Abstract
An 8-year-old male neutered Labrador Retriever was referred to the University of Wisconsin Veterinary Medical Teaching Hospital with a presumptive diagnosis of leukemia. Hematologic abnormalities included normal neutrophil count with a left shift, monocytosis, eosinophilia, thrombocytopenia, and circulating immature mononuclear cells. Bone marrow was effaced by immature hematopoietic cells of various morphologic appearances. In addition, large multinucleated cells were observed frequently. Flow cytometric analysis of nucleated cells in blood revealed 34% CD34+ cells, consistent with acute leukemia. By immunocytochemical analysis of cells in blood and bone marrow, some mononuclear cells expressed CD18, myeloperoxidase, and CD11b, indicating myeloid origin; some, but not all, large multinucleated cells expressed CD117 and CD42b, the latter supporting megakaryocytic lineage. The diagnosis was acute myeloblastic leukemia without maturation (AML-M1). To identify genetic aberrations associated with this malignancy, cells from formalin-fixed paraffin-embedded bone marrow were analyzed cytogenetically by multi-color fluorescence in situ hybridization (FISH). Co-localization of bacterial artificial chromosome (BAC) containing BCR and ABL was evident in 32% of cells. This confirmed the presence of the canine BCR-ABL translocation or Raleigh chromosome. In people, the analogous translocation or Philadelphia chromosome is characteristic of chronic myelogenous leukemia (CML) and is rarely reported in AML. BCR-ABL translocation also has been identified in dogs with CML; however, to our knowledge this is the first report of AML with a BCR-ABL translocation in a domestic animal.
Keywords: Cytogenetics, FISH, immunophenotyping, Raleigh chromosome
Case Presentation
An 8-year-old male neutered Labrador Retriever was referred to the University of Wisconsin Veterinary Medical Teaching Hospital (UW-VMTH) with a presumptive diagnosis of leukemia. The dog initially was presented to the referring veterinarian for lethargy, anorexia, soft stool, and significant polydipsia for 2 days. The dog was febrile (105°F) and had a moderate leukocytosis (49.5 × 103 leukocytes/μL, reference interval [RI] 5.5–16.9 × 103/μL) and thrombocytopenia (41.0 × 103 platelets/μL, RI 175–500 × 103/μL). A CBC performed 1 year earlier had revealed no hematologic abnormalities. The dog was started on a regimen of sucralfate, prednisone, amoxicillin, ciprofloxacin, and doxycycline. On presentation to the UW-VMTH, the dog was bright, alert, and well hydrated. Physical examination was unremarkable. Initial diagnostic procedures included a CBC, serum biochemical profile, thoracic radiographs, and abdominal ultrasonographic examination.
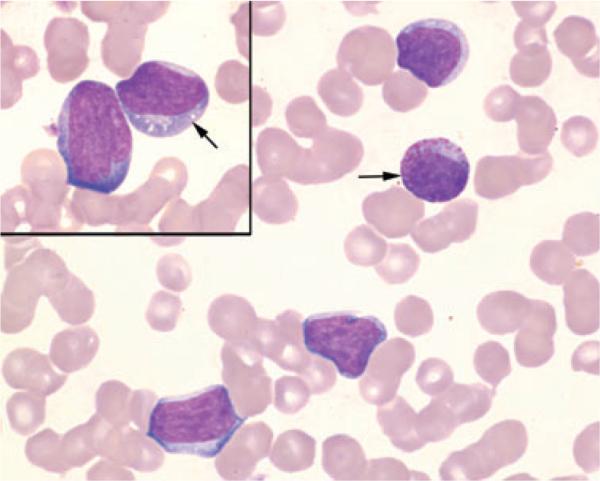
The CBC (Advia 120; Siemens Healthcare Diagnostics, Tarrytown, NY, USA), including a manual differential leukocyte count performed on a blood smear stained with modified Wright–Giemsa stain (7150 Aerospray slide stainer; Wescor Inc., Logan, UT, USA), revealed moderate thrombocytopenia, moderate leukocytosis, a neutrophil count within the RI with a degenerative left shift, monocytosis, eosinophilia, and atypical mononuclear cells (Table 1). Toxic change consisting of Döhle bodies, basophilia, and cytoplasmic vacuolation, was noted in mature and immature neutrophils. Atypical mononuclear cells (22,427/μL) were 15–20 μm in diameter with moderate to high nuclear:cytoplasmic (N:C) ratios (Figure 1). The cytoplasm was basophilic and infrequently contained coarse pink granules, vacuoles, or both. Nuclei were round and occasionally indented with coarsely stippled chromatin and, occasionally, prominent nucleoli. Large and giant platelets were observed along with rare cytoplasmic fragments. Abnormalities identified on the serum biochemical profile (Hitachi 912; Roche Diagnostics, Indianapolis, IN, USA) included mildly increased alkaline phosphatase (435 U/L, RI 13-289 U/L) and aspartate aminotransferase (114 U/L, RI 13-81 U/L) activities.
Table 1.
Hematologic results from a dog with acute myeloblastic leukemia (AML-M1).
| Variable | Result | Reference Intervals |
|---|---|---|
| Total plasma protein (g/dL) | 7.0 | 6.0–7.5 |
| RBC (×106/nU | 5.6 | 5.5–8.5 |
| HGB (g/dL) | 12 | 12–18 |
| HCT (%) | 37 | 37–55 |
| MCV(fL) | 67 | 60–77 |
| MCH (pg) | 22 | 19–24 |
| MCHC (g/dL) | 33 | 32–36 |
| Platelets (×103/μL) | 64 | 175–500 |
| Total WBC (×103/μL) | 45.8 | 6.0–17.0 |
| Segmented neutrophils (×103/μL) | 9.2 | 3.0–11.5 |
| Band neutrophils (×103/μL) | 2.8 | 0–0.3 |
| Lymphocytes (×103/μL) | 3.2 | 1.0–4.8 |
| Monocytes (×103/μU | 3.2 | 0.2–1.4 |
| Eosinophils (×103/μL) | 3.2 | 0.1–0.8 |
| Metamyelocytes (×103/μU | 1.8 | – |
| Atypical mononuclear (×103/μL) | 22.4 | – |
Figure 1.
Peripheral blood from a dog with acute myeloblastic leukemia. Note atypical mononuclear cells with high N:C ratios, basophilic cytoplasm, and round to indented nuclei with coarsely stippled chromatin and, occasionally, visible nucleoli. The cytoplasm occasionally contains pink cytoplasmic granules (arrow) or small distinct vacuoles (arrow, inset). Modified Wright–Giemsa, × 100 objective.
Thoracic radiographic imaging revealed 2 soft-tissue opacities in the cranial mediastinum, one measuring 9.2 × 7.5 cm located adjacent to and partially obscuring the cranial cardiac silhouette and another measuring 5.3 cm in diameter located ventral to the trachea. Fine-needle aspirates from one of the masses consisted of large numbers of individualized round cells similar to the atypical mononuclear cells in blood. Moderate numbers of immature granulocytes were admixed with the round cells, and binucleated and multinucleated cells were seen occasionally. There were numerous mitotic figures. Abdominal ultrasonographic examination was within normal limits.
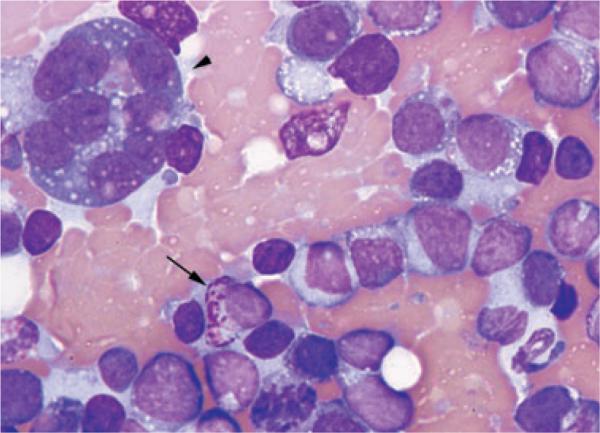
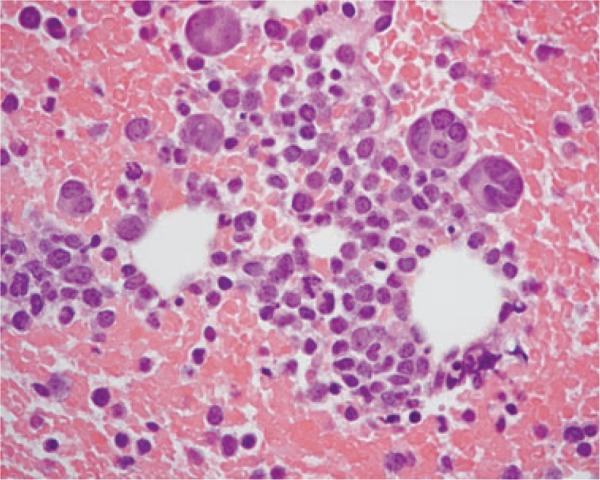
A bone marrow aspirate and core biopsy were collected from the proximal humerus. The aspirate was highly cellular and consisted mostly of immature hematopoietic cells that were 12–18 μm in diameter and had high N:C ratios (Figure 2). The cytoplasm was lightly to deeply basophilic and often contained a few fine vacuoles or, less often, fine pink granules. Nuclei were round with coarsely stippled chromatin and occasionally distinct nucleoli. Cells containing numerous coarse, pink granules were noted in small numbers. Binucleate, trinucleate, and large multinucleated cells (40–70 μm) were present in moderate numbers. The latter differed from mature megakaryocytes in that nuclei appeared individualized and had coarse chromatin, and the cytoplasm was deeply basophilic with occasional small vacuoles. There were rare erythrocytic and granulocytic precursors and rare well-differentiated, mature megakaryocytes. The findings were consistent with acute leukemia with marked erythrocytic, granulocytic, and megakaryocytic hypoplasia. Sections of the bone marrow biopsy were mildly cellular and contained focal cellular areas surrounded by numerous erythrocytes (Figure 3). Most cells were round mononuclear cells similar to the mononuclear cells in the bone marrow aspirate. Multinucleated cells were also present, and rare mitotic figure were seen. Erythrocytic and granulocytic precursors were severely reduced. The histologic interpretation was round cell neoplasm of unknown origin.
Figure 2.
Bone marrow aspirate from a dog with acute myeloblastic leukemia. Mononuclear cells predominate and have high N:C ratios and basophilic cytoplasm. Some cells contain discrete vacuoles or coarse pink granules (arrow). A large multinucleated cell (arrowhead) is present and has a high N:C ratio, basophilic cytoplasm containing small vacuoles, and 8 round to oval nuclei. Modified Wright–Giemsa, × 100 objective.
Figure 3.
Histologic section of bone marrow from a dog with acute myeloblastic leukemia. Focal cellular areas, surrounded by hemorrhage, are composed of immature mononuclear cells and a few multinucleated cells. H&E, × 60 objective.
Neoplastic cells were characterized by flow cytometric, immunohistochemical, and immunocytochemical analysis (Table 2). Flow cytometric analysis of peripheral blood nucleated cells, performed at the Clinical Immunology Laboratory at Colorado State University, demonstrated 34% CD34+ cells that did not express lymphocyte antigens (Table 2); low numbers of the cells may have expressed CD14 and CD18, but dual staining was not performed for confirmation. This result was consistent with acute leukemia.1 Immunohistochemical evaluation of formalin-fixed sections of bone marrow was performed at the University of Wisconsin School of Veterinary Medicine histology laboratory using a Lab Vision 720 2D automated stainer (Fremont, CA, USA). Mononuclear cells and multinucleated cells did not express CD3, CD20, CD79a, MHCII, or factor VIII-related antigen (von Willebrand factor). Some multinucleated cells, but not the mononuclear cells, were immunoreactive for CD117. Immunocytochemical analysis was performed by on air-dried blood smears (IHC Services, Smithville, TX, USA) and bone marrow aspirates (Leukocyte Antigen Biology Laboratory, School of Veterianry Medicine, University of California–Davis). In blood, low numbers of mononuclear cells were immunoreactive for CD18, and rare mononuclear cells weakly expressed CD61 and may have been giant platelets. In bone marrow >3% of the mononuclear cells were immunoreactive for CD11b and myeloperoxidase (MPO). Mononuclear cells did not express CD42b, but low numbers of multinucleated cells were immunoreactive for CD42b but not for other markers, supporting a megakaryocytic lineage. Based on the morphologic and immunophenotypic findings and criteria established by the Animal Leukemia Study Group of the American Society for Veterinary Clinical Pathology,2 a diagnosis of acute myeloblastic leukemia without maturation (AML-M1) was made. Multinucleated cells were hypothesized to be dysplastic megakaryocytes, but a bilineage hematopoietic malignancy was possible. The dog was treated with doxorubicin and prednisone, but subsequently lost to follow-up.
Table 2.
Immunophenotypic profile of mononuclear cells from a leukemic dog.
| ICC |
||||||
|---|---|---|---|---|---|---|
| Antigen | Lineage | FC* (Blood) | IHC† (BM) | Blood‡ | BM§ | Antibody clone |
| CD3 | Lymphocytes | Negative | Negative | NA | NA | CA17.2A12*, F7.2.38† |
| CD4 | Lymphocytes | Negative | NA | NA | NA | YKIX302.9 |
| CD5 | Lymphocytes | Negative | NA | NA | NA | YKIX322.3 |
| CD8 | Lymphocytes | Negative | NA | NA | NA | YCATE55.9 |
| CD21 | Lymphocytes | Negative | NA | NA | NA | CA2.1D6 |
| CD22 | Lymphocytes | Negative | NA | NA | NA | RFB6 |
| CD20 | Lymphocytes | NA | Negative | NA | NA | Polyclonal RB-9013-P |
| CD79a | Lymphocytes | NA | Negative | NA | NA | HM57/A9 |
| MHCII | Macrophages, dendritic cells, B cells | NA | Negative | NA | NA | HLADR |
| CD14 | Monocytes | ± | NA | NA | NA | UCHM1 |
| CD18 | Leukocytes | ± | NA | Positive | NA | CA16.3C10 |
| CD11b | Granulocytes, monocytes | NA | NA | NA | Positive | CA16.3E10 |
| MPO | Neutrophils | NA | NA | NA | Positive | CA25.10A6 |
| CD42b | Megakaryocytes | NA | NA | NA | Negative | sc-7070 |
| CD61 | Megakaryocytes | NA | NA | Negative | NA | Y2/51 |
| vWF | Megakaryocytes | NA | Negative | NA | NA | Polyclonal A0082 |
| CD34 | Hematopoietic precursors | Positive | NA | NA | NA | 1H6 |
| CD117 | Hematopoietic precursors | NA | Negative | NA | NA | Polyclonal A450229-2 |
Performed at the Clinical Immunology Laboratory, College of Veterinary Medicine and Biomedical Sciences, Colorado State University.
Performed by the Histopathology Laboratory, School of Veterinary Medicine, University of Wisconsin.
Performed by IHC Services, Smithville, Texas.
Performed at the Leukocyte Antigen Biology Laboratory, School of Veterinary Medicine, University of California-Davis.
FC indicates flow cytometry; IHC, immunohistochemistry; ICC, immunocytochemistry; BM, bone marrow; NA, not analyzed; MPO, myeloperoxidase; vWF, von Willebrand factor.
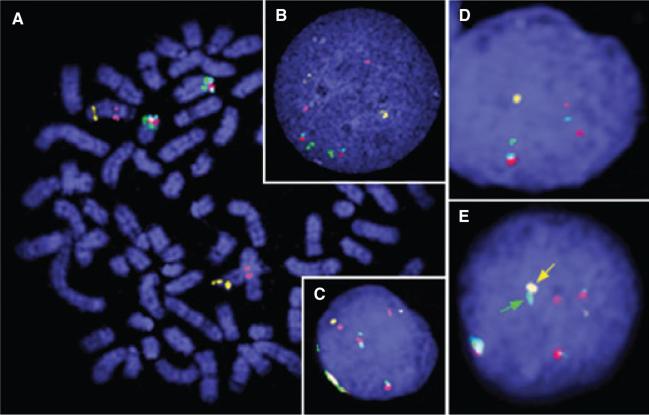
To identify and characterize possible genetic aberrations in the leukemic cells, sections of formalin-fixed paraffin-embedded bone marrow were sent to North Carolina State University where cells were extracted and analyzed by fluorescence in situ hybridization (FISH). A 5-color FISH reaction described previously3 comprised 5 bacterial artificial chromosome (BAC) clones containing BRCA1 (Breast cancer 1) and ABL (v-abl Abelson murine leukemia viral oncogene homolog 1) on Canis familiaris (CFA) chromosome 9 and BCR (breakpoint cluster region), PTEN (phosphatase and tensin homolog), and a telomeric marker on CFA 26 (Figure 4A). To extract cells from paraffin-embedded tissue, 2.25-μm sections were treated with xylene, and the tissue was shredded and treated with pepsin to yield individual cells. Cells underwent methanol:glacial acetic acid fixation prior to hybridization with fluorescently labeled probes by co-denaturation (Figures 4B and 4C).
Figure 4.
Fluorescence in situ hybridization. (A) Control dog metaphase spread prepared using conventional methods showing localization of the 5 differentially labeled BAC clones from the CHORI-82 dog genome library (http://bacpac.chori.org/library.php?id=253) used in this study: 074A02 containing BRCA1 (pink) and 326F17 containing ABL (yellow) on CFA 9 and 486K17 containing BCR (green), 521G14 containing PTEN (red), and telomeric marker 191C19 (blue) on CFA 26. (B) Control interphase nuclei prepared using conventional methods. (C) Control interphase nuclei from paraffin-embedded tissue. (D) Interphase nuclei from the Labrador Retriever with acute myeloblastic leukemia showing a hemizygous loss of BRCA1, ABL, and BCR. (E) Inter-phase nuclei from the Labrador Retriever with co-localization of BCR (green) and ABL (yellow).
Fifty cells were evaluated for the copy number of each of the 5 loci as well as for evidence of co-localization of BCR and ABL, as described previously.3,4 One or more genetic aberrations were present in 74% of cells analyzed, the most notable being co-localization of BCR and ABL that occurred in 32% of cells (Figure 4E). Copy number changes (both gains and deletions) were also evident for BRCA1, ABL, and PTEN. Hemizygous deletion of both BRCA1 and ABL was present in 20% of cells (Figure 4D). The distal end of CFA 26 (including PTEN) was found to be deleted in 14% of cells, but was also gained with the same frequency. These data indicated genomic instability.
Discussion
The current report describes unique morphologic, immunophenotypic, and cytogenetic features in a dog with acute myeloblastic leukemia. The diagnosis was based on morphologic evaluation with the finding of >30% blasts in peripheral blood and <10% differentiated granulocytes in bone marrow and on expression of CD34 antigen without expression of lymphoid, histiocytic, and megakaryocytic antigens. Myeloid differentiation was further supported by immunoreactivity for CD18, CD11b (the β2 integrin chain of Mac-1 and CR3 preferentially expressed in granulocytes), and MPO, with CD11b and MPO expressed by >3% of cells, leading to the final diagnosis of AML-M1.2,5,6 Cytogenetic detection of BCR-ABL translocation associated with AML provides significant information that may have an impact on future diagnosis and treatment of this disease in dogs.
Acute myeloid leukemia is a hematopoietic malignancy characterized by clonal expansion of cells of granulocytic, monocytic, erythrocytic, or megakaryocytic lineages. In dogs, the true incidence of AML is not known; however, in 2 retrospective studies of canine hematopoietic malignancies with circulating neoplastic cells, including the leukemic stage of lymphoma, 16–34% were AML.7,8 Classification of AML in dogs and cats proposed by the Animal Leukemia Study Group adapted criteria and definitions of the French-American-British (FAB) group and the National Cancer Institute workshop.7,8 The FAB system provides common terminology for AML by classifying the disease into 8 categories (M0–M7), based on morphologic and cytochemical characteristics of blast cells.9,10 Cytochemical staining has been superseded by immunophenotypic characterization of cell lineage.7 In people, AML classification is based on the 2008 World Health Organization (WHO) system, which includes a modification of the FAB system and expands the number of entities by incorporating additional information, including cytogenetic features and previous history of myelodysplastic syndrome or anticancer therapy, and others with their own diagnostic categories.11–13 Although, cytogenetic analysis of leukemic cells from the dog reported was performed, the WHO classification was not applied because, in the authors’ opinion, larger studies of cytogenetic abnormalities in canine AML are needed to address similarities and differences in AML between people and dogs.
The presence of multinucleated cells that expressed CD117 and CD42b (platelet glycoprotein 1b) in bone marrow was intriguing and has not been described previously in canine AML. These atypical multinucleated cells, likely megakaryocytes, associated with low numbers of mature megakaryocytes in the bone marrow suggest dysmegakaryocytopoiesis2; dys-plastic megakaryocytes have been reported in dogs with subsequent development of AML.14 Similarly, people with myelodysplastic syndrome have higher risk of progression to AML.15 Hematologic abnormalities had not been detected 1 year earlier in the dog reported here, but a pre-existing myelodysplastic process that transformed to AML could not be excluded. In a previous report of a dog with megakaryocytic leukemia, similar multinucleated cells were identified as abnormal multinucleated megakaryoblasts.16 In the present case, however, acute megakaryocytic leukemia (AML-M7) was excluded because the mononuclear cells did not express megakaryocytic antigens. Expression of CD117 by multinucleated cells suggests that these cells were early precursors based on the fact that CD117 is normally expressed by hematopoietic progenitor cells17; however, expression of CD117 by leukemic cells in dogs has not been evaluated frequently, which limits interpretation of this finding.
In people, translocation between the ABL and BCR genes located on chromosome 9 and 22, respectively, is observed in approximately 95% of cases of chronic myelogenous leukemia (CML)18,19 and results in the derivative Philadelphia chromosome considered to be the hallmark of CML.20 This aberration, however, is also observed in 20–30% of patients with acute lymphoblastic leukemia (ALL)21 and in 0.32–0.9% of patients with AML.22,23 The BCR gene encodes a cytoplasmic and nuclear phosphoprotein24 that is primarily expressed in the early stages of myeloid development with reduced expression in mature leukocytes.25 Similarly, ABL encodes a nuclear and cytoplasmic tyrosine kinase of hematopoietic cells that normally has little constitutive kinase activity. Translocation of BCR and ABL genes on the Philadelphia chromosome produces a fusion gene under the regulation of the BCR promoter.26 The new transcript encodes a chimeric protein with constitutively increased tyrosine kinase activity, due to dimerization or tetramerization of the fusion molecule and subsequent autophosphorylation. The BCR-ABL oncoprotein remains in the cytoplasm where it tyrosine-phosphorylates other proteins, including those associated with cell proliferation and transcriptional activity.26 Understanding the molecular pathogenesis of Philadelphia chromosome-positive (Ph+) CML allowed scientists to develop a drug that targets the BCR-ABL protein. Imatinib mesylate (Gleevec; Novartis, East Hanover, NJ, USA) is a competitive inhibitor of the active site of the BCR-ABL kinase. The drug has been shown to inhibit cellular growth and induce apoptosis of the neoplastic cells and has substantially increased survival of people with CML.27
Completion of the canine genome sequence28,29 and advancement in cytogenetic methodologies30 have facilitated the cytogenetic characterization of specific hematopoietic diseases in dogs. Using FISH, a recent study demonstrated translocation of ABL located on chromosome 9 and BCR located on chromosome 26 in 5 cases of canine CML and one case of ALL4; this alteration, termed the Raleigh chromosome, corresponds to the Philadelphia chromosome in people. This translocation also has been identified in chronic monocytic leukemia (CMoL)3; however, to our knowledge, this is the first report of the Raleigh chromosome in a dog with AML. Our study also demonstrated genetic instability involving the PTEN gene, which has been previously reported in a dog with CMoL.3 The significance of this finding regarding the pathogenesis of either AML or CMoL is still unknown.
In human medicine, clinical criteria are applied to differentiate cases of Ph+ AML from Ph+ CML in myeloid blast crisis in order to establish treatment protocol and prognosis. These criteria include absence of a previous hematologic disorder, lack of evidence of chronic-phase or accelerated-phase CML after induction chemotherapy, and lack of clinical and laboratory features of CML, such as splenomegaly and basophilia.31 Such criteria are not established in veterinary medicine. In dogs with CML in blast crisis, reported abnormalities included leukocyte counts ranging from 41,000 to 169,000/μL, myeloid hyperplasia in the bone marrow, splenomegaly, and, in some cases, hepatomegaly and association with previous chemotherapy.32 The dog of this report had no history of previous chemotherapy or hematologic disorders and had no evidence of lymphadenopathy or organomegaly. Thus, CML in blast crisis was not considered, although previous occult CML with no clinical symptoms cannot be excluded.
Cytogenetic characterization of canine leukemia is anticipated to be critical as we continue to improve classification of hematopoietic neoplasms in dogs. As exemplified by the advancements in therapeutic strategies for human CML, genetic characterization of leukemia provides an understanding of the molecular pathogenesis of the neoplasm, fuels the development of molecular therapies that target specific genes, and validates treatment options for patients with similar genetic alterations. The present report represents only a single case of canine AML with distinct morphologic features and cytogenetic aberrations; however, it elucidates the similarities between canine and human leukemias and encourages further comparative studies of hematopoietic diseases to elucidate molecular mechanisms and improve treatment options.
Acknowledgments
The authors would like to thank Drs. William Vernau (University of California–Davis) and Anne Avery (Colorado State University) for their expertise and assistance in interpreting the immunophenotyping results.
References
- 1.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 2.Jain NC, Blue JT, Grindem CB, et al. Proposed criteria for classification of acute myeloid leukemia in dogs and cats. Vet Clin Pathol. 1991;20:63–82. doi: 10.1111/j.1939-165x.1991.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 3.Cruz Cardona JA, Milner R, Alleman AR, et al. BCRABL translocation in a dog with chronic monocytic leukemia. Vet Clin Pathol. 2011;40:40–47. doi: 10.1111/j.1939-165X.2010.00277.x. [DOI] [PubMed] [Google Scholar]
- 4.Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans–man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 5.Villiers E, Baines S, Law AM, Mallows V. Identification of acute myeloid leukemia in dogs using flow cytometry with myeloperoxidase, MAC387, and a canine neutro-phil-specific antibody. Vet Clin Pathol. 2006;35:55–71. doi: 10.1111/j.1939-165x.2006.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 6.Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69:145–164. doi: 10.1016/s0165-2427(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 7.Tasca S, Carli E, Caldin M, Menegazzo L, Furlanello T, Gallego LS. Hematologic abnormalities and flow cytometric immunophenotyping results in dogs with hematopoietic neoplasia: 210 cases (2002–2006). Vet Clin Pathol. 2009;38:2–12. doi: 10.1111/j.1939-165X.2008.00099.x. [DOI] [PubMed] [Google Scholar]
- 8.Adam F, Villiers E, Watson S, Coyne K, Blackwood L. Clinical pathological and epidemiological assessment of morphologically and immunologically confirmed canine leukaemia. Vet Comp Oncol. 2009;7:181–195. doi: 10.1111/j.1476-5829.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 10.Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 11.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;30114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 12.Heerema-McKenney A, Arber DA. Acute myeloid leukemia. Hematol Oncol Clin North Am. 2009;23:633–654. doi: 10.1016/j.hoc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact. 2010;19184:16–20. doi: 10.1016/j.cbi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 14.McManus PM, Hess RS. Myelodysplastic changes in a dog with subsequent acute myeloid leukemia. Vet Clin Pathol. 1998;27:112–115. doi: 10.1111/j.1939-165x.1998.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 15.Juopperi TA, Bienzle D, Bernreuter DC, Vernau W, Thrall MA, McManus PM. Prognostic markers for myeloid neoplasms: a comparative review of the literature and goals for future investigation. Vet Pathol. 2011;48:182–197. doi: 10.1177/0300985810389317. [DOI] [PubMed] [Google Scholar]
- 16.Ameri M, Wilkerson MJ, Stockham SL, Almes KM, Patton KM, Jackson T. Acute megakaryoblastic leukemia in a German Shepherd dog. Vet Clin Pathol. 2010;39:39–45. doi: 10.1111/j.1939-165X.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 17.Reuss-Borst MA, Buhring HJ, Schmidt H, Muller CA. AML: immunophenotypic heterogeneity and prognostic significance of c-kit expression. Leukemia. 1994;8:258–263. [PubMed] [Google Scholar]
- 18.Rowley J. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 19.Nowell PC, Hungerford DA. A minute chromosome in chronic granulocytic leukemia [abstract]. Science. 1960;132:1497. [Google Scholar]
- 20.Kurzrock R, Kantarjian HM, Druker BJ, Talpaz M. Philadelphia chromosome-positive leukemias: from basic mechanisms to molecular therapeutics. Ann Intern Med. 2003;138:819–830. doi: 10.7326/0003-4819-138-10-200305200-00010. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DA. Philadelphia chromosome positive acute lymphocytic leukemia: a new era of challenges. Hematology Am Soc Hematol Educ Program. 2007;2007:435–443. doi: 10.1182/asheducation-2007.1.435. [DOI] [PubMed] [Google Scholar]
- 22.Soupir CP, Vergilio JA, Dal Cin P, et al. Philadelphia chromosome-positive acute myeloid leukemia: a rare aggressive leukemia with clinicopathologic features distinct from chronic myeloid leukemia in myeloid blast crisis. Am J Clin Pathol. 2007;127:642–650. doi: 10.1309/B4NVER1AJJ84CTUU. [DOI] [PubMed] [Google Scholar]
- 23.Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88:2375–2384. [PubMed] [Google Scholar]
- 24.Maru Y, Witte ON. The BCR gene encodes a novel serine/threonine kinase activity within a single exon. Cell. 1991;67:459–468. doi: 10.1016/0092-8674(91)90521-y. [DOI] [PubMed] [Google Scholar]
- 25.Wetzler M, Talpaz M, Van Etten RA, Hirsh-Ginsberg C, Beran M, Kurzrock R. Subcellular localization of Bcr, Abl, and Bcr-Abl proteins in normal and leukemic cells and correlation of expression with myeloid differentiation. J Clin Invest. 1993;92:1925–1939. doi: 10.1172/JCI116786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cilloni D, Saglio G. CML: a model for targeted therapy. Best Pract Res Clin Haematol. 2009;22:285–294. doi: 10.1016/j.beha.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 28.Kirkness EF, Bafna V, Halpern AL, et al. The dog genome: survey sequencing and comparative analysis. Science. 2003;301:1898–1903. doi: 10.1126/science.1086432. [DOI] [PubMed] [Google Scholar]
- 29.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplo-type structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 30.Breen M. Canine cytogenetics–from band to basepair. Cytogenet Genome Res. 2008;120:50–60. doi: 10.1159/000118740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuneo A, Ferrant A, Michaux JL, et al. Philadelphia chromosome-positive acute myeloid leukemia: cytoimmunologic and cytogenetic features. Haematologica. 1996;81:423–427. [PubMed] [Google Scholar]
- 32.Tarrant JM, Stokol T, Blue JT, McDonough SP, Farrell P. Diagnosis of chronic myelogenous leukemia in a dog using morphologic, cytochemical, and flow cytometric techniques. Vet Clin Pathol. 2001;30:19–24. doi: 10.1111/j.1939-165x.2001.tb00251.x. [DOI] [PubMed] [Google Scholar]