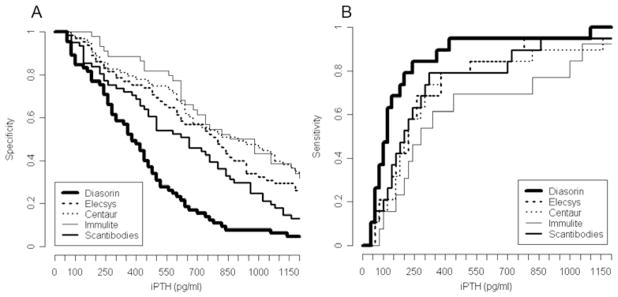
Specificities (A) and sensitivities (B) of five iPTH assays for the outcome low bone turnover.
Figure 3.
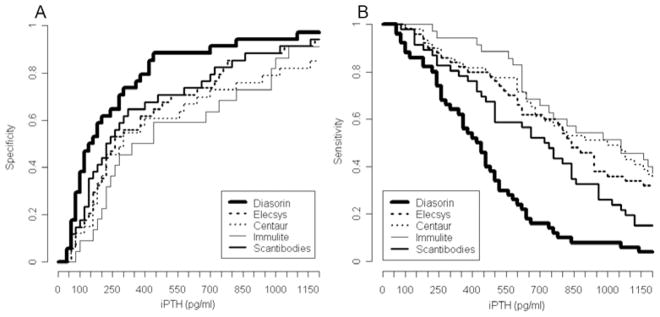
Specificities (A) and sensitivities (B) and of five iPTH assays for the outcome high bone turnover.
Serum total alkaline phosphatase levels were significantly higher in patients with high bone turnover versus patients with normal or low bone turnover (p < 0.05) (Table 1). However, addition of serum total alkaline phosphatase measurements to iPTH results did not increase the value of the model for predicting a specific bone turnover abnormality (p > 0.05 for all assays).
Discussion
Data on differences between iPTH assays were published before using pooled plasma as well as individual plasma samples from CKD-5 patients [Cantor et al. 2006, Souberbielle et al. 2006]. The present study compares results obtained by the five most commonly used iPTH assays for the same individual CKD-5 patient plasma sample and provides the novel information of comparing the different iPTH assay results with bone histology.
The demonstrated differences in absolute numbers between the iPTH assays highlight that the type of assay must be considered when therapeutic decisions are made based on serum iPTH results. The observed numerical disparities between assay results might be related to differences in detection of iPTH fragments accumulating in CKD-5 patients and/or to methodological disparities between assays. The studied iPTH assays have different capabilities to detect N-terminally truncated fragments [Cantor et al. 2006, Souberbielle et al. 2006, Whitham et al. 2000], and it was shown that these PTH fragments are antagonistic to PTH 1-84 effects on bone turnover [Langub et al. 2003]. In the specific case of the Diasorin assay, our results confirm previously published data showing a consistently proportional bias in comparison to other iPTH assays that appears to be related to assay calibration [Cantor 2005, Joly et al. 2008].
Ac.f. and BFR/BS are the preferred comprehensive parameters for determination of bone turnover. As shown previously, Ac.f. and BFR/BS are well correlated, and both variables correlate well with iPTH results [Malluche et al. 2008, Monier-Faugere et al. 2001, Qi et al. 1995]. Data on correlation between static cellular parameters of bone resorption/formation and PTH measurements were published before [Malluche et al. 1976, 1984, 1986, Ritz et al. 1973] and were shown not to be superior to these dynamic parameters. Therefore, they are not presented in our study.
When evaluating test performance, sensitivity reflects the test’s ability to correctly identify individuals with an outcome, while specificity provides information on correctly excluding individuals without the outcome. An ideal test would incorporate both high degrees of sensitivity and specificity. When screening CKD-5 patients at risk for bone turnover abnormalities, nephrologists attempt to make therapeutic decisions to avoid high bone turnover (i.e. intensifying suppression therapy) or low bone turnover (i.e. decreasing suppression therapy). Accordingly, we studied the value of different iPTH assays for the assessment of low and high bone turnover. Our results emphasize that the uniform K/DOQI recommendation of 150 – 300 pg/ml for iPTH assays results in poor sensitivity for ruling in the diagnosis of low bone turnover at ranges less than 150 pg/ml and poor specificity for ruling out patients with low or normal bone turnover at ranges of 300 pg/ml or higher (Table 2), and it is apparent that different cut-off values for each assay are needed. When a specificity level of at least 80% was required, the overall low sensitivities of iPTH measurements revealed that all studied iPTH assays perform poorly to diagnose a specific bone turnover category. The specificity level of ≥ 80%, however, allows using these cut-off values to rule out specific bone turnover categories, that is, to screen for bone turnover abnormalities (Table 3). A measurement that falls between the low and the high cut-offs for a specific iPTH assay conveys the message that no therapeutic changes are needed. Despite differences between study populations, poor assay-related performances for diagnosing bone turnover abnormalities were recently reported for the Immulite assay in Brazilian CKD-5 patients [Barreto et al. 2008] and for the Biosource Europe SA PTH assay in Macedonian CKD-5 patients [Bervoets et al. 2003].
The inability of iPTH measurements to reliably diagnose bone turnover is not surprising given the fact that iPTH is only one of several effectors on bone turnover. It would be desirable to add measurements of markers reflecting effects of bone turnover rather than measuring concentrations of a single effector. Our results demonstrate that the common clinical practice of measuring serum total alkaline phosphatase does not increase the predictive value of the classification rule for diagnosing bone turnover abnormalities in CKD-5 patients. Further studies are needed to determine whether the additional measurements of PTH fragments or other parameters such as markers of bone formation and resorption will increase diagnostic accuracy. For this purpose, bone-specific alkaline phosphatase and tartrate-resistant acid phosphatase 5b were identified as promising candidates [Bervoets et al. 2003, Fahrleitner-Pammer et al. 2008, Urena et al. 1999].
Our study was not designed to address factors that may affect bone turnover in CKD-5 patients but to assess the performance of widely used 2nd generation iPTH assays for predicting bone turnover abnormalities in this patient population. Similarly, evaluation of the usefulness of the PTH 1-84/large C-terminal PTH fragment ratio was not within the scope of our study. To address this important question, a larger scale study is currently underway. While serial iPTH measurements might change the value of a classification rule for screening CKD-5 patients at risk for bone turnover abnormalities, future studies will have to determine if the use of statistical models accounting for the within and between subjects variance, the time varying nature and changes in the predictive accuracy of iPTH measurements represents an improvement over a single measurement. Although we acknowledge that the applied selection criteria might influence external validity, demographic variables, the range of iPTH levels, and the spectrum of observed bone turnover abnormalities in our study cover the most commonly encountered clinical situations. Also, there were no statistically significant differences in patient characteristics regarding age, gender, race, duration of dialysis, diabetes mellitus, and vitamin D treatment status between bone turnover groups; this allowed focusing on the performance of iPTH measurements for assessment of bone turnover.
Our results call for larger scale studies with the goal of arriving at a classification rule that will add established and novel noninvasive bone markers to PTH results to allow noninvasive characterization of bone turnover in a more precise manner than is currently available.
Acknowledgments
The study was supported by the Dean’s Clinical Research Scholar Program, University of Kentucky, # 1012112710 (JH); by NIH RO1 DK51530 (HHM), and by the Kentucky Nephrology Research Trust (M-CMF). The authors would like to thank Ms. Juliana Van Willigen and Mr. Richard Wheaton for their technical assistance. This study was presented in abstract form at the American Society of Nephrology 40th Annual Meeting, San Francisco, CA on November 04, 2007.
Tom Cantor, Bsc. is president of Scanti-bodies Laboratory, Inc. Hartmut H. Malluche, MD provided occasional consulting to Scanti-bodies Laboratory, Inc.
Footnotes
All other authors have no competing financial interest.
References
- Barreto FC, Barreto DV, Moyses RM, Neves KR, Canziani ME, Draibe SA, Jorgetti V, Carvalho AB. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73:771–777. doi: 10.1038/sj.ki.5002769. [DOI] [PubMed] [Google Scholar]
- Berson SA, Yalow RS. Immunochemical heterogeneity of parathyroid hormone in plasma. J Clin Endocrinol Metab. 1968;28:1037–1047. doi: 10.1210/jcem-28-7-1037. [DOI] [PubMed] [Google Scholar]
- Bervoets AR, Spasovski GB, Behets GJ, Dams G, Polenakovic MH, Zafirovska K, Van Hoof VO, De Broe ME, D’Haese PC. Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. Am J Kidney Dis. 2003;41:997–1007. doi: 10.1016/s0272-6386(03)00197-5. [DOI] [PubMed] [Google Scholar]
- Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barre M, D’Amour P. Accumulation of a non-(1-84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab. 1996;81:3923–3929. doi: 10.1210/jcem.81.11.8923839. [DOI] [PubMed] [Google Scholar]
- Brossard JH, Yamamoto LN, D’Amour P. Parathyroid hormone metabolites in renal failure: bioactivity and clinical implications. Semin Dial. 2002;15:196–201. doi: 10.1046/j.1525-139x.2002.00053.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Aston JP, Weeks I, Woodhead JS. Circulating intact parathyroid hormone measured by a two-site immunochemiluminometric assay. J Clin Endocrinol Metab. 1987;65:407–414. doi: 10.1210/jcem-65-3-407. [DOI] [PubMed] [Google Scholar]
- Cantor T. Parathyroid hormone assay drift: an unappreciated problem in dialysis patient management. Semin Dial. 2005;18:359–564. doi: 10.1111/j.1525-139X.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- Cantor T, Yang Z, Caraiani N, Ilamathi E. Lack of comparability of intact parathyroid hormone measurements among commercial assays for end-stage renal disease patients: implication for treatment decisions. Clin Chem. 2006;52:1771–1776. doi: 10.1373/clinchem.2006.071589. [DOI] [PubMed] [Google Scholar]
- Donadio C, Ardini M, Lucchesi A, Donadio E, Cantor T. Parathyroid hormone and large related C-terminal fragments increase at different rates with worsening of renal function in chronic kidney disease patients. A possible indicator of bone turnover status? Clin Nephrol. 2007;67:131–139. doi: 10.5414/cnp67131. [DOI] [PubMed] [Google Scholar]
- Fahrleitner-Pammer A, Herberth J, Browning SR, Obermayer-Pietsch B, Wirnsberger G, Holzer H, Dobnig H, Malluche HH. Bone markers predict cardiovascular events in chronic kidney disease. J Bone Miner Res. 2008;23(11):1850–1858. doi: 10.1359/jbmr.080610. [DOI] [PubMed] [Google Scholar]
- Fischer JA, Binswanger U, Dietrich FM. Human parathyroid hormone. Immunological characterization of antibodies against a glandular extract and the synthetic amino-terminal fragments 1–12 and 1–34 and their use in the determination of immunoreactive hormone in human sera. J Clin Invest. 1974;54:1382–1394. doi: 10.1172/JCI107885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberth J, Fahrleitner-Pammer A, Obermayer-Pietsch B, Krisper P, Holzer H, Malluche HH, Dobnig H. Changes in total parathyroid hormone (pth), pth-(1-84) and large c-pth fragments in different stages of chronic kidney disease. Clin Nephrol. 2006;65:328–334. doi: 10.5414/cnp65328. [DOI] [PubMed] [Google Scholar]
- Hruska KA, Teitelbaum SL. Renal osteodystrophy. N Engl J Med. 1995;33:166–174. doi: 10.1056/NEJM199507203330307. [DOI] [PubMed] [Google Scholar]
- Joly D, Drueke TB, Alberti C, Houillier P, Lawson-Body E, Martin KJ, Massart C, Moe SM, Monge M, Souberbielle JC. Variation in serum and plasma PTH levels in second-generation assays in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2008;51:987–995. doi: 10.1053/j.ajkd.2008.01.017. [DOI] [PubMed] [Google Scholar]
- K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- Langub MC, Monier-Faugere MC, Wang G, Williams JP, Koszewski NJ, Malluche HH. Administration of PTH-(7-84) antagonizes the effects of PTH-(1-84) on bone in rats with moderate renal failure. Endocrinology. 2003;144:1135–1138. doi: 10.1210/en.2002-221026. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Faugere MC. Atlas of mineralized bone histology. New York: Karger; 1986. [Google Scholar]
- Malluche HH, Faugere MC. Renal bone disease 1990: an unmet challenge for the nephrologist. Kidney Int. 1990;38:193–211. doi: 10.1038/ki.1990.187. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Ritz E, Lange H, Kutschera K, Hodgson M, Seiffert U, Schoeppe W. Bone histology in incipient and advanced renal failure. Kidney Int. 1976;9:355–362. doi: 10.1038/ki.1976.42. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Meyer W, Sherman D, Massry SG. Quantitative bone histology in 84 normal American subjects. Micromorphometric analysis and evaluation of variance in iliac bone. Calcif Tissue Int. 1982;34:449–455. doi: 10.1007/BF02411283. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Faugere MC, Fanti P, Price PA. Plasma levels of bone Gla-protein reflect bone formation in patients on chronic maintenance dialysis. Kidney Int. 1984;26:869–874. doi: 10.1038/ki.1984.230. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Monier-Faugere MC. Renal osteodystrophy: what’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol. 2006;65:235–242. doi: 10.5414/cnp65235. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Monier-Faugere MC. The role of bone biopsy in the management of patients with renal osteodystrophy. J Am Soc Nephrol. 1994;4:1631–1642. doi: 10.1681/ASN.V491631. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Monier-Faugere MC, Wang G, Fraza OJ, Charytan C, Coburn JW, Coyne DW, Kaplan MR, Baker N, McCary LC, Turner SA, Goodman WG. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2008;69:269–278. doi: 10.5414/cnp69269. [DOI] [PubMed] [Google Scholar]
- Manning RM, Hendy GN, Papapoulos SE, O’Riordan JL. Development of homologous immunological assays for human parathyroid hormone. J Endocrinol. 1980;85:161–170. doi: 10.1677/joe.0.0850161. [DOI] [PubMed] [Google Scholar]
- Martin KJ, Hruska KA, Freitag JJ, Klahr S, Slatopolsky E. The peripheral metabolism of parathyroid hormone. N Engl J Med. 1979;301:1092–1098. doi: 10.1056/NEJM197911153012005. [DOI] [PubMed] [Google Scholar]
- Martin KJ, Olgaard K, Coburn JW, Coen GM, Fukagawa M, Langman C, Malluche HH, McCarthy JT, Massry SG, Mehls O, Salusky IB, Silver JM, Smogorzewski MT, Slatopolsky EM, McCann L. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004;43:558–565. doi: 10.1053/j.ajkd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Mayer GP, Keaton JA, Hurst JG, Habener JF. Effects of plasma calcium concentration on the relative proportion of hormone and carboxyl fragments in parathyroid venous blood. Endocrinology. 1979;104:1778–1784. doi: 10.1210/endo-104-6-1778. [DOI] [PubMed] [Google Scholar]
- Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, Malluche HH. Improved assessment of bone turnover by the PTH-(1-84)/large C-PTH fragments ratio in ESRD patients. Kidney Int. 2001;60:1460–1468. doi: 10.1046/j.1523-1755.2001.00949.x. [DOI] [PubMed] [Google Scholar]
- Nussbaum SR, Zahradnik RJ, Lavigne JR, Brennan GL, Nazawa-Ung K, Kim LY, HTK, Wang CA, Potts JT, Segre GV. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1988;33:1364–1367. [PubMed] [Google Scholar]
- Qi Q, Monier-Faugere MC, Geng Z, Malluche HH. Predictive value of serum parathyroid hormone levels for bone turnover in patients on chronic maintenance dialysis. Am J Kidney Dis. 1995;26:622–631. doi: 10.1016/0272-6386(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Ritz E, Krempien B, Mehls O, Malluche H. Skeletal abnormalities in chronic renal insufficiency before and during maintenance hemodialysis. Kidney Int. 1973;4:116–127. doi: 10.1038/ki.1973.90. [DOI] [PubMed] [Google Scholar]
- Segre GV, Niall HD, Sauer RT, Potts JT., Jr Edman degradation of radioiodinated parathyroid hormone: application to sequence analysis and hormone metabolism in vivo. Biochemistry. 1977;16:2417–2427. doi: 10.1021/bi00630a017. [DOI] [PubMed] [Google Scholar]
- Souberbielle JC, Boutten A, Carlier MC, Chevenne D, Coumaros G, Lawson-Body E, Massart C, Monge M, Myara J, Parent X, Plouvier E, Houillier P. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int. 2006;70:345–350. doi: 10.1038/sj.ki.5001606. [DOI] [PubMed] [Google Scholar]
- Urena P, De Vernejoul MC. Circulating biochemical markers of bone remodeling in uremic patients. Kidney Int. 1999;55:2141–2156. doi: 10.1046/j.1523-1755.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- Wang M, Hercz G, Sherrard DJ, Maloney NA, Segre GV, Pei Y. Relationship between intact 1-84 parathyroid hormone and bone histomorphometric parameters in dialysis patients without aluminum toxicity. Am J Kidney Dis. 1995;26:836–844. doi: 10.1016/0272-6386(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Whitham KM, Milford-Ward A. External quality assessment of bone metabolism marker assays. initial experiences in a UK NEQAS programme. Clin Chem Lab Med. 2000;38:1121–1124. doi: 10.1515/CCLM.2000.168. [DOI] [PubMed] [Google Scholar]


