Abstract
Summary
We evaluated the associations between dual energy X-ray absorptiometry (DXA) and histologically determined cancellous and cortical bone volume by controlling for vascular calcifications and demographic variables in hemodialysis (HD) patients. Femoral bone mineral density (f-BMD) was associated with cortical porosity.
Introduction
Assessment of bone mass in chronic kidney disease patients is of clinical importance because of the association between low bone volume, fractures, and vascular calcifications. DXA is used for noninvasive assessment of bone mass whereby vertebral results reflect mainly cancellous bone and femoral results reflect mainly cortical bone. Bone histology allows direct measurements of cancellous and cortical bone volume. The present study evaluates the association between DXA and histologically determined cancellous and cortical bone volumes in HD patients.
Methods
In 38 HD patients, DXA was performed for assessment of bone mass, anterior iliac crest bone biopsies for bone volume, and multislice computed tomography for vascular calcifications.
Results
While lumbar bone mineral density (l-BMD) by DXA was not associated with histologically measured cancellous bone volume, coronary Agatson score showed a borderline statistically significant association (P=0.055). When controlled for age and dialysis duration, f-BMD by DXA was associated with cortical porosity determined by histology (P=0.005).
Conclusions
The usefulness of l-BMD for predicting bone volume is limited most probably because of interference by soft tissue calcifications. In contrast, f-BMD shows significant association with cortical porosity.
Keywords: Bone biopsy, Bone mineral density, Bone volume, Chronic kidney disease, Cortical porosity, Vascular calcifications
Introduction
Low bone mass is a common complication of chronic kidney disease (CKD), and the most prominent clinical complications of low bone mass are fractures. Compared to the general population, the incidence of fractures was reported to be 3.6–9.8 times higher for stage 5 CKD patients on dialysis (CKD-5 patients) [1]. In the Dialysis Outcomes and Practice Patterns Study, the authors found a 7% increased risk of hip fracture per year of dialysis treatment [2]. Most recently, low bone mass has also been shown to be associated with increased cardiovascular calcifications in non-CKD and CKD patients [3, 4]. In light of the high mortality associated with fractures and cardiovascular disease in CKD [5, 6], noninvasive methods for assessing bone mass in this patient population is of great clinical importance.
Dual energy X-ray absorptiometry (DXA) is the most widely used tool for assessment of bone mass in the general population [7, 8]. However, DXA measures not only the mineral content of bone but also of the surrounding soft tissue [9, 10] limiting the interpretation of results [11]. CKD-5 patients represent a unique population particularly prone to developing soft tissue and vascular calcifications [12, 13]. Therefore, overestimation of bone mineral density (BMD) by anterioposterior (AP) lumbar DXA represents a great problem in these patients; this limitation should be less pronounced but not excluded in femoral DXA measurements [14]. Overall, studies evaluating the role of BMD determination by DXA for assessment of fracture risk in CKD-5 patients report conflicting results [15–18].
In order to guide appropriate therapeutic interventions, characterization of the relationship between DXA measurements and histologically determined bone volume in CKD-5 patients is desirable. In an early study, Lindergard and colleagues could not find a correlation between BMD measured at the radius (consisting primarily of cortical bone) and histologically determined bone volume at the iliac crest of CKD-5 patients (cancellous bone) [19]. In contrast, a more recent study in CKD-5 patients reported that hip and spine T-scores were associated with histologically determined bone volume at the iliac crest [20]. In light of these conflicting results, we evaluated the associations between BMD measured by DXA at the spine and femur and histologically determined bone volume in cancellous bone (bone volume/tissue volume) and in cortical bone (cortical width and cortical porosity) in CKD-5 patients. Moreover, vascular calcifications were assessed by multislice computed tomography (MSCT) and adjusted for in statistical analyses.
Materials and methods
Study design
This study investigates the association between BMD measured by DXA at the hip and spine and histologically determined parameters of bone volume in cancellous and cortical bone in a cross-sectional study design. In addition, vascular calcifications were measured by multislice computed tomography at the thoracic aorta and the coronary and iliac arteries and were adjusted for in the analyses. The protocol was approved by the Institutional Review Boards of participating institutions. The study has been conducted in adherence to the Declaration of Helsinki, and all patients provided informed consent.
Patients
Thirty-eight white stage 5 CKD patients on hemodialysis were recruited from 11 medical centers in Portugal. All patients provided informed consent for performing DXA measurements, bone biopsies, and multislice computed tomography. DXA measurements were performed at time of bone biopsy and patients underwent multislice computed tomography on average 3.8±1.9 months after the bone biopsy.
Inclusion criteria
These are age 18 years or older, dialysis duration of at least 3 months, mental competence, and willingness to participate in the study.
Exclusion criteria
These are kidney transplant; pregnancy; uncontrolled systemic illnesses or organic diseases with potential influence on bone metabolism such as diabetes mellitus, active or chronic liver disease, malabsorption, malignancy, and thyroid dysfunction; history of or present treatment with bisphosphonates, fluoride, calcitonin, glucocorticoids, or other immunosuppressive agents, hormone replacement therapy, and selective estrogen receptor modulators; and chronic alcoholism and/or drug addiction.
Bone biopsies
Anterior iliac crest bone biopsies were performed under local anesthesia and conscious sedation. Bone samples were obtained with the one-step electrical drill technique (Straumann Medical, Waldenburg, Switzerland). Bone samples were processed undecalcified and cut avoiding cracks or overlaps of bone tissue. Sections were stained with the modified Masson–Goldner trichrome stain [21], the aurin tricarboxylic acid stain [22], and solochrome azurin [23], assessment of stainable aluminum, and modified Gomori stain for detection of iron [24]. Unstained sections were prepared for phase contrast and fluorescent light microscopy. Histomorphometric analysis of bone was done at standardized sites in cancellous (×200 magnification) and cortical bone (×80 magnification) using the semi-automatic method (Osteoplan II, Kontron, Munich, Germany). For cancellous bone, the volume of bone trabecules (BV) and total tissue volume (TV) were traced and bone volume/total volume (BV/TV) was calculated for assessment of mineralized trabecular bone volume. For cortical bone, cortical width was measured; cortical porosity was determined by tracing the total cortex and all Haversian canals and computing the ratio between canal area over total cortical tissue area.
Results were compared to our normative database consisting of histomorphometric results of age- and gender-matched healthy individuals [25, 26]. “Low” cancellous bone volume was defined as BV/TV <16.8%, “normal” as BV/TV between 16.8% and 22.9%, and “high” as BV/TV >22.9%. Cortical width was considered “low” for values <0.52 mm, “normal” for values between 0.52 and 1.65 mm, and “high” for values >1.65 mm. Cortical porosity was classified “low” for values <1.9%, “normal” for values between 1.9% and 12%, and “high” for values >12%. All bone samples were processed and analyzed without knowledge of the clinical data at the Bone Diagnostic and Research Laboratory, University of Kentucky, Lexington, KY, USA.
Bone mineral density
DXA was performed by the same operator on the same Hologic QDR Discovery scanner according to the manufacturer’s recommendations for patient positioning, scan protocols, and scan analysis. Measurements of the spine and hip were obtained from the AP projection. For AP lumbar spine, lumbar vertebrae 1 to 4 were measured and BMD results were analyzed for mean measurements of L1–L4. For proximal femur scans, BMD was measured at the femoral neck. The coefficients of variation for these BMD measurements are AP spine 1.2% and femur 0.9%.
Assessment of vascular calcifications
Vascular calcifications were assessed at the thoracic aorta and coronary and iliac arteries by a quantitative score using MSCT. MSCT scans were performed on the model Somatom Volume Zoom (Siemens AG, Erlangen, Ger-many). Slices of 2.5 mm thickness were acquired under the following conditions: 120 kVp, 130 mAs, and 0.5 gantry rotation time. All images were transferred to a workstation and analyzed with calcium scoring software (HeartView CT, Siemens AG, Erlangen, Germany). Quantification of vascular calcifications was performed by calculating the Agatston score based on the maximum X-ray attenuation coefficient (measured in Hounsfield units) [27].
Biochemical measurements
Blood was drawn at the time of the bone biopsy after an overnight fast. The following biochemical parameters were measured: serum calcium and phosphorus by an autoanalyzer (Hitachi 747, Globe Scientific Inc, USA), intact parathyroid hormone (iPTH) by DPC IMMULITE® PTH IRMA from Diagnostics Products Corporation (Los Angeles, CA, USA; normal range 16–87 pg/ml; intra- and interassay coefficients of variation <7% and <9%), and 25-(OH)-vitamin D by LIAISON® 25-OH Vitamin D assay (Diasorin, Saluggia, Italy; normal range 25–100 ng/ml; intra- and interassay coefficients of variation 4.1% and 7%).
Statistical analysis
Descriptive statistics are presented as means, medians, minimums, maximums, and standard deviations (SDs). The variables iPTH and hemodialysis (HD) duration were log-transformed for analysis. Boxplots were used to characterize the distributions of BV/TV, cortical width, and cortical porosity. Bivariate associations were assessed using scatter plots, nonparametric Spearman rank correlations, locally weighted regression, and generalized additive models to characterize the associations between femoral/lumbar BMD and cortical porosity, cortical width, and BV/TV. Linear regression analyses were performed to evaluate possible relationships while controlling for relevant measured correlates. All calculations were performed using the R statistical package (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the study population are presented in Table 1. All patients were receiving phosphate binder therapy (58% sevelamer hydrochloride, 42% calcium acetate) and 19 patients (50%) were treated with active vitamin D analogs. None of the patients were treated with a calcimimetic agent. There were no clinically symptomatic fractures prior and during the study.
Table 1.
Characteristics of the study population
| Mean (SD) | Median (min–max) | |
|---|---|---|
| Age (years) | 45.2 (15.2) | 45 (21–74) |
| Gender | ||
| Male (N, %) | 20 (52.6) | |
| Female (N, %) | 18 (47.4) | |
| Dialysis duration (months) | 73.1 (56.6) | 48.3 (21–206) |
| Calcium (mg/dl) | 96 (6.6) | 95.4 (87–111) |
| Phosphorus (mg/dl) | 5.4 (0.9) | 5.5 (3.9–7.2) |
| iPTH (pg/ml) | 620 (614) | 353.4 (50–2,164) |
| VIT D25 (ng/ml) | 21.2 (7.8) | 21.5 (7.8–37.6) |
| Agatston scores | ||
| Coronary arteries | 958.3 (1,888.4) | 99.25 (0.0–6,726.0) |
| Thoracic aorta | 1,391.0 (3,026.9) | 3.2 (0.0–12,576.2) |
| Iliac arteries | 2,916.0 (5,430.6) | 848.2 (0.0–28,670.0) |
iPTH intact parathyroid hormone, vit D25 25(OH)-hydroxy vitamin D
In cancellous bone, BV/TV was low in 16%, normal in 24%, and high in 60% patients. In cortical bone, cortical porosity was low in 3%, normal 40%, and high in 57%, and cortical width was low in 8%, normal 89%, and high in 3%. None of the biopsies showed positive staining for aluminum or iron.
DXA results are reported as “measured bone mineral density” (in grams per centimeter squared). The correlation between lumbar and femoral BMD measurements was r= 0.49 (P=0.01).
Femoral bone mineral density
Unadjusted analysis of the association between femoral BMD and cortical porosity revealed a correlation coefficient r=−0.20 (P=0.24). When adjusted for different Agatston score groups, no statistically significant effects of the different vascular calcifications sites were found. In the final model, a forward predictor selection routine identified femoral BMD (point estimate −157.49; P= 0.005), age >50 years (point estimate −8.37; P=0.001), and HD duration (point estimate −25.44; P=0.005) as being associated with cortical porosity (Table 2). Further statistical analysis recognized a statistically significant interaction between femoral BMD and HD duration (point estimate 32.48; P=0.01): independent of age group, cortical porosity increased as femoral BMD decreased, and this increase in cortical porosity was more rapid for patients with shorter HD duration (Fig. 1).
Table 2.
Predictor variables of cortical porosity (from linear regression analysis)
| Estimate | Standard error | P value | |
|---|---|---|---|
| fBMD | −157.49 | 52.36 | 0.005 |
| Age >50 years | −8.37 | 2.18 | 0.0005 |
| HD | −25.44 | 8.40 | 0.005 |
| fBMD/HD | 32.48 | 12.09 | 0.01 |
fBMD femoral bone mineral density, HD hemodialysis duration, fBMD/HD interaction term between femoral bone mineral density and hemodialysis duration
Fig. 1.
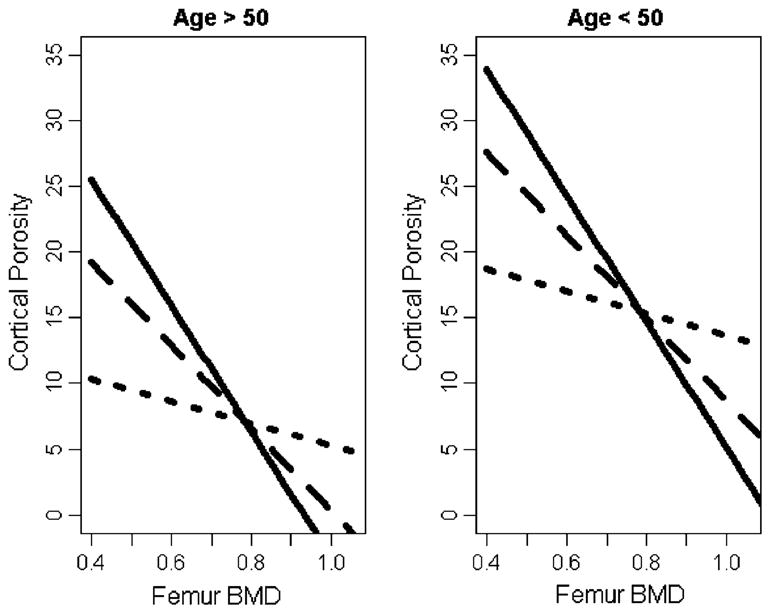
Estimated mean cortical porosity based on age group and HD duration. The three lines in each plot correspond to patients with dialysis durations equal to the 25th (solid line), 50th (dashed line), and 75th (dotted line) empirical percentiles
Unadjusted correlation analysis of femoral BMD and cancellous bone volume (BV/TV) yielded r=−0.06 (P= 0.73). While age, gender, and HD duration were not predictive of cancellous bone volume (BV/TV), the model containing femoral BMD and coronary Agatston score >100 revealed a statistically significant negative association (point estimate −4.93; P=0.03); this association was, however, lost when the model was adjusted for age and gender.
When examining a possible association between femoral BMD and cortical width, unadjusted (r=0.004; P=0.98) and adjusted analyses (models containing age, gender, HD duration, and vascular calcifications) did not yield any significant predictors.
Lumbar bone mineral density
In unadjusted analysis, the correlation between lumbar BMD and cancellous bone volume (BV/TV) was not statistically significant (r=−0.1; P=0.53) and remained statistically nonsignificant when the model was adjusted for gender, age, and HD duration (P>0.05 for all variables). Similar results were found for the correlations between lumbar DXA and cortical width/porosity (r=−0.09, P= 0.60; r=−0.15, P=0.39, respectively) and adjusted models (gender, age, HD duration, all variables P>0.05).
In the next analytical step, the model was adjusted for Agatston scores measured at the thoracic aorta, coronary artery, or iliac artery. For this purpose, patients were classified according to an Agatston score cutoff of 100 (≤100 versus >100). Regression modeling revealed that only coronary Agatston score >100 showed borderline significant negative association with lower cancellous bone volume (BV/TV) (point estimate −4.14; P=0.055) but this association was lost when the model was also adjusted for gender and age.
Conclusions
The National Institutes of Health consensus conference on “Osteoporosis prevention, diagnosis and therapy” defines osteoporosis as a systemic disease of impaired bone strength [28]. Clinically and in specific populations, the diagnosis “osteoporosis” is defined on the basis of standardized BMD levels (T-score≤−2.5 SD) determined by DXA at the spine, hip, or forearm [29]. In the case of patients suffering from CKD, the use of this traditional approach to diagnose osteoporosis is problematic since all forms of renal bone disease may be accompanied by low BMD [30, 31], and erroneously high BMD measurements due to vascular/soft tissue calcifications—which are commonly encountered pathologic findings in CKD patients—are known problems of anterioposterior DXA projections [32]. Accordingly, it has been proposed that, currently, the only way to establish the diagnosis of osteoporosis in patients with stage 5 CKD is the histomorphometrical finding of low bone volume [33]. Based on these observations, we selected not to classify our patients according to specific scores but to evaluate the association between measured bone mineral density (expressed in grams per centimeter squared) at the lumbar and femoral sites and histomorphometric determinants of cancellous bone volume (BV/TV) and cortical bone volume (cortical width and cortical porosity). Furthermore, we adjusted our models for Agatston scores measured by multislice computed tomography.
Our study results corroborate previous observations regarding lack of associations between histologically determined cancellous bone volume (BV/TV) and BMD measurements reported in CKD patients (creatinine clearance 10–78 ml/min) [34] and expand those findings to stage 5 CKD patients on hemodialysis. The usefulness of iliac crest bone biopsies for assessment of bone changes in the femur has been reported in patients requiring total hip replacement [35]. Although there is paucity of data regarding the association between cortical bone and fractures in CKD patients, the role of increased cortical porosity and decreased cortical thickness in femoral neck fractures has been described in the general population [36–38]. A novel finding of our study is the statistically highly significant association between cortical porosity and femoral BMD in stage 5 CKD patients that persisted after statistical adjustments. Since cortical changes of the femoral neck contribute to the risk of hip fractures [38] and fractures of the axial skeleton are highly prevalent in stage 5 CKD patients [39], our findings suggest a possible clinical role of femoral DXA measurements for identifying stage 5 CKD patients on hemodialysis at risk for fracture. The stronger association between femoral BMD and cortical porosity at shorter HD durations implies that additional factors present after longer HD vintage such as more extensive soft tissue calcifications will limit the value of femoral BMD for assessing cortical porosity as dialysis vintage progresses.
We would like to acknowledge the following limitations of our study: Goal of our study was not the comprehensive evaluation of all risk factors for hip fractures in stage 5 CKD patients but to investigate the role of DXA measurements for assessing histological parameters of cancellous (BV/TV) and cortical bone volume (cortical porosity and cortical width). In order to limit the influence of potentially confounding variables of bone metabolism and vascular calcifications, we imposed strict inclusion and exclusion criteria for study participation; accordingly, our findings call for larger population-based studies to validate a possible role of femoral BMD measurements by DXA including hip structural analysis for assessing bone fracture risk not only in stage 5 CKD patients on different dialysis modalities but also to include patients suffering from different levels of CKD as well as CKD patients with previous fractures. Future large age-matched population-based studies will also need to answer the question on differences in bone volume between healthy and CKD patients.
In summary, our data suggest a role for femoral DXA measurements to assess cortical porosity in stage 5 CKD patients on hemodialysis. Anterioposterior lumbar measurements by DXA do not yield information useful for assessment of cancellous bone volume (BV/TV) that has been shown to be associated with coronary calcifications [4]. In light of the high morbidity and mortality of stage 5 CKD patients, future prospective clinical studies will need to further characterize the role of this relatively inexpensive and widely available clinical tool (DXA) for assessment of clinical outcomes such as fractures.
Acknowledgments
The following colleagues have participated in the study: Célia Gil, Carlos Oliveira, José Galvão, António Morais Sarmento, Silvia Ribeiro, Jorge Dickson, Berta Carvalho, Ilídio Rodrigues, Jorge Baldaia, and Odete Pereira. This study was supported by grants National Institutes of Health NIH RO1 DK51530 (H.H.M.), by the Kentucky Nephrology Research Trust (M-C.M-F., H.H.M.), by the Dean’s Clinical Research Scholar Program, University of Kentucky, no 1012112710 (J.H.), and by a grant from Genzyme. The authors would like to thank ISNI, Instituto Nefrológico de Investigação, for the assistance in the organization of this study and Ms. Juliana Van Willigen and Richard Wheaton for their technical assistance.
Footnotes
Conflicts of interest None.
Contributor Information
T. Adragao, Nephrology Department, Santa Cruz Hospital, Lisbon, Portugal
J. Herberth, Division of Nephrology, Bone and Mineral Metabolism, University of Kentucky, Lexington, KY, USA
M.-C. Monier-Faugere, Division of Nephrology, Bone and Mineral Metabolism, University of Kentucky, Lexington, KY, USA
A. J. Branscum, Departments of Biostatistics, Statistics, and Epidemiology, University of Kentucky, Lexington, KY, USA
A. Ferreira, Nephrology Department, Curry Cabral Hospital, Lisbon, Portugal
J. M. Frazao, Nephrology Department, Hospital de S. João, Medical School and Nephrology Research and Development Unit, University of Porto, Porto, Portugal
H. H. Malluche, Email: hhmall@uky.edu, Division of Nephrology, Bone and Mineral Metabolism, University of Kentucky, Lexington, KY, USA. Division of Nephrology, Bone & Mineral Metabolism, UK Medical Center, Room MN 564, 800 Rose Street, Lexington 40536-0084 KY, USA
References
- 1.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 2.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 3.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 4.Adragao T, Herberth J, Monier-Faugere MC, Branscum AJ, Ferreira A, Frazao JM, Dias Curto J, Malluche HH. Low bone volume—a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:450–455. doi: 10.2215/CJN.01870408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36:1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 6.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47:149–156. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Hans DB, Shepherd JA, Schwartz EN, Reid DM, Blake GM, Fordham JN, Fuerst T, Hadji P, Itabashi A, Krieg MA, Lewiecki EM. Peripheral dual-energy X-ray absorptiometry in the management of osteoporosis: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:188–206. doi: 10.1016/j.jocd.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Writing Group for the ISCD Position Development Conference. Indications and reporting for dual-energy X-ray absorptiometry. J Clin Densitom. 2004;7:37–44. doi: 10.1385/jcd:7:1:37. [DOI] [PubMed] [Google Scholar]
- 9.Hangartner TN, Johnston CC. Influence of fat on bone measurements with dual-energy absorptiometry. Bone Miner. 1990;9:71–81. doi: 10.1016/0169-6009(90)90101-k. [DOI] [PubMed] [Google Scholar]
- 10.Formica C, Loro ML, Gilsanz V, Seeman E. Inhomogeneity in body fat distribution may result in inaccuracy in the measurement of vertebral bone mass. J Bone Miner Res. 1995;10:1504–1511. doi: 10.1002/jbmr.5650101011. [DOI] [PubMed] [Google Scholar]
- 11.Wren TA, Kim PS, Janicka A, Sanchez M, Gilsanz V. Timing of peak bone mass: discrepancies between CT and DXA. J Clin Endocrinol Metab. 2007;92:938–941. doi: 10.1210/jc.2006-1570. [DOI] [PubMed] [Google Scholar]
- 12.Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 13.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 14.Taal MW, Masud T, Green D, Cassidy MJ. Risk factors for reduced bone density in haemodialysis patients. Nephrol Dial Transplant. 1999;14:1922–1928. doi: 10.1093/ndt/14.8.1922. [DOI] [PubMed] [Google Scholar]
- 15.Jamal SA, Chase C, Goh YI, Richardson R, Hawker GA. Bone density and heel ultrasound testing do not identify patients with dialysis-dependent renal failure who have had fractures. Am J Kidney Dis. 2002;39:843–849. doi: 10.1053/ajkd.2002.32006. [DOI] [PubMed] [Google Scholar]
- 16.Piraino B, Chen T, Cooperstein L, Segre G, Puschett J. Fractures and vertebral bone mineral density in patients with renal osteodystrophy. Clin Nephrol. 1988;30:57–62. [PubMed] [Google Scholar]
- 17.Urena P, Bernard-Poenaru O, Ostertag A, Baudoin C, Cohen-Solal M, Cantor T, de Vernejoul MC. Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol Dial Transplant. 2003;18:2325–2331. doi: 10.1093/ndt/gfg403. [DOI] [PubMed] [Google Scholar]
- 18.Inaba M, Okuno S, Kumeda Y, Yamakawa T, Ishimura E, Nishizawa Y. Increased incidence of vertebral fracture in older female hemodialyzed patients with type 2 diabetes mellitus. Calcif Tissue Int. 2005;76:256–260. doi: 10.1007/s00223-004-0094-0. [DOI] [PubMed] [Google Scholar]
- 19.Lindergard B, Johnell O, Nilsson BE, Wiklund PE. Studies of bone morphology, bone densitometry and laboratory data in patients on maintenance hemodialysis treatment. Nephron. 1985;39:122–129. doi: 10.1159/000183355. [DOI] [PubMed] [Google Scholar]
- 20.Van Eps CL, Jeffries JK, Anderson JA, Bergin PT, Johnson DW, Campbell SB, Carpenter SM, Isbel NM, Mudge DW, Hawley CM. Mineral metabolism, bone histomorphometry and vascular calcification in alternate night nocturnal haemodialysis. Nephrology (Carlton) 2007;12:224–233. doi: 10.1111/j.1440-1797.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 21.Goldner J. A modification of the Masson trichrome technique for routine laboratory purposes. Am J Pathol. 1938;14:237–243. [PMC free article] [PubMed] [Google Scholar]
- 22.Lillie PD, Fullmer HM. Histopathologic technique and practical histochemistry. McGraw Hill; New York: 1976. [Google Scholar]
- 23.Denton J, Freemont AJ, Ball J. Detection of distribution of aluminum in bone. J Clin Pathol. 1984;37:136–142. doi: 10.1136/jcp.37.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomori G. Microtechnical demonstration: a criticism of its methods. Am J Pathol. 1936;12:655–663. [PMC free article] [PubMed] [Google Scholar]
- 25.Malluche HH, Meyer W, Sherman D, Massry SG. Quantitative bone histology in 84 normal American subjects. Micromorphometric analysis and evaluation of variance in iliac bone. Calcif Tissue Int. 1982;34:449–455. doi: 10.1007/BF02411283. [DOI] [PubMed] [Google Scholar]
- 26.Malluche HH, Faugere MC. Atlas of mineralized bone histology. Karger; New York: 1986. [Google Scholar]
- 27.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 28.NIH Consensus Development Panel . Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 29.Miller PD, Bonnick SL, Rosen CJ. Consensus of an international panel on the clinical utility of bone mass measurements in the detection of low bone mass in the adult population. Calcif Tissue Int. 1996;58:207–214. doi: 10.1007/BF02508636. [DOI] [PubMed] [Google Scholar]
- 30.Lindberg JS, Moe SM. Osteoporosis in end-state renal disease. Semin Nephrol. 1999;19:115–122. [PubMed] [Google Scholar]
- 31.Hruska KA, Teitelbaum SL. Renal osteodystrophy. N Engl J Med. 1995;33:166–174. doi: 10.1056/NEJM199507203330307. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham J, Sprague SM, Cannata-Andia J, Coco M, Cohen-Solal M, Fitzpatrick L, Goltzmann D, Lafage-Proust MH, Leonard M, Ott S, Rodriguez M, Stehman-Breen C, Stern P, Weisinger J. Osteoporosis in chronic kidney disease. Am J Kidney Dis. 2004;43:566–571. doi: 10.1053/j.ajkd.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Miller PD. Treatment of osteoporosis in chronic kidney disease and end-stage renal disease. Curr Osteoporos Rep. 2005;3:5–12. doi: 10.1007/s11914-005-0021-y. [DOI] [PubMed] [Google Scholar]
- 34.Lobao R, Carvalho AB, Cuppari L, Ventura R, Lazaretti-Castro M, Jorgetti V, Vieira JG, Cendoroglo M, Draibe SA. High prevalence of low bone mineral density in pre-dialysis chronic kidney disease patients: bone histomorphometric analysis. Clin Nephrol. 2004;62:432–439. doi: 10.5414/cnp62432. [DOI] [PubMed] [Google Scholar]
- 35.Dorr LD, Arnala I, Faugere MC, Malluche HH. Five-year postoperative results of cemented femoral arthroplasty in patients with systemic bone disease. Clin Orthop Relat Res. 1990;259:114–121. [PubMed] [Google Scholar]
- 36.Boyce TM, Bloebaum RD. Cortical aging differences and fracture implications for the human femoral neck. Bone. 1993;14:769–778. doi: 10.1016/8756-3282(93)90209-s. [DOI] [PubMed] [Google Scholar]
- 37.Bell KL, Loveridge N, Power J, Garrahan N, Meggitt BF, Reeve J. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999;24:57–64. doi: 10.1016/s8756-3282(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 38.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–135. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 39.Ersoy FF, Passadakis SP, Tam P, Memmos ED, Katopodis PK, Ozener C, Akcicek F, Camsari T, Ates K, Ataman R, Vlachojannis JG, Dombros AN, Utas C, Akpolat T, Bozfakioglu S, Wu G, Karayaylali I, Arinsoy T, Stathakis PC, Yavuz M, Tsakiris JD, Dimitriades CA, Yilmaz ME, Gultekin M, Karayalcin B, Yardimsever M, Oreopoulos DG. Bone mineral density and its correlation with clinical and laboratory factors in chronic peritoneal dialysis patients. J Bone Miner Metab. 2006;24:79–86. doi: 10.1007/s00774-005-0650-3. [DOI] [PubMed] [Google Scholar]


