Abstract
Genetic aberrations linked to tumorigenesis have been identified in both canine and human hematopoietic malignancies. While the response of human patients to cancer treatments is often evaluated using cytogenetic techniques, this approach has not been used for dogs with comparable neoplasias. The aim of this study was to demonstrate the applicability of cytogenetic techniques to evaluate the cytogenetic response of canine leukemia to chemotherapy. Cytology and flow cytometric techniques were used to diagnose chronic myelomonocytic leukemia in a dog. High-resolution oligonucleotide array comparative genomic hybridization (oaCGH) and multicolor fluorescence in situ hybridization (FISH) were performed to identify and characterize DNA copy number aberrations (CNAs) and targeted structural chromosome aberrations in peripheral blood WBC at the time of diagnosis and following one week of chemotherapy. At the time of diagnosis, oaCGH indicated the presence of 22 distinct CNAs, of which trisomy of dog chromosome 7 (CFA 7) was the most evident. FISH analysis revealed that this CNA was present in 42% of leukemic cells; in addition, a breakpoint cluster region-Abelson murine leukemia viral oncogene homolog (BCR-ABL) translocation was evident in 17.3% of cells. After one week of treatment, the percentage of cells affected by trisomy of CFA7 and BCR-ABL translocation was reduced to 2% and 3.3%, respectively. Chromosome aberrations in canine leukemic cells may be monitored by molecular cytogenetic techniques to demonstrate cytogenetic remission following treatment. Further understanding of the genetic aberrations involved in canine leukemia may be crucial to improve treatment protocols.
Keywords: Chromosome, cytogenetics, hematopoietic cells, remission, vincristine
Case Presentation
A 12-year-old neutered mixed-breed male dog was presented with stiffness, right forelimb lameness, and neck pain to Freshwater Veterinary Hospital, Enfield, CT. Tests for Lyme disease, anaplasmosis, ehrlichiosis, and heartworm disease were all negative (SNAP 4Dx Plus Test; IDEXX Laboratories, Inc., Westbrook, ME, USA). At presentation, the submandibular lymph nodes were slightly enlarged and firm. Initial empirical treatment included a nonsteroidal anti-inflammatory drug (Rimadyl 50 mg, 2 mg/kg twice a day) and antimicrobial therapy (Doxycycline 150 mg, 6 mg/kg twice a day), with minimal improvement of the right forelimb lameness.
Three weeks later, the dog had a decreased appetite and showed intermittent vomiting. All lymph nodes were still slightly enlarged and firm, no cytologic examination was performed. Radiographic examination of the thorax and all affected limbs revealed no abnormalities. A CBC (Antech Diagnostics, Lake Success, NY, USA) indicated a normocytic, normochromic anemia (HCT 25%, Reference Interval [RI] 36–60%; Hemoglobin = 15.8, RI 12.1–20.3 g/dL; RBC = 3.46, RI 4.8–9.3 106/μL; MCV = 72, RI 58–79 fL; MCH = 25.1, RI 19–28 pg; MCHC = 34.8, RI 30–38 g/dL) and an absolute leukocytosis (69,700/μL, RI 6000–17,000/μL) due moderate neutrophilia (20,213/μL, RI 3000–11,400/μL) with a marked left shift (2788/μL RI 0–300/μL) and severe monocytosis (41,820/μL, RI 150–1350/μL; Table 1). Blood smears revealed moderate anisocytosis and anisokaryosis and confirmed the presence of large numbers of round cells that resembled immature monocytes (Figure 1). These cells had elongated oval, reniform, or irregularly lobulated nuclei that were approximately 2–3 red cells in diameter and had abundant, finely granulated basophilic cytoplasm, which occasionally contained small clear vacuoles. These cells exhibited several criteria of malignancy, including multiple prominent nucleoli, and anisocytosis and anisokaryosis. In addition, dysplastic features in the neutrophil series were observed, such as donut-shaped nuclei, and giant metamyelocytes and bands. The morphologic characteristics together with the marked monocytosis (Table 1) were consistent with the presumptive hematologic diagnosis of chronic myelomonocytic leukemia (CMML).1 Bone marrow was not assessed.
Table 1.
CBC data from a dog with chronic myelomonocytic leukemia. Blood samples taken on day 1 prior to and on day 7 after chemotherapy were used for cytogenetic evaluation by oligonucleotide array comparative genomic hybridization (oaCGH) and fluorescence in situ hybridization (FISH).
| Reference Interval (Count/μl) | Day 1* | Day 7* | Day 14 | Day 18 | Day 22 | Day 24 | Day 29 | Day 31 | Day 37 | |
|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 6000–17,000 | 69,700 | 3770 | 11,500 | 18,200 | 13,720 | 15,100 | 12,800 | 22,200 | 106,700 |
| Neutrophils | 3000–11,400 | 20,213 | 720 | 4945 | 4368 | 2760 | 1963 | 1024 | 15,350 | 11,737 |
| Bands | 0–300 | 2788 | 0 | 0 | 182 | 0 | 0 | 0 | 0 | 0 |
| Lymphocytes | 1000–4800 | 4879 | 1170 | 2070 | 2002 | 2270 | 2567 | 2048 | 3180 | |
| Monocytes | 150–1350 | 41,820 | 1780 | 4485 | 10,738 | 6950 | 7248 | 4480 | 2530 | 59,752 |
| Eosinophils | 100–7500 | 0 | 100 | 0 | 910 | 1730 | 3171 | 5248 | 1100 | 1067 |
| Basophils | Rare | 0 | 0 | 0 | 0 | 10 | 151 | 0 | 40 | 0 |
| Atypical Mononuclear Cells | 34,144 | |||||||||
| Hematocrit | 36–60(%) | 25.0 | 25.3 | 25.4 | 28.0 | 27.4 | 24.6 | 25.0 | 23.0 | 21.7 |
| Reticulocytes | (%) | N/A | 0.4 | N/A | N/A | 0.7 | 0.8 | N/A | 1.8 | N/A |
| nRBC | 0–1 (#/100WBC) | N/A | N/A | 33 | 11 | 2 | N/A | 4 | N/A | 4 |
| Thrombocytes | 170,000–400,000 | 197,000 | 148,000 | 368,000 | 232,000 | 173,000 | 230,000 | 215,000 | 158,000 | 46,000 |
| Treatment | VP | P | P | PH | PH | PH | H | H | VP |
oaCGH analysis and FISH.
V indicates Vincristine; P, Prednisolone; H, Hydroxyurea.
Figure 1.
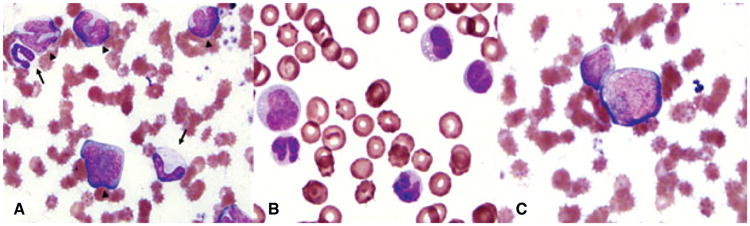
Peripheral blood smears from a dog with chronic myelomonocytic leukemia. Wright–Giemsa stain, ×100 objective. (A) Blood sampled prior to treatment. Note the increased numbers of atypical monocytoid cells (arrow heads), the nuclei of which have atypical nuclear contour and chromatin patterns, and prominent nucleoli. Note immature cells in the neutrophil series (large metamyelocyte and band neutrophil, arrows). (B) Blood sampled one week after chemotherapy. Note the presence of large atypical monocytoid cells as well as smaller mature monocytes. (C) Faint magenta granules can be observed in the cytoplasm of the central large atypical monocytoid cell.
Leukemic cells (ie, peripheral blood mononuclear cells, PBMCs) were isolated from a blood sample using Ficoll and stained for flow cytometric analysis as described elsewhere (Appendix S1).2 Flow cytometric analysis (Becton Dickinson LSR II flow cytometer; BD Biosciences, San Jose, CA, USA) showed a relatively uniform population of cells with regard to size (forward angle light scatter) and granularity (right angle or side scatter), consistent with monocytes (Figure 2A). The cells expressed the panleukocyte antigen CD45 and > 97% of them were negative for CD34, suggesting a monomorphic neoplastic population (Figure 2B). This was further supported by the expression of the myeloid differentiation antigen CD11b (Figure 2C), with approximately 60% at low levels (CD11bdim population) and another 15% at higher levels (CD11bintermediate and CD11bbright). Approximately 25% of the cells coexpressed CD11b and CD4, which is characteristic of mature canine neutrophils (Figure 2C). About 50% of the cells also showed robust expression of the monocytic differentiation antigen CD14 (Figure 2D). Only a few lymphocytes (< 5%) that expressed lymphoid differentiation antigens, such as CD4 (without CD11b and CD14), CD5, CD8, and CD21, were observed. Altogether, the immunophenotyping data showed that the affected dog's PBMCs expressed a mixture of granulocytic and monocytic cell markers at various stages of differentiation with a negligible population of CD34+ blast cells, consistent with a diagnosis of CMML. Treatment was initiated with 0.6 mg Vincristine IV (0.7 mg/m2), and 50 mg (2 mg/kg) oral prednisone once a day.
Figure 2.
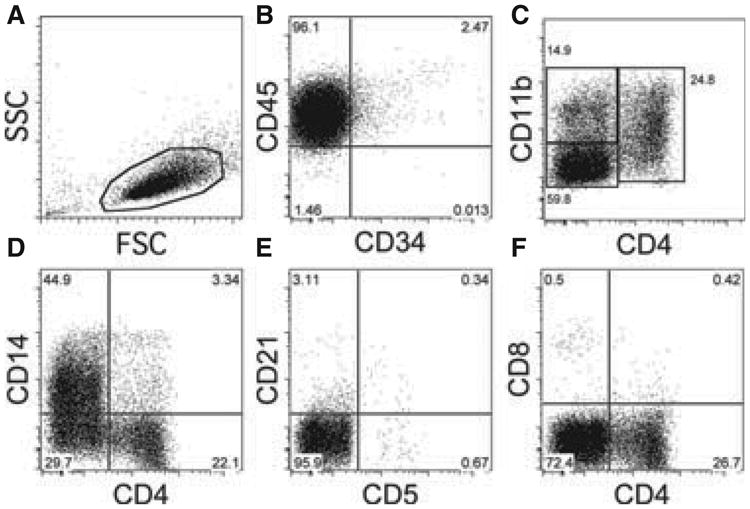
Flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) from a dog with chronic myelomonocytic leukemia prior to treatment. 7-AAD–positive dead cells were excluded from the analysis. PBMCs were gated based on forward and side scatter characteristics. Labels on the x- and y-axis indicate the respective anti-CD antibodies used for staining of the cells.
One week later, the dog was eating well and no longer vomiting. A CBC revealed nonregenerative anemia (hematocrit 25.3%, RI 36–60%; 0.4% reticulocytes) moderate leukopenia (3770/μL, RI 6000–17,000/μL) due to marked neutropenia (720/μL, RI 3000–11,400/μL), mild monocytosis (1780/μL, RI 150–1350/μL), and mild thrombocytopenia (148,000/μL, RI 170,000–400,000, Table 1).
Oligonucleotide array comparative genomic hybridization (oaCGH) was performed using Sure Print G3 Canine Genome 180K microarrays (Agilent Technologies, Santa Clara, CA, USA), which contain 171,534 coding and noncoding 60-mer oligonucleotide sequences spaced at approximately 13 kb intervals. DNA from cryopreserved PBMCs sampled before treatment was isolated using a DNeasy Blood & Tissue Kit (QIAGEN, Inc., Valencia, CA, USA). A sex-matched equimolar pool of genomic DNA from 16 healthy dogs (8 different breeds, 2 of each breed) was used as the reference to prevent detection of natural constitutional copy number variations. Oligonucleotide array CGH (Appendices S2, S3) of the leukemic cell DNA sample revealed 22 regions of copy number aberrations (CNAs) on 13 chromosomes, including 7 gains ranging in size from 49 kb to 80 Mb, and 15 losses ranging in size from 31 kb to 1.9 Mb (Figure 3A). The most apparent aberration in the whole genome profile was a gain of 5951 contiguous probes covering the entire length of dog chromosome 7 (Canis familiaris [CFA] 7; Figure 3A). The average log2 ratio across these probes was 0.31.
Figure 3.
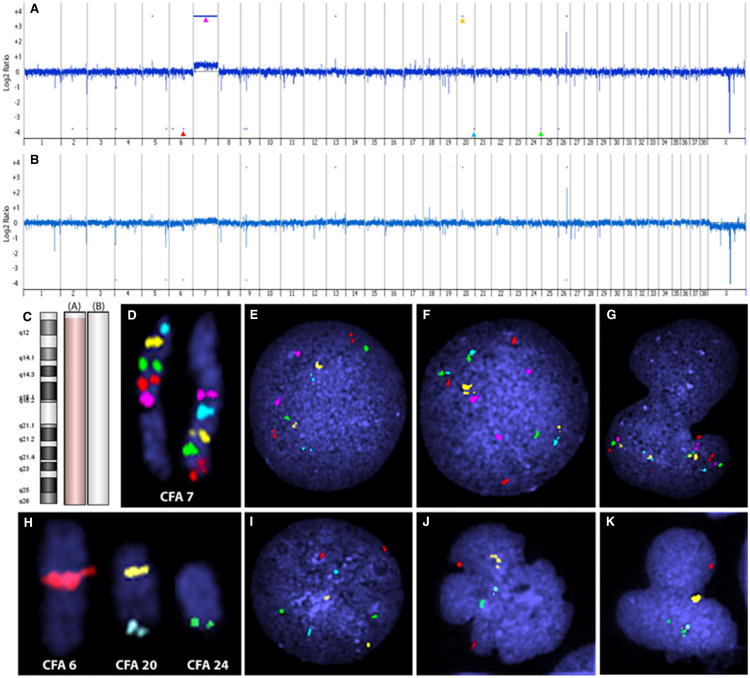
Oligo array comparative genomic hybridization (oaCGH) and fluorescence in situ hybridization (FISH) verification of peripheral blood mononuclear cells from a dog with chronic myelomonocytic leukemia. (A) Genomic profile of oaCGH of pretreatment DNA viewed at 100 kb window. Dash marks above and below the profile indicate regions of copy number aberration as determined by the ADM-2 algorithm. Arrowheads indicate regions further verified by FISH analysis. (B) Genomic profile of oaCGH of posttreatment DNA viewed at 100 kb window. Dash marks above and below the profile indicate regions of copy number aberration as determined by the ADM-2 algorithm. (C) Aberration summary of Canis familiaris (CFA) 7 from oaCGH corresponding to pretreatment (A) and posttreatment (B) DNA. Light red shading indicates copy number gain and light green shading indicates copy number loss (not evident). (D) Tiling of CFA 7 with bacterial artificial chromosome (BAC) clones at a 10-Mb interval (362E04,126M10, 182C02, 335A22, 326O12, 029J03, 334P01, 332H21, and 122I21). (E) Interphase nuclei from control dog showing BAC clones for proximal end of CFA 7 with normal copy number of two. (F) and (G) Trisomy of CFA 7 in interphase nuclei from a leukemic cell. (H) Localization of BAC clones to verify other oaCGH copy number changes; red (307I06) at 49 Mb on CFA 6, yellow (215F17) and aqua (136N17) at 19 Mb and 60 Mb, respectively, on CFA 20, and green (182B05) at 49 Mb on CFA 24. (I) Interphase nuclei from a control dog showing array verification BAC clones with normal copy number of 2. (J) Interphase nuclei from a leukemic cell showing hemizygous deletion of aqua and green, and gain of yellow. (K) Interphase nuclei from a leukemic cell showing hemizygous deletion of red, aqua, and gain of yellow.
To verify key aberrations indicated by oaCGH and identify targeted structural changes, multicolor fluorescence in situ hybridization (FISH) was performed as described previously (Appendix S4)3 using PBMCs from heparinized peripheral blood drawn from the dog prior to the first dose of vincristine and 7 days after chemotherapy. In addition, cell nuclei from clinically healthy dogs (n = 10) were assessed in each reaction to verify the precise chromosomal location of each clone (Figure 3H) and to confirm that each had a copy number of 2. To verify and quantify gain of CFA 7, 9 bacterial artificial chromosome (BAC) probes were tiled along CFA 7 at ∼10 Mb intervals in 2 separate multicolor FISH reactions; group 1 comprised the most proximal 5 probes and group 2 comprised the 5 most distal probes, with the middle probe overlapping between the 2 reactions (Figure 3D). Trisomy of CFA 7 was identified in 42–44% of cells. Of the 21 other CNAs in the pretreatment data, only 7 were large enough (> 200 kb) to contain an entire BAC clone. Four of these regions were selected for assessment by FISH; 3 copy number losses located at CFA 6: 48.2–50 Mb (log2 ratio –0.30), CFA 20: 59.9–60.1 Mb (log2 ratio –0.22), and CFA 24: 49.2–49.9 Mb (log2 ratio –0.25), and one copy number gain located at CFA 20: 19.2–19.4 Mb (log2 ratio 0.54, Figure 3A) were found. The copy number status obtained from FISH analysis of each of the regions verified array results (Figure 3J,K). The probe representing the region at 19.2 Mb on CFA 20 (colored yellow in Figures 3H–K) resulted in a signal of more intensity than normal in normal canine DNA (Figures 3J,K), suggesting a tandem duplication of the respective sequence in the leukemic cells. Likewise, the other 3 regions were found to have hemizygous deletions.
The presence of the Raleigh chromosome, ie, colocalization of the breakpoint cluster region and Abelson murine leukemia viral oncogene homolog (BCR-ABL) and other targeted aberrations were simultaneously assessed with 2 5-color FISH reactions on PBMCs collected prior to chemotherapy and cells from control dogs. The first probe set comprised differentially labeled BAC clones representing breast cancer 1 (BRCA1) and ABL on CFA 9 and BCR, phosphatase and tensin homolog (PTEN), and a telomeric single locus probe (SLP) on CFA 26 (Figure 4). The second probe set comprised 5 probes that tile the proximal half of CFA 22, including a BAC representing retinoblastoma 1 (RB1) (Figure 5). When hybridized to interphase nuclei of cells from healthy dogs (n = 10), all probes used revealed a copy number of 2 and mapped uniquely to the correct chromosomal location (Figures 4A,B,C and 5A,B,C). Examples of the hybridization patterns of these 2 probe sets in interphase nuclei of leukemic cells of the dog presented in this report are shown in Figures 4D–H and 5D–H. The number of cells containing aberrations involving these probes is presented in Table 2. Essentially, 39.3% (59/150) of scored cells had one or more aberrations involving the BACs used. Colocalization of BCR and ABL (indicating the presence of the Raleigh chromosome) was evident in 17.3% (26/150) of the cells. Deletions of BRCA1 and PTEN were also present (Figure 4D–H), as was a heterozygous deletion of RB1 (Figure 5D–H).
Figure 4.
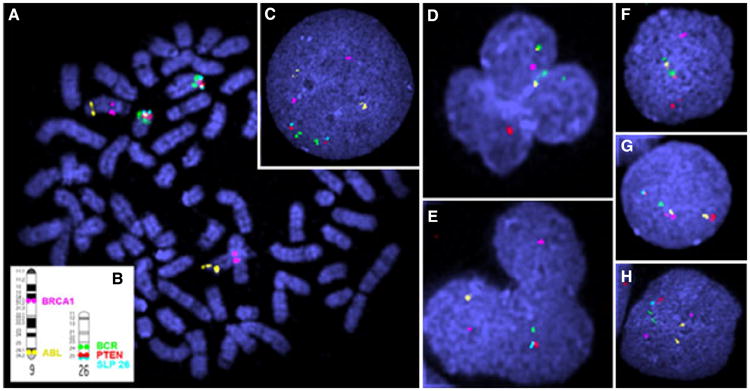
Fluorescence in situ hybridization of Canis familiaris (CFA) 9 and CFA 26. A–C Control dog (A) Metaphase spread showing localization of fluorescently labeled bacterial artificial chromosome (BACs) clones. (B) Ideogram showing accurate location of BAC clones. (C) Interphase nuclei. D–H Dog with chronic myelomonocytic leukemia, peripheral blood mononuclear cells collected prior to treatment, note breakpoint cluster region (BCR; labeled green; 486K17) and Abelson murine leukemia (ABL; labeled yellow; 326F17) colocalization (D, F) and deletions of breast cancer 1 (BRCA1; labeled pink; 074A02) (D, F, G), phosphatase and tensin homolog (PTEN; labeled red; 521G14) (D, E, H), ABL (yellow) (E, F), BCR (green) (E, G), and telomeric single locus probe (light blue; 191C19) (D, E, F, G, H).
Figure 5.
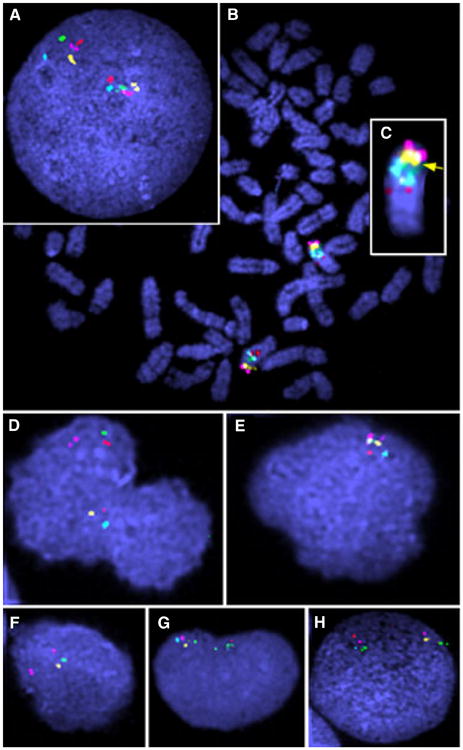
Fluorescence in situ hybridization (FISH) of Canis familiaris (CFA) 22. A–C Control dog CFA (A) Interphase nuclei. (B) Metaphase spread. (C) Zoomed view showing probe placement and order. D–H Dog with chronic myelomonocytic leukemia, peripheral blood mononuclear cells prior to treatment. Note retinoblastoma 1 (RB1; yellow; 521E11) deletion in addition to other deletions.
Table 2.
Fluorescence in situ hybridization verification of oligonucleotide array comparative genomic hybridization (oaCGH) and targeted structural aberrations in interphase nuclei of peripheral blood mononuclear cells from a dog with chronic myelomonocytic leukemia prior to (day 1) and one week after vincristine and prednisone therapy (day 7).
| Affected Cells | |||
|---|---|---|---|
|
| |||
| Day 1 (Pretreatment) | Day 7 (Posttreatment) | P-Value | |
| Locus in Array Verification | |||
| CFA 7 – Gain of Proximal Five BACs | 42% (21/50) | 2% (1/50) | 9.4 × 10−7*** |
| CFA7 – Gain of Distal Five BACs | 44% (22/50) | 4% (2/50) | 2.8 × 10−6*** |
| CFA 6 (48.2 Mb) Deletion | 16% (8/50) | 10% (5/50) | 0.55 |
| CFA 20 (19.2 Mb) Gain | 44% (22/50) | 12% (6/50) | 6.7 × 10−4** |
| CFA 20 (59.9 Mb) Deletion | 12% (6/50) | 8% (4/50) | 0.74 |
| CFA 24 (49.2 Mb) Deletion | 10% (5/50) | 18% (9/50) | 0.38 |
| Targeted Aberrations | |||
| Total | 39.3% (59/150) | 14.6% (22/150) | 2.1 × 10−6*** |
| BCR-ABL Translocation | 17.3% (26/150) | 3.3% (5/150) | 8.6 × 10−5*** |
| PTEN Deletion | 9.3% (14/150) | 2% (3/150) | 1.0 × 10−2* |
| BRCA1 Deletion | 8.7% (13/150) | 5.3% (8/150) | 0.37 |
| RB1 Deletion | 10.6% (16/150) | 0% (0/0) | 2.0 × 10−5*** |
Data are percentages (absolute number per total number investigated).
P < .01,
P < .001,
P < .0001.
CFA indicates Canis familiaris; BAC, bacterial artificial chromosome; BCR-ABL, breakpoint cluster region and Abelson murine leukemia viral oncogene homolog; PTEN, phosphatase and tensin homolog; BRCA1, breast cancer 1; RB1, retinoblastoma 1.
Oligonucleotide array CGH analysis of DNA isolated from PBMCs sampled one week following chemotherapy identified 14 aberrant regions on 11 chromosomes comprised of 5 gains (range 49–757 kb) and 9 losses (range 45 kb–1.9 Mb), including 12 regions maintained from before treatment (Figure 3B). A subtle increase in copy number of CFA 7 was evident on the whole genome profile (Figure 3B), but was not statistically significant (Figures 3B,C). Two regions were identified that were not determined to be aberrant prior to treatment, a 757 kb gain at CFA 9q14 and a 1.2 Mb loss at CFA 26q24. FISH was performed on interphase nuclei from PBMCs obtained one week following chemotherapy to evaluate all regions assessed in pretreatment cells. Differences in the frequency of aberrations in pretreatment and posttreatment cells were analyzed using Fisher's exact test. FISH analysis revealed a decrease of 10–20 fold in the percentage of cells with trisomy 7, which was statistically significant when compared with day one results (Table 2). Of the other 4 oaCGH regions targeted by FISH, only the gain on CFA 20 showed a significant difference compared with the pretreatment status. Overall, cells at 7 days treatment presented with significant decreases in the proportion of targeted aberrations, including BCR-ABL translocation, and PTEN and RB1 deletions (Table 2). While the other 4 probes on CFA22 were deleted in 1–3 cells each, these losses did not deviate significantly from controls, indicating that the deletion of CFA22 was focused on the region containingRB1.
The prednisone dose was lowered to 40 mg (1.6 mg/kg) once a day for the second treatment week, at the end of which the CBC was characterized by an inefficient regenerative erythropoietic response with a stable hematocrit at 25.4% and 33 nucleated red blood cells/lL (Table 1). WBC counts were within the RI, but marked monocytosis (4485/μL, RI 150–1350) persisted. The lymph nodes appeared a little softer and less prominent. The prednisone dose was reduced to 25 mg (1 mg/kg) orally once a day for the third treatment week. At the end of the third treatment week (ie, 2 weeks following initial treatment with vincristine), hydroxyurea therapy was initiated at 1200 mg (50 mg/kg) 3 times a week. The dog continued to improve clinically, although it remained mildly anemic (Hematocrit 27.4%). At 4 weeks after initial presentation, the WBC count slowly increased to 22,200/μL due to a persistent moderate monocytosis (2530/μL, RI150–1350). Aweek later, the dog suffered a relapse of leukemia (WBC count of 106,700/μL) characterized by severe monocytosis (59,752/μL) and a large number (34,144/μL) of intermediate-to-large atypical mononuclear cells as per the hematology instrument classification and confirmed in a differential count by a board-certified clinical pathologist. The dog received another dose of 0.63 mg vincristine (0.71 mg/m2) intravenously, prednisoneat 50 mg (2 mg/kg) once a day, 500 mg (19 mg/kg) amoxicillin twice a day, and 112.5 mg (4.2 mg/kg) marbofloxacin once a day, while the hydroxyurea was discontinued. Despite treatment efforts, the dog continued to deteriorate and was humanely euthanized 6 days later. At that time, a full CBC was not performed, but a manual hematocrit was 10%, and there was an impressive buffy coat comprising 15% of the microhematocrit tube (usually < 1%). Additional samples for cytogenetic analysis were not available.
Discussion
Since 2005, a high-quality genome sequence of the domestic dog is available.4 Tools accompanying the genome assembly have become helpful genomic resources and allow the definition of genetic abnormalities in a variety of canine diseases, including hematopoietic neoplasias; it is hoped that this would lead to the identification of new therapeutic approaches in veterinary medicine.5,6 Chromosome aberrations associated with human neoplasias have also been identified in dogs using FISH and oaCGH.7,8 The BCR-ABL fusion gene is evolutionarily conserved in canine chronic myeloid leukemia (CML). Termed the “Raleigh” chromosome, it has been identified using multicolor FISH, not only in several cases of canine CML,8 but also in one case of canine chronic monocytic leukemia (CMoL)9 and in one case of canine acute myeloid leukemia.10 In people, CML is a myeloproliferative disease characterized by uncontrolled proliferation of granulocytes and the presence of the Philadelphia chromosome. The latter results from a reciprocal translocation between human chromosomes 9 and 22, designated t (9;22)(q34;q11). This aberration juxtaposes the BCR gene to the gene encoding the nonreceptor tyrosine kinase ABL,11,12 resulting in the generation of a 210-kD chimeric BCR-ABL fusion protein (p210) with constitutive tyrosine kinase activity. Treatment of CML with the tyrosine kinase inhibitor (TKI) imatinib mesylate (Gleevec), results in significant improvement of outcomes of CML patients (some response in 96% of CML patients in the chronic phase, complete cytogenetic response in 57% at a median time of 8.3 months).13,14 In long-term studies, 41% of patients show a complete cytogenetic response (0% BCR-ABL–positive cells assessed via conventional cytogenetics) after 5 years with continuous imatinib treatment. The progression to accelerated phase was delayed in 61% of patients, and progression to blast phase was delayed in 76% of patients.15 In contrast, the BCR-ABL fusion is rarely seen in human CMML and when present, the disease pattern seems to be intermediate between CML and CMML.16 The disease is marked by monocytosis and some left-shifting of granulocytes in the peripheral blood, which can progress to blast phase quickly and generally has a poor prognosis.17
While conventional cytogenetics is still considered a reliable method for detecting and monitoring the presence of BCR-ABL, FISH allows for analysis of a much greater cell count than the 20 metaphases evaluated in conventional practices and can be performed using samples of peripheral blood as well as bone marrow. Traditionally, only 2 single locus probes were used, one for BCR and one for ABL, which resulted in up to 10% false positives.18 Using multiple probes and counting a larger number of cells has reduced the number of false positives to < 5%.18 Recently, FISH has been incorporated into the monitoring standard procedures of human studies on the effectiveness of TKIs in addition to conventional cytogenetics.19 Reverse-transcriptase PCR (RT-PCR), and more specifically real-time RT-PCR, has recently been developed to quantitatively determine the BCR-ABL transformation. The high sensitivity allows the detection of very low levels of residual disease.18
In this case, a dog was diagnosed with CMML, a rarely reported canine myeloproliferative disease,20 based on conventional hematologic and cytologic techniques, and flow cytometric analysis. A genome-wide assessment of DNA copy number aberrations was performed on the leukemic cells by cytogenetic techniques prior to and after one week of chemotherapy, documenting the treatment-related elimination of neoplastic cells with cytogenetic mutations, including the Raleigh chromosome. This provides evidence that cytogenetic techniques can assist clinical assessment in the evaluation of a therapeutic response.
High-resolution oaCGH of DNA isolated from cells at the time of diagnosis revealed the presence of trisomy CFA 7, which was verified by FISH analysis. CFA 7 (∼84 Mb) is evolutionarily conserved with segments of human chromosome (HSA) 1q and various sections of HSA 18.21 We have observed gain of CFA 7 also in ∼10% of dogs with leukemia (Culver, unpublished data), which is orthologous to reported recurrent gain of HSA 1q25-q32 in human CML.22 This region of CFA 7 is also conserved with regions of mouse chromosomes (MMU) 1 and 3, both of which have been reported to be trisomic in murine models of induced myeloid leukemia.23,24 Data from all 3 species suggest that there may be a conserved pathogenic mechanism linked with genome organization in these shared regions. The 31 Mb region of CFA 7 that is evolutionarily conserved with HSA 1q25-q32 contains hundreds of genes. Candidate genes for this region have been previously listed,25 but it is beyond the scope of this report to speculate on their involvement in this dog's leukemia.
RB1 deletion is present in approximately 50% of human chronic lymphocytic leukemias.1 Loss of RB1 has also been linked to neoplastic transformation in human retinoblastoma, osteogenic sarcoma, breast carcinoma, and small-cell lung cancer. It is associated with cell cycle control and has been suggested to play a role in the oncogenesis of certain hematologic malignancies.26 Deletion of RB1 has been documented previously in human patients with CMML.26,27
In this case of canine CMML, we assessed colocalization of fluorescently labeled BAC clones containing BCR and ABL as well as copy number status of RB. BCR-ABL colocalization was recorded in 17.3% of pretreatment cells and deletion of RB1 was evident in 10.6% of pretreated cells. Posttreatment, the proportion of cells with these 2 cytogenetic aberrations had decreased significantly, BCR-ABL to 3.3% of cells, and RB1 deletion to 0%. As these 2 aberrations were assessed independently, it is not possible from this study to determine if the aberrations were present simultaneously in the same cells. Interestingly, the presence of the BCR-ABL translocation would suggest a CML according to the most recent WHO classifications in people.1 Human CML treated with vincristine and prednisone results in complete or partial hematologic remission in only 30% of patients, with the Philadelphia chromosome remaining present throughout the course of disease.28 Human CMML treated with vincristine and prednisone results in a rapid decrease in circulating WBC counts followed by remission to a normal leukogram lasting for 2–5 months.29 The dog presented here showed a partial hematologic response with Raleigh chromosome-positive cells remaining after treatment.
To our knowledge, this is the first time that a treatment response has been documented by molecular cytogenetic techniques in a domestic animal with spontaneous cancer. This case report shows that molecular techniques, including flow cytometry, high-resolution oaCGH, and FISH can be of additional value in characterizing, diagnosing, and potentially monitoring canine CMML and treatment effects.
Supplementary Material
Appendix S1. Flow cytometric analysis
Appendix S2. Comparative genomic hybridization
Appendix S3. oaCGH Analysis
Appendix S4. Fluorescence in situ hybridization
Acknowledgments
This work was supported in part by funds from the AKC Canine Health Foundation awarded to MB/JM. SC was supported by an NCSU Comparative Biomedical Sciences DVM-PhD Training Fellowship. Additional funds were provided by the NCSU Cancer Genomics Fund (MB). The authors thank the dog's owners for the donation of samples for this research effort.
Footnotes
Disclosure: The authors have indicated that they have no affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in this article.
Supporting Information: Additional Supporting Information may be found in the online version of this article:
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th. Vol. 2. Lyon, France: IARC Press; 2008. [Google Scholar]
- 2.Ito D, Endicott M, Jubala C, et al. A tumor-related lymphoid progenitor population supports hierarchical tumor organization in canine B-cell lymphoma. J Vet Intern Med. 2011;25:890–896. doi: 10.1111/j.1939-1676.2011.0756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas R, Duke S, Bloom S, et al. A cytogenetically characterized, genome-anchored 10-MbBAC set and CGH array for the domestic dog. J Hered. 2007;98:474–484. doi: 10.1093/jhered/esm053. [DOI] [PubMed] [Google Scholar]
- 4.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 5.Chen WK, Swartz JD, Rush LJ, Alvarez CE. Mapping DNA structural variation in dogs. Genome Res. 2009;19:500–509. doi: 10.1101/gr.083741.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson PN. Using the canine genome to cure cancer and other diseases. Theriogenology. 2007;68:378–381. doi: 10.1016/j.theriogenology.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Thomas R, Seiser EL, Motsinger-Reif A, et al. Refining tumor-associated aneuploidy through “genomic recoding” of recurrent DNA copy number aberrations in 150 canine non-Hodgkin's lymphomas. Leukemia and Lymphoma. 2011;52:1321–1335. doi: 10.3109/10428194.2011.559802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans – man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 9.Cruz C, Milner R, Alleman A, et al. BCR-ABL translocation in a dog with chronic monocytic leukemia. Veterinary Clinical Pathology. 2011;40:40–47. doi: 10.1111/j.1939-165X.2010.00277.x. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo JF, Culver S, Behling-Kelly E, Breen M, Friedrichs K. Acute myeloblastic leukemia with associated BCR-ABL translocation in a dog. Veterinary Clinical Pathology. 2012;41:362–368. doi: 10.1111/j.1939-165X.2012.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowley JD. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 12.Juopperi T, Bienzle D, Bernreuter D, Vernau W, Thrall M, McManus P. Prognostic markers for myeloid neoplasms. Veterinary Pathology Online. 2011;48:182–197. doi: 10.1177/0300985810389317. [DOI] [PubMed] [Google Scholar]
- 13.Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. The New England Journal of Medicine. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 14.Melo J, Hughes T, Apperley J. Chronic myeloid leukemia. Hematology. 2003;2003:132–152. doi: 10.1182/asheducation-2003.1.132. [DOI] [PubMed] [Google Scholar]
- 15.Hochhaus A, Druker B, Sawyers C, et al. Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-{alpha} treatment. Blood. 2008;111:1039–1043. doi: 10.1182/blood-2007-07-103523. [DOI] [PubMed] [Google Scholar]
- 16.Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype [editorial; comment] Blood. 1996;88:2375–2384. [PubMed] [Google Scholar]
- 17.McManus PM. Classification of myeloid neoplasms: a comparative review. Veterinary Clinical Pathology. 2008;34:189–212. doi: 10.1111/j.1939-165x.2005.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Schiffer C, Jones D, Cortes J. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: practical advice on the use and interpretation of monitoring methods. Blood. 2008;111:1774–1780. doi: 10.1182/blood-2007-09-110189. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 20.Hiraoka H, Hisasue M, Nagashima N, et al. A dog with myelodysplastic syndrome: chronic myelomonocytic leukemia. The Journal of veterinary medical science/the Japanese Society of Veterinary Science. 2007;69:665–668. doi: 10.1292/jvms.69.665. [DOI] [PubMed] [Google Scholar]
- 21.Derrien T, André C, Galibert F, Hitte C. AutoGRAPH: an interactive web server for automating and visualizing comparative genome maps. Bioinformatics. 2007;23:498–499. doi: 10.1093/bioinformatics/btl618. [DOI] [PubMed] [Google Scholar]
- 22.Brazma D, Grace C, Howard J, et al. Genomic profile of chronic myelogenous leukemia: imbalances associated with disease progression. Genes Chromosom Cancer. 2007;46:1039–1050. doi: 10.1002/gcc.20487. [DOI] [PubMed] [Google Scholar]
- 23.Hayata I, Seki M, Yoshida K, et al. Chromosomal aberrations observed in 52 mouse myeloid leukemias. Cancer Res. 1983;43:367–373. [PubMed] [Google Scholar]
- 24.Bouffler SD, Meijne EIM, Huiskamp R, Cox R. Chromosomal abnormalities in neutron-induced acute myeloid leukemias in CBA/H mice. Radiat Res. 1996;146:349–352. [PubMed] [Google Scholar]
- 25.Makishima H, Jankowska A, McDevitt M, et al. CBL, CBLB, TET2, ASXL1, and IDH1/2 mutations and additional chromosomal aberrations constitute molecular events in chronic myelogenous leukemia. Blood. 2011;117:e198–e206. doi: 10.1182/blood-2010-06-292433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YC, Chen PJ, Yeh SH, et al. Deletion of the human retinoblastoma gene in primary leukemias. Blood. 1990;76:2060–2064. [PubMed] [Google Scholar]
- 27.Gelsi-Boyer V, Trouplin V, Adélaïde J, et al. Genome profiling of chronic myelomonocytic leukemia: frequent alterations of RAS and RUNX1 genes. BMC Cancer. 2008;8:299–313. doi: 10.1186/1471-2407-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canellos GP, Devita VT, Whang-Peng J, Carbone PP. Hematologic and cytogenetic remission of blastic transformation in chronic granulocytic leukemia. Blood. 1971;38:671–679. [PubMed] [Google Scholar]
- 29.Taetle R, Haerr R. Treatment of chronic myelomonocytic leukemia. Vincristine and prednisone therapy during symptomatic phase or after transformation to acute leukemia. West J Med. 1985;143:524–527. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Flow cytometric analysis
Appendix S2. Comparative genomic hybridization
Appendix S3. oaCGH Analysis
Appendix S4. Fluorescence in situ hybridization


