Abstract
Background
Varenicline has been shown to reduce cigarette craving during a quit attempt.
Objectives
Use BOLD fMRI to explore differences in smoking cue reactivity at baseline and after five weeks of varenicline smoking cessation treatment.
Methods
Treatment-seeking nicotine-dependent adult smokers underwent BOLD fMRI scans with block presentation of visual smoking, neutral, and rest cues under two conditions: craving or resisting the urge to smoke at baseline and following 5 weeks of standard varenicline therapy. Data were analyzed using FMRI Expert Analysis Tool, version 5.98 of Functional Magnetic Imaging of the Brain Software Library focused on the smoking vs. neutral cue contrast at the individual and group level, Z>2.3 with cluster threshold p=0.05.
Results
Twenty-one participants were scanned at baseline and 16 completed the study; 10 were abstinent at the 2nd session, confirmed with urinary cotinine. In the Crave Condition no significant differences were found between the abstinent and non-abstinent groups at either time point. During the baseline Resist Condition, the abstinent group compared to the non-abstinent group demonstrated activation in a distributed network involved in alertness, learning and memory. Additionally, within the abstinent group, increased activation of the superior frontal gyrus was found at baseline compared to week 5.
Conclusion
Successful smoking cessation with varenicline is associated with increased activation, prior to a quit attempt, in brain areas related to attentiveness and memory while resisting the urge to smoke
Scientific Significance
Varenicline may exert effects by both reducing craving and enhancing resistance to smoking urges during cue-elicited craving.
INTRODUCTION
Current clinical guidelines recommend the use of evidence-based medications during smoking quit attempts (1). Varenicline, one of the first-line medications, has been associated with significantly higher continuous abstinence rates at one year compared to placebo and other first-line therapies including nicotine replacement therapy, and bupropion (2). Varenicline is a partial agonist at the α4β2 nicotinic acetylcholine receptor (nAChR) with both nicotine-like agonist effects leading to relief of withdrawal symptoms and craving as well as receptor blockade attenuating the rewarding effects of smoking (3). For example, compared to bupropion or placebo, varenicline has been shown to significantly reduce subjective measures of satisfaction with smoking the first cigarette after the target quit date (TQD) (4).
Craving during a quit attempt is a challenge for most nicotine-dependent smokers and is a factor associated with relapse (5). In addition to the pharmacological rewards from nicotine administration, smoking is also maintained by the associations of the pharmacological action of nicotine with cues such as the sight of favored cigarette pack or lighter and the sight or smell of cigarette smoke (6). In both laboratory and real-world studies, these smoking-related cues readily induce craving (7,8). Neuroimaging studies have examined regional areas of brain activation associated with craving during presentation of smoking-related cues. Exposure to smoking-related cues commonly provokes activation in regions subserving attention such as the anterior cingulate cortex (ACC), precuneus, and cuneus (9,10); the mesolimbic dopamine reward system, known to be activated by addictive drugs including the right posterior amygdala, posterior hippocampus, ventral tegmental area, nucleus accumbens (NaC); the medial (m) thalamus (11); and areas involved in decision making and goal directed behavior such as the prefrontal cortex (PFC) (12). To date, only two studies have reported on areas of regional brain activation, while smokers actively resisted the urge to smoke during presentation of smoking cues. Brady and colleagues (9) found higher activation in the left posterior cingulate cortex (PCC), bilateral precuneus, bilateral retrospenial area, bilateral superior frontal gyrus (SFG), and left dorsal ACC contrasting resisting to neutral epochs. In contrast, our group did not find significant differences in neural activation between craving and resisting without taking into account the specific strategy employed to counter the urge to smoke (12).
Prior research has indicated that first-line medications are more efficacious in attenuating the ambient craving associated with withdrawal and deprivation than the minimal effect on periodic cue-elicited craving (13,14). However, varenicline, compared to placebo, has previously been shown in one study to reduce smoking cue-induced response in the ventral striatum (VS) and medial orbitofrontal cortex (mOFC) in non-treatment seeking nicotine-dependent smokers (15). While important, the reduction of craving is only one potential strategy in combating nicotine dependence in cigarette smokers. Enhancing smokers’ ability to resist the urge to smoke during craving and following cue exposure has the potential to improve treatment outcomes. To our knowledge, no studies have previously described the effects of varenicline on brain activation patterns while resisting the urge to smoke during smoking-related cue exposure.
We report on the subjective measures and changes in regional activation patterns during cue-elicited craving and resisting the urge to smoke before and during a smoking quit attempt with varenicline. As a result of varenicline’s partial activation of the nAChR, we hypothesized that smokers on varenicline would evidence reduced smoking satisfaction and craving to smoking cues by subjective measures and reduction in BOLD fMRI activation in areas associated with craving during the presentation of smoking cues after 5 weeks of treatment with varenicline as compared to baseline. The a priori analysis plan included comparison between participants who were able to quit smoking and those who continued to smoke to determine if abstinence impacted the effect of varenicline on activation patterns.
MATERIALS AND METHODS
Subjects
Twenty-one right-handed, treatment-seeking nicotine-dependent smokers averaging 35.2 (SD = 12.1) years of age (12 women and 9 men) and smoking on average 20 (SD = 2.0) cigarettes/day were recruited through local community advertisements. All participants gave written informed consent as approved by the Medical University of South Carolina (MUSC) Institutional Review Board. A subset of participants’ baseline data (n = 15) has been previously reported in a description of regional activation patterns in craving and resisting the urge to smoke during smoking-related cue exposure prior to any type of smoking cessation treatment (12).
Eligible participants met the criteria for nicotine dependence, were motivated to quit smoking, and were willing to set a target quit date. Exclusion criteria included use of other tobacco products or pharmacotherapy for smoking cessation, previous varenicline failure, medical conditions or medications affecting brain function, pregnancy, current significant Axis I disorders, other lifetime substance dependence, or substance abuse within the past 30 days. The Fagerström Test for Nicotine Dependence (FTND) (16), Questionnaire of Smoking Urges-Brief (QSU-B) (17), Modified Cigarette Evaluation Questionnaire (mCEQ) (18), and Tobacco Use History were administered. Exhaled carbon monoxide levels (≥10 ppm) were measured with a MicroSmokelyzer (Bedfont Scientific Ltd., Kent, United Kingdom) to confirm recent smoking. Subjects participated in two scanning sessions, one at baseline and the other at week 5, approximately 1 month after the TQD. Following the initial fMRI session and setting a TQD, standard treatment with varenicline was initiated with a titration phase at .5 mg per day for 3 days followed by .5 mg twice a day for 4 days. On the TQD, varenicline was increased to 1 mg twice a day for the remainder of the study. Participants were seen weekly to monitor smoking measures, review smoking diary, medication log, and pill count, obtain CO levels, assess adverse effects associated with varenicline, and for brief supportive smoking cessation counseling. At week 5, the abstinent subgroup included participants with at least a 7-day point-prevalence abstinence rate, CO in non-smoking range (≤3 ppm), and negative cotinine levels (<200 ng/ml). The non-abstinent subgroup included individuals who completed the study and reported smoking, provided CO levels (> 10 ppm) or those who were lost to follow-up with presumed return to smoking. Participants were reimbursed a nominal amount for study participation, and received two additional months of varenicline treatment following study completion to comply with current recommendations for length of therapy.
fMRI Procedures
The cue-elicited craving paradigm, image acquisition parameters, procedures, and processing methods were fully described previously (12). Briefly, participants were exposed to two separate fMRI runs with 24s blocks of five smoking-related pictures or five neutral pictures, interleaved with rest blocks (24s) and self-rating of craving (6s) using a handpad at baseline and following each block. Each run had a total of 24 blocks, eight smoking-cue, eight rest, and eight neutral blocks; no blocks were repeated in immediate succession. All participants received the same order of blocks and pictures. During the initial fMRI run, participants were instructed to “allow yourself to crave when you see the smoking related pictures.” In a separate immediately subsequent run, participants were instructed to “resist the urge to smoke when you see the smoking pictures by any means you find helpful.”
Functional scanning was performed with a 3.0-T Siemens Trio scanner utilizing a standard multislice single-shot gradient echo EPI sequence with the following parameters: TR = 2.2 s, TE = 35 ms, 64 × 64 matrix, 3×3×3 mm voxels, 328 volumes, and 36 ascending transverse slices with ascending transverse slices with approximate AC-PC alignment.
fMRI Data Analysis
Functional MRI analysis was completed using FMRI Expert Analysis Tool (FEAT), version 5.98 of Functional Magnetic Imaging of the Brain (FMRIB) Software Library using standard statistical approaches focusing on the smoking versus neutral cues contrast from the crave and resist conditions (12). Prior to analysis, FSL pre-statistics processing included removal of non-brain with Brain Extraction Tool (BET), motion correction with Motion Correction FMRIB Linear Image Registration Tool (MCFLIRT) (19), spatial smoothing with a Gaussian kernel of FWHM 8 mm, and grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0s). Participant data were excluded due to excessive movement (greater than 3 mm) during the scanning runs. Based on this criterion, one participant was excluded entirely from data analysis. An additional participant’s data was excluded from the resist condition as participant appeared to fall asleep or kept eyes closed and did not display any visual activation comparing smoke versus rest contrast.
A first-level analysis was performed to examine each individual’s data from the crave and resist conditions, comparing blocks of smoking-related and neutral cue exposure. Imaging data gathered during the self-rating and rest conditions were excluded from the analysis. Voxels were thresholded at Z > 2.3 with cluster threshold set at p = .05.
Upper-level analyses were completed using FEAT FMRIB’s Local Analysis of Mixed Effects (FLAME 12) using the individual feat directories with the same statistical threshold to estimate inter-session and inter-subject random-effects component of the mixed effects variance (20). FILM with local autocorrelation correction was used to complete the time-series statistical analysis (21). Upper-level analyses focused on the smoking versus neutral contrast in both the crave and resist conditions, including the group at large at baseline, the abstinent versus non-abstinent subgroups at baseline and at week 5 of a smoking quit attempt with varenicline, and between the two time points in each subgroup.
RESULTS
Smoking Outcomes
Varenicline was well tolerated by all of the participants. The most common adverse effects were nausea (38%) and insomnia (19%). One participant required a dosage reduction following titration to 1 mg in the morning and .5 mg in the evening due to insomnia. At week 5, 47.6% (n 10) were included in the abstinent subgroup. The remaining participants who either continued to smoke at a reduced level (n = 6) or dropped out of the study (n = 5) with presumed return to smoking were considered non-abstinent As seen in Table 1, at baseline no significant differences were found between the abstinent and non-abstinent subgroups in age, gender, cigarettes/day, and education. A racial difference was found with more Caucasians in the abstinent subgroup compared to the non-abstinent subgroup (p = .03); the small number of participants limits the ability to infer that race accounted for any of the group differences. To note, some previous research, but not all (22), has indicated that African-American smokers may have a more difficult time quitting smoking and are more likely to drop out of smoking cessation trials (23,24).
TABLE 1.
Demographic characteristics and baseline measures.
| Characteristics | Abstinent group, (n = 10) | Non-abstinent group, (n = 11) | p-Value |
|---|---|---|---|
| Age, mean (SD) | 34.8(13.07) | 35.55(11.72) | .89 |
| Gender, % male | 30.0 | 54.5 | .26 |
| Cigarettes/day, mean (SD) | 20.45(5.83) | 17.27(4.29) | .18 |
| Education, % some college | 70.0 | 81.8 | .92 |
| Race, % Caucasian | 100 | 63.6 | .03 |
At baseline, both subgroups were moderately nicotine-dependent as measured by the FTND and experienced similar levels of ambient craving and smoking satisfaction as measured by the mCEQ (Table 2). Likewise, no statistically significant differences were seen between the two subgroups in the QSU-B factor 1, a measure of positive reinforcement for smoking, QSU-B factor 2, a measure of negative reinforcement for smoking or the total QSU-B. After 5 weeks of varenicline treatment during a smoking quit attempt, both the abstinent and non-abstinent subgroups experienced a significant decrease compared to baseline in the level of nicotine dependence, smoking satisfaction, ambient craving, and QSU-B measures (Table 2). A significant decline in CO levels was seen in the abstinent subgroup but not the non-abstinent subgroup. Both subgroups had significant declines in smoking satisfaction following treatment; however, the significance was greater in the abstinent smokers. As seen in Table 2, comparison between the two subgroups at week 5 revealed a significant reduction in smoking satisfaction of the last cigarette, based on the mCEQ questionnaire, and marginally significant reduction ambient craving in the abstainers compared to the non-abstainers. No other differences in smoking measures were seen following 5 weeks of varenicline treatment between the two subgroups.
TABLE 2.
Smoking measures.
| Baseline
|
Week 5
|
Significance between Baseline and Week 5
|
||||||
|---|---|---|---|---|---|---|---|---|
| Abstinent | Non- abstinent | p-value | Abstinent | Non- abstinent | p-value | Abstinent | Non Abstinent | |
| QSU-B Factor 1 | 29.8 (5.25) | 28.72 (3.90) | 0.604 | 5.10 (0.31) | 6.83 (2.79) | 0.188 | <0.001 | <0.001 |
| QSU-B Factor 2 | 13.4 (6.53) | 12.18 (7.25) | 0.690 | 5 (0) | 5 (0) | NS | 0.002 | 0.008 |
| QSU-B Total | 43.2 (10.74) | 40.91 (7.23) | 0.578 | 10.1 (0.32) | 11.83 (2.78) | 0.188 | <0.001 | <0.001 |
| FTND | 5.7 (1.25) | 5.09 (1.81) | 0.379 | 0.1 (0.31) | 1.67 (2.65) | 0.209 | <0.001 | 0.023 |
| mCEQ | 47.3 (11.57) | 42.7 (10.90) | 0.364 | 1.2 (3.79) | 18.83 (12.20) | 0.003 | <0.001 | 0.002 |
| Ambient Craving | 6 (2.64) | 5.54 (2.69) | 0.709 | 0.11 (0.33) | 1.67 (1.51) | 0.052 | <0.001 | 0.001 |
| CO Level | 16.6 (6.65) | 15.55 (8.19) | 0.748 | 1.80 (1.03) | 12.17 (17.87) | 0.214 | <0.001 | 0.675 |
Craving and Resisting the Urge to Smoke Neuroimaging Findings
Overall Group
During the crave condition at baseline, activation was seen in regions associated with craving including the PFC, ACC, PCC, precuneus, and left lingual cortex supporting our previous work (12). At week 5, similar regions of the PFC, ACC, PCC, and precuneus were found to be activated while on varenicline. When comparing the crave condition at baseline to week 5, no significant differences were discovered. During the baseline resist condition, activation was found in the PFC and left OFC. At week 5, the overall group demonstrated activation in the bilateral thalamus; however, no statistically significant differences were found when comparing baseline and week 5 of the entire group.
Abstainers and Continued Smokers
During the crave condition at baseline, no statistically significant differences were noted between those who were later able to abstain and the non-abstainers. At baseline during the resist condition, the abstinent subgroup compared to the non-abstinent subgroup demonstrated activation in the right insular cortex and possibly right putamen, left anterior thalamus, bilateral middle cingulate, and PCC part of the distributed network involved in alertnes, learning, and memory as seen in Figure 1 and Table 3.
FIGURE 1.
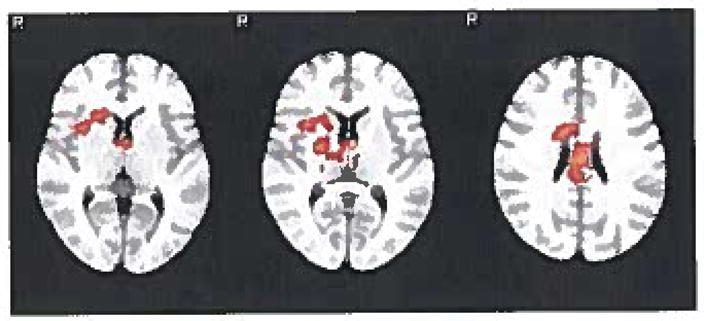
Resist condition at baseline (smoke-neutral contrast): areas of regional activation in the abstinent group greater than the non-abstinent group.
TABLE 3.
Cluster table.
| Contrast | Cluster | Z Max | p-Value | Voxels | MNI (x, y, z) | Anatomy | ||
|---|---|---|---|---|---|---|---|---|
| Abstinent > non-abstinent at baseline while resisting the urge to smoke (smoke–neutral contrast) | ||||||||
| Smoke > neutral | ||||||||
| 1 | 3.85 | .0009 | 2048 | 24 | −6 | 10 | Right putamen, right insular cortex1 | |
| 3.85 | −4 | −4 | 8 | Left anterior thalmus1 | ||||
| 3.7 | −2 | −12 | 26 | Posterior cingulate gyrus | ||||
| 3.67 | 6 | −18 | 28 | Posterior cingulate gyrus | ||||
| 3.6 | 14 | 6 | 24 | Middle cingulate gyrus1 | ||||
| 3.54 | 6 | −26 | 26 | Posterior cingulate gyrus | ||||
| Abstinent group: baseline > week 5 while resisting the urge to smoke | ||||||||
| Smoke > neutral | ||||||||
| 1 | 3.48 | .0246 | 1434 | 18 | 16 | 60 | Superior frontal gyrus | |
| 3.48 | 0 | 14 | 56 | Superior frontal gyrus | ||||
| 3.42 | 10 | 38 | 52 | Superior frontal gyrus | ||||
| 3.38 | 6 | 42 | 54 | Prefrontal cortex | ||||
| 3.33 | 0 | 20 | 56 | Prefrontal cortex | ||||
| 3.33 | 8 | 20 | 62 | Superior frontal gyrus | ||||
Notes: All analyses were completed using cluster thresholding (z > 2.3 and corrected cluster threshold of p = .05) at individual and group levels, unless otherwise specified. Voxels is the number of activated voxel within each cluster. Z Max is the local maximum z value. MNI (x, y, z) are the MNI coordinates for the local maximum. Anatomy structures were found using FSL View by inputting MNI coordinates utilizing the Harvard–Oxford Subcortical Structural Atlas.
Z max found in white matter, within the activation the closest subcortical structure listed.
In the abstinent subgroup, no significant differences were found between baseline and week 5 on varenicline in crave condition. Of particular interest, in the resist condition, greater activation was found in bilateral SFG extending into the PFC at baseline compared to week 5 in the abstinent subgroup, supporting the view that the SFG is involved in the inhibitory modulation of cue-elicited craving. (Figure 2; coordinates reported in Table 3). No areas of greater activation were found comparing week 5 to baseline in the abstinent subgroup. No statistically significant differences were found in either the crave or resist condition between the two time points in the non-abstinent subgroup.
FIGURE 2.
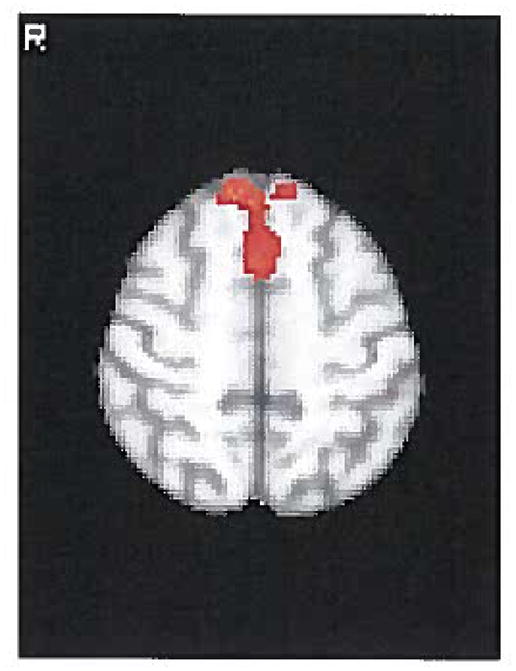
Resist condition in the abstinent group (smoke-neutral contrast): areas of regional activation that were greater at baseline compared to week 5.
DISCUSSION
Our findings indicated several differences between crave and resist conditions and abstainers and non-abstainers in conjunction with varenicline treatment. After 5 weeks on varenicline, both subgroups reported a statistically significant decline in ambient craving. Mechanistically, varenicline’s action on dopamine may account for the decrease in subjective craving. Low dopamine levels have been associated with craving and withdrawal symptoms from nicotine, both associated with relapse (25). Varenicline, a partial agonist at α4β2 nAChR, produces a sustained moderate increase in mesolimbic dopamine levels alleviating the reduced dopamine levels found during abstinence and quit attempts (26). We were unable to detect significant differences in the brain regional activation pattern in the crave condition between the abstinent and non-abstinent subgroups or between the two time points in either subgroup. These results suggest that while both subgroups reported less subjective craving, areas associated with attention and reward continued to be activated. This unconscious activation to smoking-related cues may be a key factor for the frequent slips and relapses during smoking quit attempts.
Limited research has examined the effect of medications on resisting the urge to smoke during cue exposure. A recent laboratory study found that among heavy, automatic smokers (within the first 5 minutes of waking), varenicline enhanced the ability to resist the urge to smoke and decreased subsequent smoking when presented with the opportunity to smoke (27). McClernon and colleagues demonstrated reduced activation of the SFG during cue-elicited craving following treatments aimed at reducing or extinguishing cue responsiveness (28,29). Our finding of increased activation of the SFG while resisting the urge to smoke at baseline compared to week 5 in successful quitters is of particular interest. To our knowledge, this is the first report of reduced activation of the SFG while resisting the urge to smoke during a successful quit attempt with varenicline. This finding supports recent research into the role of the SFG in both excitatory and inhibitory modulations of craving and cue reactivity (30).
Although exploratory, these preliminary findings suggest that varenicline’s potential to enhance cognition may be beneficial to a subset of smokers during a quit attempt. In chronic smokers, abstinence has been shown to impair cognitive function, which is restored with nicotine administration (31). During abstinence from smoking, varenicline, compared to placebo, has been shown to improve cognitive performance, including working memory and sustained attention (32). In addition to its α4β2 nAChR activity, varenicline is a full agonist of α7 nAChR. In animal studies, selective α7 nAChR agonists activate neurons in the PFC and nucleus accumbens shell, areas important for cognitive function (33). As such, the significant reduction in SFG and distributed network of insula, anterior thalamus, and cingulated cortex following smoking cessation/reduction may represent the cognitive enhancing properties of varenicline. By week 5, even in the presence of cue-elicited craving, resisting the urge to smoke is easier to modulate and no regional areas of activation were greater compared to baseline.
Our study has both strengths and limitations. Strengths include the within-subject design and assessment at two time points during a smoking quit attempt on varenicline. The study utilized subjective measures of smoking behavior combined with functional imaging of craving and resisting the urge to smoke. The majority of previous neuroimaging research has focused on cue-elicited craving only; examination of underlying neural substrates of resisting urges to smoke is also critical to the development of more effective treatments. Adherence was confirmed by self-report, a medication diary, and pill count. However, it is possible that not all of the participants took the medication as prescribed. An additional limitation is CO measure which is relatively imprecise in confirming abstinence and it is possible that participants smoked between visits. At the week 5 imaging visit, abstinence was verified by both CO monitoring and urinary cotinine, increasingly the likelihood of detecting smoking. The small sample size may have restricted our ability to detect subtle group changes in the BOLD signal, and the lack of a control group inhibits our ability to distinguish between the effects of abstinence and action of varenicline. The truest control group would be an unaided quit attempt without the use of any medication; however, this is impractical as the majority of unaided quit attempts result in a return to smoking. Small sample size in the subgroup analysis necessitates replication with a larger sample size. The addition of cognitive measures would also be beneficial.
In sum, varenicline’s combination of pharmacological effects as a partial agonist may not only ameliorate withdrawal symptoms and craving but also improve cognition, thus aiding in employing strategies to cope with craving and not giving into the urge to smoke.
Acknowledgments
The authors thank the individuals who participated in the study and acknowledge the contributions of the clinical research team, including Kat Giarla, Ann Frampton, and Max Owens.
Funding: GRAND GA30523K with additional support from 5R21DA026085-02, National Institute of Child Health and Human Development K12HD055885 awarded to Dr. Hartwell, & MUSC’s Clinical and Translational Science Awards grant UL1 RR029882 National Center for Research Resources, National Institutes of Health.
Footnotes
Declaration of Interest
Dr. Hartwell has received research funding from Pfizer for an unrelated multisite clinical trial examining the safety and efficacy of varenicline in adolescent smokers. Dr. Gray has received research funding from Merck, Inc., and Supernus Pharmaceuticals for unrelated studies. The other authors report no conflicts of interest.
References
- 1.Fiore MC, Jaen CR, Baker TI, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: Public Health Services; 2008. [Google Scholar]
- 2.Garrison GD, Dugan SE. Varenicline: A first-line treatment option for smoking cessation. Clin Ther. 2009;31:463–491. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 4.West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- 5.Killen JD, Fortmann SP. Craving is associated with smoking relapse: Findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- 6.Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD. Temptations to smoke after quitting: A comparison of lapsers and maintainers. Health Psychol. 1996;15:455–461. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- 7.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 8.Warthen MW, Tiffany ST. Evaluation of cue reactivity in the natural environment of smokers using ecological momentary assessment. Exp Clin Psychopharmacol. 2009;17:70–77. doi: 10.1037/a0015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- 11.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 12.Hartwell KJ, Johnson KA, Xingbao Li, Myrick H, LeMatty T, George MS, Brady KT. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16:654–666. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- 15.Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 17.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 18.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32:912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuro Image. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 20.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuro Image. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 21.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuro Image. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 22.Fu SS, Burgess DJ, Hatsukami DK, Noorbaloochi S, Clothier BA, Nugent S, van Ryn M. Race and nicotine replacement treatment outcomes among low-income smokers. Am J Prev Med. 2008;35:S442–S448. doi: 10.1016/j.amepre.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12:647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covey LS, Botello-Harbaum M, Glassman AH, Masmela J, LoDuca C, Salzman V, Fried J. Smokers’ response to combination bupropion, nicotine patch, and counseling treatment by race/ethnicity. Ethn Dis. 2008;18:59–64. [PubMed] [Google Scholar]
- 25.Malin DH. Nicotine dependence: Studies with a laboratory model. Pharmacol Biochem Behav. 2001;70:551–559. doi: 10.1016/s0091-3057(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 26.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: An alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 27.McKee SA, Weinberger AH, Shi J, Tetrault J, Developing CS. Validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14(11):1362–1371. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClernon F, Kozink Rachel, Lutz Avery, Rose Jed. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacol. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClernon F, Kozink R, Lutz A, Rose J. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacol. 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud’homme X, Krystal AD. Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry. 2011;70:794–799. doi: 10.1016/j.biopsych.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacol. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- 32.Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of α7 nicotinic acetylcholine receptors: From animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–343. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]


