Abstract
Human immunodeficiency virus 1 (HIV) latency remains a significant obstacle to curing infected patients. One promising therapeutic strategy is to purge the latent cellular reservoir by activating latent HIV with latency-reversing agents (LRAs). In some cases, co-drugging with multiple LRAs is necessary to activate latent infections, but few studies have established quantitative criteria for determining when co-drugging is required. Here we systematically quantified drug interactions between histone deacetylase inhibitors and transcriptional activators of HIV and found that the need for co-drugging is determined by the proximity of latent infections to the chromatin-regulated viral gene activation threshold at the viral promoter. Our results suggest two classes of latent viral integrations: those far from the activation threshold that benefit from co-drugging, and those close to the threshold that are efficiently activated by a single drug. Using a primary T cell model of latency, we further demonstrated that the requirement for co-drugging was donor dependent, suggesting that the host may set the level of repression of latent infections. Finally, we showed that single drug or co-drugging doses could be optimized, via repeat stimulations, to minimize unwanted side effects while maintaining robust viral activation. Our results motivate further study of patient-specific latency-reversing strategies.
Introduction
HIV establishes latent infections that persist even after successful treatment with antiretroviral therapy (ART) [1]. The primary latent viral reservoir is in long-lived CD4+ resting memory T cells [2] from which viral replication reemerges rapidly if antiretroviral treatment is interrupted. One potential therapeutic strategy to cure HIV infection is to purge the latent reservoir by activating the latent proviruses with latency reversing agents (LRAs) in combination with ART [3]. Ideally, ART will prevent the establishment of new infections, and latently infected cells will be cleared by viral cytopathic effects and/or via targeting by cytotoxic immune cells.
A major complication facing strategies to purge latent reservoirs is that there are multiple obstacles to HIV transcriptional activation that contribute to the establishment and maintenance of latency [4]. First, in resting memory T cells, host transcription factors that activate the HIV-1 promoter, including nuclear factor-κB (NF-κB) and nuclear factor of activated T cells (NFAT), are present at low levels in the nucleus, resulting in inefficient initiation of viral gene expression [5–11]. In addition, low levels of the HIV transcriptional transactivator protein Tat also limit gene expression efficiency by inhibiting transcriptional elongation [12–14]. Finally, silencing of the HIV promoter via chromatin repression has been demonstrated both in vitro and in vivo, mediated by recruitment of histone deacetylase I (HDACI) [15–18] and CpG methylation [19,20]. Thus, the state of the cell and the site of viral integration could produce different subsets of latent infections that may require different LRAs to activate the latent pool.
Recently, the use of multiple drugs targeting at least two of these mechanisms has emerged as an attractive therapeutic strategy to counteract latency, and multiple studies have reported that co-treatment with two LRAs increases the overall level of viral activation. In some cases the drugs interact synergistically, such that activation with two drugs is more than would be expected from a simple model of independent drug action. For example, treatment with HDAC inhibitors, including trichostatin A (TSA), suberoylanilide hydroxamic acid (SAHA) or valproic acid (VPA), in combination with tumor necrosis factor-α (TNF), a canonical activator of NF-κB, results in synergistic activation of the latent provirus in some transformed cell line models of HIV latency [20–23]. Synergy between HDAC inhibitors and prostratin, an activator of NF-κB and protein kinase C, has also been observed in primary T cell models and patient samples [22,24]. Synergistic activation has also been observed for a DNA methyltransferase inhibitor, 5-aza-2-deoxycytidine (Aza), and NF-κB-activators prostratin or TNF [19,20,25]. However, evidence of drug interactions is variable across experimental HIV latency models. Therefore, there is a need to identify quantitative criteria associated with the subsets of latent infections for which synergistic interactions are observed and that would benefit from co-drugging.
We recently reported that chromatin accessibility at the HIV latent viral integration site sets a threshold for NF-κB-mediated activation of HIV gene expression, and once the activation threshold is crossed, HIV gene expression exhibits non-linear increases with nuclear levels of NF-κB p65 [23]. This threshold provides an explanation for drug synergy observed in anti-latency therapy strategies that combine an activator of NF-κB with a chromatin modifier such as an HDAC inhibitor. Specifically, the chromatin-modifying enzyme lowers the activation threshold via relieving chromatin repression, and the NF-κB activator leads to non-linear increases in gene expression. However, if a subset of latent infections is sufficiently close to the activation threshold, then we hypothesize that no drug synergy would be observed, and that co-drugging may not be necessary to purge this reservoir. Thus, we propose that evaluating chromatin repression of latent HIV integrations relative to this activation threshold will be useful for determining quantitative criteria under which we would expect to observe synergistic drug interactions.
A “shock and kill” anti-latency strategy is further complicated by the non-specific nature of LRAs. Because there is currently no way to specifically target latently infected cells, in vivo treatment with LRAs might cause toxicity in uninfected cells and/or lead to unwanted activation of other immune system cells [3]. Chemotherapeutic agents cause similar problems in cancer, and co-drugging has been one strategy to lower overall drug exposure in order to limit off-target toxicity while maintaining drug efficacy [26]. To apply a similar strategy to anti-latency therapy, we must first establish when co-drugging is beneficial, and then determine if toxicity can be reduced while maintaining viral activation.
In this study, we explore therapeutic strategies associated with co-drugging by quantifying experimental contexts for observing synergistic drug interactions between HDAC inhibitors and transcriptional activators. Using clonal Jurkat T cell line models of HIV latency, we find that the extent of synergistic interactions between these classes of drugs depend on chromatin accessibility at the promoter, with one subset requiring multiple drugs for activation while another subset does not. Further, in a polyclonal primary T cell model of latency, we observed that the requirement for co-drugging was donor dependent, suggesting that genetic or epigenetic differences between the host T cells may be an additional regulatory layer that sets the threshold of repression for latent infections and therefore determines whether HDACs will act synergistically with transcriptional activators. Finally, we demonstrated that drug doses could be optimized to lower off-target toxicity while maintaining viral activation via repeat stimulations. Overall, we conclude that a more quantitative evaluation of the underlying molecular mechanisms leading to synergistic drug interactions across different subsets of infections will improve design of anti-latency therapy.
Results
Synergistic activation of HIV with TNF and HDAC inhibitors is a function of chromatin accessibility at the integration site
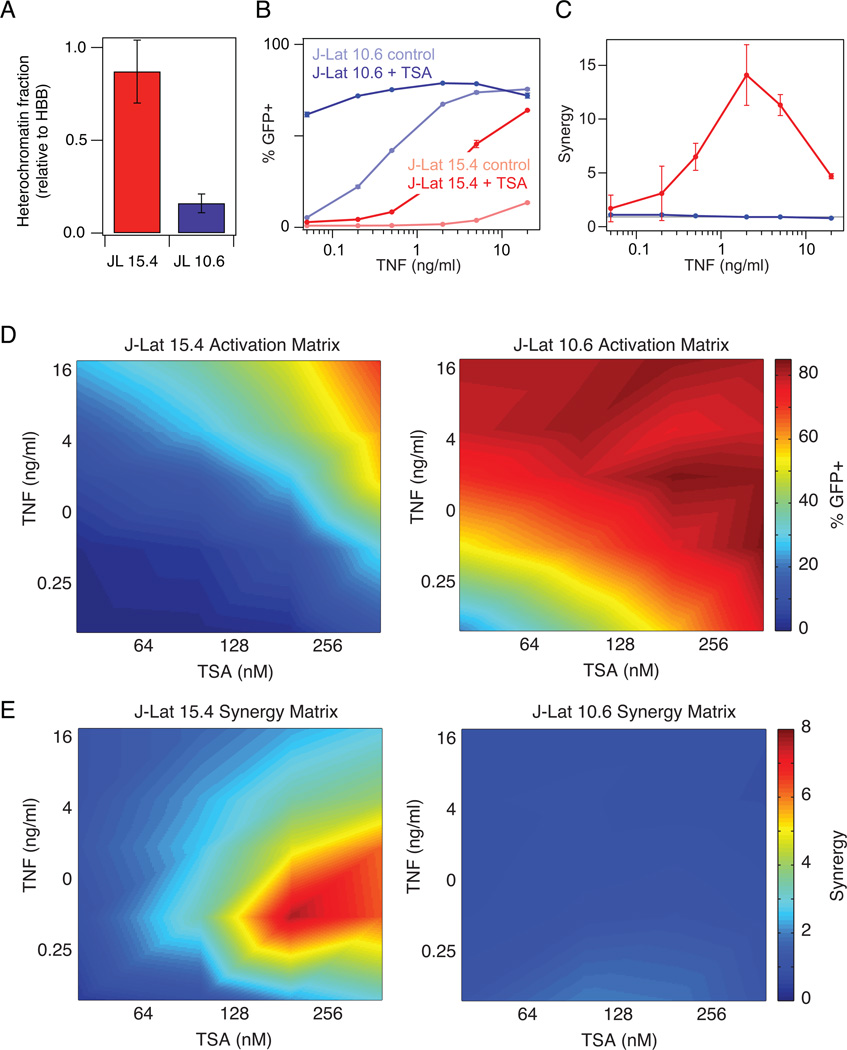
Since HIV gene activation increases non-linearly with NF-κB, we hypothesized that synergistic interactions between an activator of NF-κB and an HDAC inhibitor would depend on the extent of chromatin repression at the latent virus integration site [23]. For example, if promoter accessibility at the latent viral integration were much lower than the activation threshold due to high chromatin repression, then we would expect to see synergistic interactions between these two drug classes. However, if chromatin accessibility at the latent promoter were close to the activation threshold, then no drug synergy would be observed (Supp. Fig. S1). To test this, we compared two in vitro Jurkat T cell line models of HIV latency with significantly different levels of chromatin repression: J-Lats 15.4 and 10.6. These cell line models were established by infecting Jurkat T cells with a full-length, replication-incompetent HIV virus in which the viral protein Nef was replaced with GFP in order to easily measure reactivation [27]. Clonal populations were selected from initial, individual cells containing a single latent viral integration. Both clones have negligible HIV gene expression in the basal state, but J-Lat 15.4 has a much higher level of chromatin repression than J-Lat 10.6 as measured by a nuclease sensitivity/chromatin accessibility assay (Fig. 1A). Therefore we hypothesized that activation of J-Lat 15.4 would benefit from co-drugging more than J-Lat 10.6.
Figure 1. Comparison of TNF-TSA synergy in activating latent HIV proviruses subject to different levels of chromatin repression.
(A) Nuclease sensitivity at the HIV LTR was measured relative to the hemoglobin B (HBB) reference gene. Data are reported as the mean ± standard deviation of two measurements. (B) Dose response of activation by TNF of J-Lat 15.4 (red) and J-Lat 10.6 (blue) in the presence and absence of 400 nM TSA. GFP expression was measured by flow cytometry. Data are reported as the mean ± standard deviation of 3 biological replicates (note that some error bars are not visible). (C) Quantification of drug synergy between TNF and TSA using the Bliss independence model of drug interactions. Gray line at Synergy = 1 indicates no detectable drug interaction. (D-E) Heat map of activation (D) and drug synergy (E) of J-Lat 15.4 (left) and J-Lat 10.6 (right) for a matrix of TNF and TSA doses. Activation and synergy values were linearly interpolated to produce a continuous plot.
We treated both clones with a range of doses of TNF either alone or in combination with a saturating dose of trichostatin A (TSA; 400 nM), an inhibitor of class I and II HDACs. J-Lat clone 10.6 was activated more than 50% by either TNF or TSA alone, while J-Lat 15.4 required both drugs for more than 50% activation (Fig. 1B). We then calculated drug synergy between TNF and TSA at every dose according to the Bliss independence model of drug action, which assumes that the two drugs act through independent mechanisms [28]. Interestingly, for J-Lat 15.4, TNF–TSA co-drugging resulted in 2 to 10 times the latency activation expected for two drugs acting independently, while there was no drug synergy observed for J-Lat 10.6 (Fig. 1C). We observed similar trends in activation of gene expression and drug synergy when we directly overexpressed NF-κB p65, the transcriptional target of TNF, in combination with TSA (Supp. Fig. S2).
We also tested the DMT inhibitor Aza, which we had previously shown increases chromatin accessibility at the LTR [23]. Consistent with the TSA results, we observed synergistic benefits from co-drugging with TNF (Supp. Fig. S3A-B) and when we directly overexpressed NF-κB p65 (Supp. Fig. S3C-D) for J-Lat 15.4, but no significant drug synergy for J-Lat 10.6. Overall, these results support our hypothesis that co-drugging with an activator of NF-κB and an HDAC inhibitor (or DMT inhibitor) will result in synergistic latency activation for latent integrations with repressed chromatin environments, such as J-Lat 15.4. However, for a subset of viral integrations that are close to the activation threshold, such as J-Lat 10.6, no synergistic interactions were observed and co-drugging may not be required.
It is possible that saturating levels of TSA (400 nM) prevented us from observing TNF–TSA drug synergy in J-Lat 10.6. To test this, we varied TSA dose in addition to varying TNF dose. Specifically, we treated both clones with a matrix of combinatorial drug doses ranging from 40–400 nM TSA and 0.1–20 ng/ml TNF. The results are summarized in heat maps of HIV activation and drug synergy. Both clones were significantly activated (>60%) at the highest doses of TNF and TSA (Fig. 1D). However, we only observed synergistic activation for J-Lat 15.4, with maximum TNF-TSA synergy occurring at intermediate doses (Fig. 1E). In contrast, TNF and TSA appeared to act independently to activate latent infections in J-Lat 10.6. Thus, we conclude that latent infections such as J-Lat 10.6, which are transcriptionally silent but not subject to significant chromatin repression at the promoter, do not exhibit synergistic activation by co-drugging with an NF-κB activator and an epigenetic modifier.
Synergy between prostratin and SAHA in latent HIV activation also depends on chromatin environment and drug dose
TNF and TSA efficiently activate latent HIV in many in vitro experimental systems, but they are not clinically viable drugs. In contrast, prostratin–an activator of the PKC–NF-κB pathway–is in pre-clinical development as an LRA, and SAHA (vorinostat)–a class I HDAC inhibitor–has already been tested in clinical trials for HIV latency reactivation [29]. Several studies have suggested that combining prostratin with SAHA results in synergistic activation of latent infections in some, but not all experimental models [22,24]. Therefore, we next sought to determine if drug synergy between prostratin and SAHA would also vary significantly with the extent of chromatin repression at the site of latent viral integration and if this could explain differences in prostratin–SAHA synergy observed in previous studies.
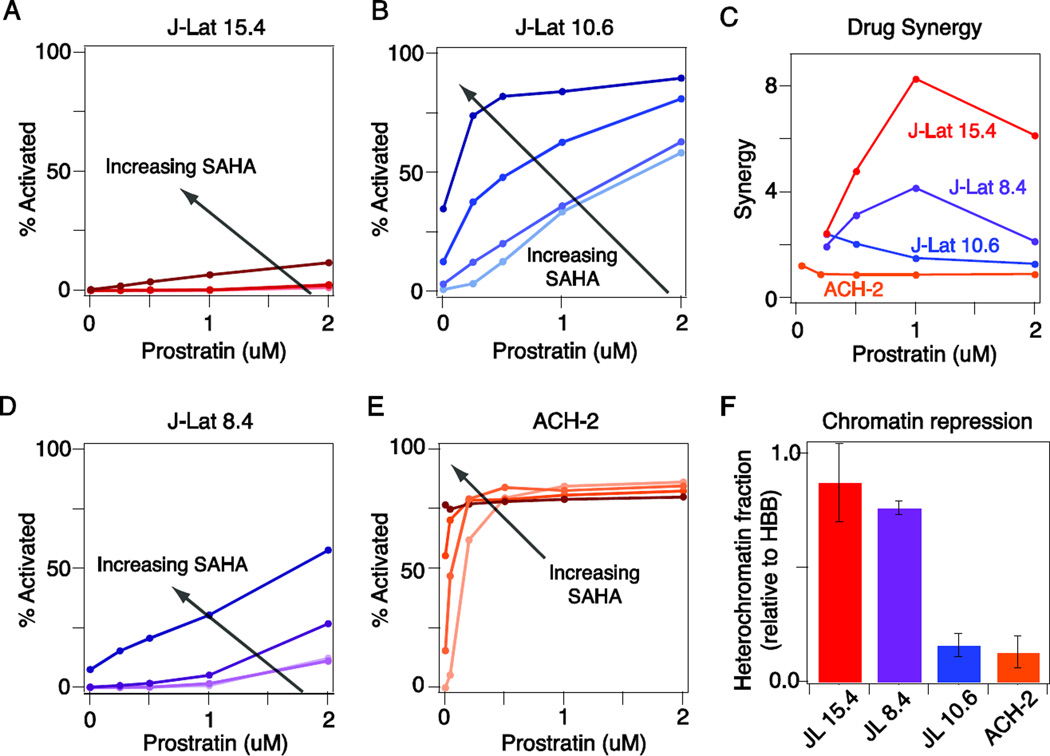
We treated J-Lat clones 15.4 and 10.6 with a matrix of combinatorial drug doses ranging from 1–4 µM SAHA and 0.25–2 µM prostratin. The maximum activation observed for J-Lat clone 15.4 was significantly less than that for clone 10.6 (12% versus 90%; Fig. 2A-B). Moreover, activation of J-Lat 15.4 required co-drugging with prostratin and SAHA, with significant drug synergy observed (Fig. 2C). In contrast, J-Lat 10.6 required only one drug for activation, with modest drug synergy observed at the lowest drug doses (Fig. 2C). Thus, our observations with prostratin–SAHA are consistent with our TNF–TSA observations.
Figure 2. Comparison of prostratin-SAHA synergy in activating latent HIV proviruses subject to different levels of chromatin repression.
A-B) Dose response of activation by prostratin of J-Lat 15.4 (A) and J-Lat 10.6 (B) in the presence of increasing doses of SAHA (0, 1, 2, and 4 uM with darker shade indicating higher dose). Viral activation was assessed by GFP expression, which was measured by flow cytometry. C) Calculation of drug synergy in the presence of 2 uM SAHA. D-E) Dose response of activation by prostratin of J-Lat 8.4 (D) and ACH-2 (E) in the presence of increasing doses of SAHA (same as in A-B). Viral activation was assessed by GFP expression (J-Lat 8.4) or anti-HIV-1 core antigen staining (ACH-2) and measured by flow cytometry. F) Nuclease sensitivity at the HIV LTR relative to the HBB reference gene. Data are reported as the mean ± standard deviation of two measurements.
To further verify our findings, we tested two other commonly used experimental cell line models of HIV latency: J-Lat 8.4 and ACH-2, a leukemic T-cell line harboring a single integration of a replication-competent virus. Chromatin repression at the LTR was comparable between J-Lat 8.4 and J-Lat 15.4, and between ACH-2 and J-Lat 10.6, as measured by a nuclease sensitivity assay (Fig. 2F). Consistently, J-Lat clone 8.4, behaved similarly to clone 15.4, with co-drugging required to achieve significant activation (Fig. 2D), while treatment with either prostratin or SAHA alone robustly activated ACH-2 cells, similar to J-Lat 10.6 (Fig. 2E). The ACH-2 results are consistent with the fact that latency in ACH-2 cells primarily results from a mutation in the TAR region, rather than significant chromatin repression [30]. Interestingly, we did observe modest prostratin–SAHA synergy in J-Lat 10.6 and ACH-2 cells at the lowest drug doses (Fig. 2C), suggesting an advantageous drug interaction, consistent with previous reports [22,24]. Overall, however, we conclude that co-drugging with prostratin and SAHA is only required for a subset of latent integrations with significant chromatin repression, while the remaining latent infections could be efficiently activated with either drug alone.
Synergy between TNF and a drug targeting transcriptional elongation does not show a dependence on chromatin environment
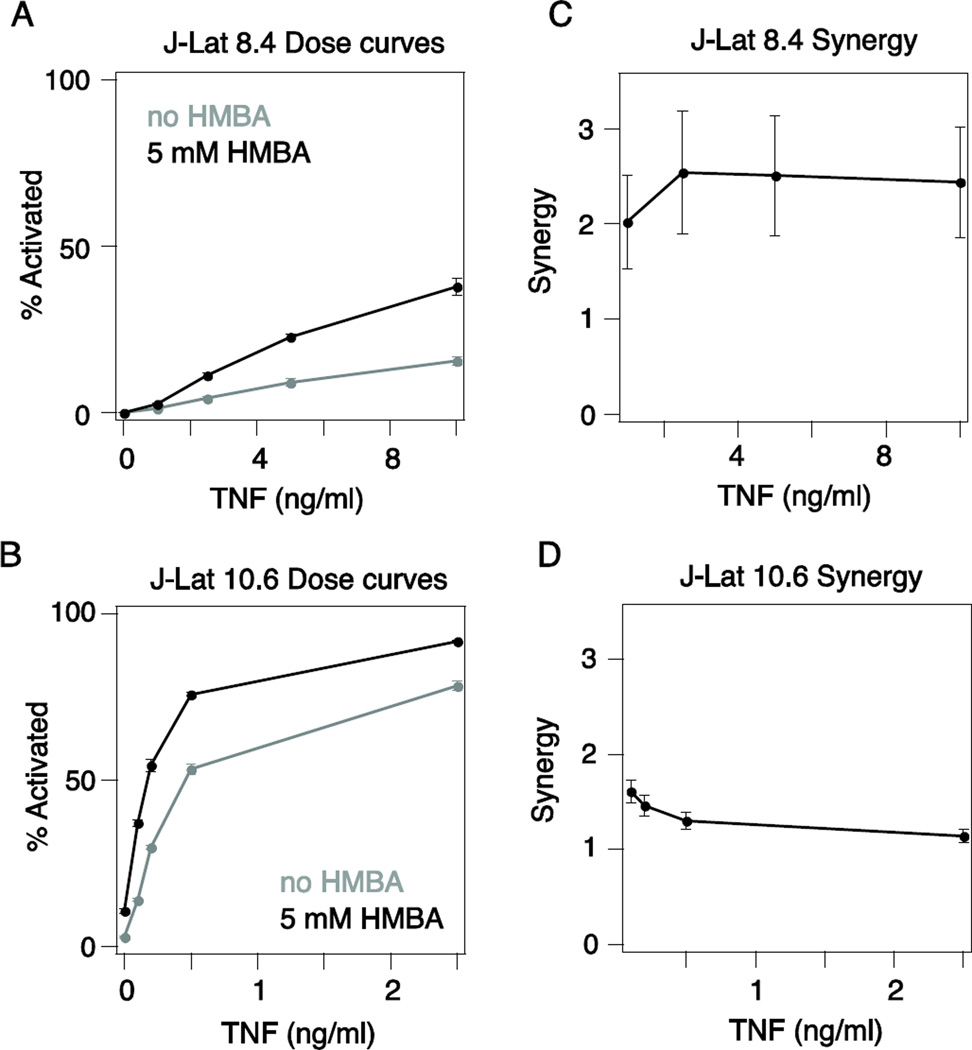
Another potential mechanism for maintaining latency is inefficient viral elongation due to low levels of the HIV transactivator Tat, which recruits the positive transcriptional elongation factor B (P-TEFb) [31]. The clinically tested chemotherapeutic hexamethylene bisacetamide (HMBA) [32] has been demonstrated to alleviate this transcriptional block by promoting the localization of P-TEFb to the LTR to enhance viral transcriptional elongation [33–35]. We hypothesized that any drug synergy observed between TNF and HMBA would not show a strong dependence on the chromatin environment of the latent viral integration because P-TEFb levels should be similar across Jurkat cells regardless of the viral integration site. To test this, we compared TNF–HMBA drug synergy for J-Lat 8.4 and J-Lat 10.6 (Fig. 3A-B). We used a lower TNF dose for J-Lat 10.6 in order to better capture any synergistic activity between TNF and HMBA. Only a low level of drug synergy was observed, likely because a limiting amount of P-TEFb is not a major barrier to activating latency in Jurkat cells. However, we found that TNF–HMBA drug synergy was similar between these two clones, consistent with a mechanism that is independent of chromatin (Fig. 3C-D). This result supports our assertion that understanding how molecular mechanisms targeted by LRAs vary across subsets of latent infections will aid in predicting differential benefits of co-drugging.
Figure 3. Level of chromatin repression does not affect drug synergy between a TNF and a pTEF-b inhibitor.
A-B) TNF dose response curves for (A) J-Lat 8.4 and (B) J-Lat 10.6 alone (gray line) and with 5mM HMBA (black line). GFP expression was measured by flow cytometry. Data are reported as the mean ± standard deviation of three biological replicates. C-D) Quantification of drug synergy between TNF and HMBA for (C) J-Lat 8.4 and (D) J-Lat 10.6 using the Bliss indepence model of drug interactions.
Polyclonal latent infections in Jurkat cell lines do not exhibit synergy between TNF and TSA
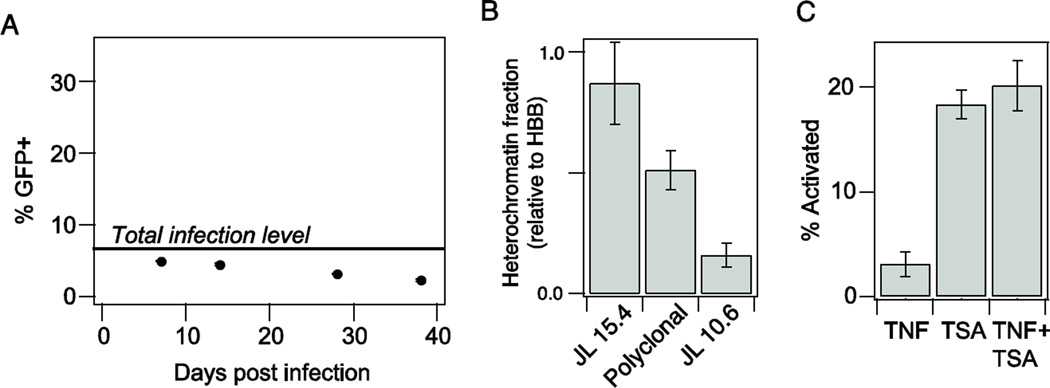
Unlike the clonal Jurkat cell line models used above, T cells harboring latent HIV in vivo will contain a mixture of integration positions. Therefore, the requirement for co-drugging to activate these latent proviruses will depend on the degree of chromatin repression observed across the entire polyclonal population. To test the degree of repression observed in a polyclonal latent population, we infected Jurkat cells with a full-length HIV virus containing stop codons in all viral proteins except Tat [36] at a low multiplicity of infection (MOI < 0.1). After 7 days, we treated the polyclonal population with TSA and phorbol myristate acetate (PMA) to activate all infections, and estimated a total infection level of approximately 7% (Fig. 4A). The polyclonal population was cultured for an additional month to allow the latent infections to return to an undetectable level, similar to the methods used to isolate J-Lat clones [27].
Figure 4. TNF-TSA drug synergy was not observed for a polyclonal latently infected population.
A) Quantification of latent population over time. Gray line indicates the total level of infection as measured by activation with 10 mM PMA and 400 nM TSA. B) Nuclease sensitivity at the HIV LTR relative to the hemoglobin B (HBB) reference gene. Data are reported as the mean ± standard deviation of two measurements. C) Activation of the polyclonal latent population with 10 ng/ml TNF and/or 400 nM TSA. GFP expression was measured by flow cytometry. % activation was normalized to the total observed infection level (indicated in A). Data are reported as the mean ± standard deviation of three biological replicates.
After establishing the latent infections, we measured chromatin repression in the polyclonal population and found that the level was between that of our experimental models with high and low repression (J-Lat 15.4 and J-Lat 10.6, respectively; Fig. 4B). We then measured activation of the latent population in response to 10 ng/ml TNF, 400 nM TSA, and the combination. Interestingly, TNF alone did not significantly activate latent gene expression, while TSA alone induced robust activation (Fig. 4C). Moreover, co-drugging did not synergistically activate or even significantly enhance overall activation at these doses. Our TNF–TSA results are consistent with published observations for TNF and the DMT inhibitor Aza–namely, that a synergistic drug interaction between TNF and Aza was not observed for a polyclonal population of latent HIV-infected Jurkat cells, despite TNF–Aza activation synergy for J-Lat clones 8.4 and 15.4 [20]. Therefore, data from our study and others suggest that the majority of latent integrations harbored in a polyclonal population of latent HIV-infected Jurkat cells may not require co-drugging for full activation.
Donor-dependent requirements for co-drugging with prostratin and SAHA is observed in a primary cell model of latent HIV infection
Experimental latency models established in quiescent primary CD4+ T cells show significant differences from the cell line models, and may provide a more physiological model of latency [37,38]. Similar to results in cell line models, previous studies demonstrated synergy between prostratin and SAHA in reactivation of latent infections in primary cultured central memory CD4+ T cells and in HIV-infected patient cells, but drug interactions were not observed across all donors [22,24]. We therefore asked if donor-to-donor differences in chromatin repression of the latent infections could contribute to differences in activation observed when co-drugging with prostratin and SAHA.
We established latent infections in cultured primary T cells by isolating naïve CD4+ T cells from whole blood of healthy donor as previously described for other studies [22,37]. Briefly, cells were activated and infected during the cell proliferation phase with a full-length virus based on the reference strain HIV-1 NL4-3, which was rendered replication-deficient by introducing a frame-shit mutation in the envelope glycoprotein gene [37,38]. Following infection, cells were cultured 7–9 days into a quiescent, central-memory T cell (TCM)-like phenotype (referred to as non-polarized cells) that harbor latent infections. Specifically, little viral gene expression was detected in the resting state, but activation with CD3/CD28 resulted in significant viral gene expression as measured by staining for intracellular p24Gag (Supp. Fig. S4).
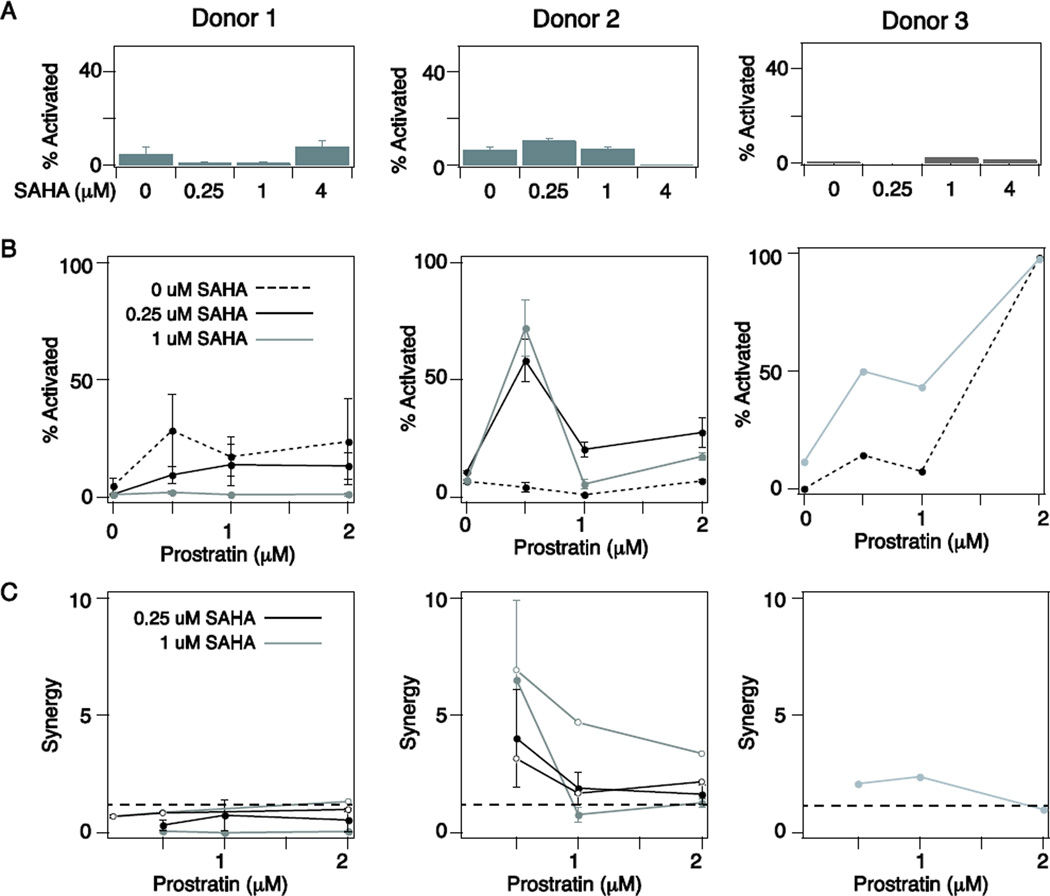
We saw no significant activation of latent infections in cultures of primary TCM-like cells with SAHA treatment alone across a range of doses and donors (Fig. 5A), consistent with previous studies [22,37]. However, we observed a significant increase in the toxicity induced by SAHA at 4 µM (Supp. Fig. S5), and therefore all subsequent experiments were performed at 1 µM SAHA or lower. We next tested latency reactivation as a function of prostratin dose in the presence of 0, 0.25 or 1 µM SAHA. We found that activation by prostratin varied considerably across donors, with Donor 1 activated 25–30% by prostratin alone, Donor 2 requiring SAHA for activation, and Donor 3 showing activation enhancement in the presence of SAHA (Fig. 5B). Notably, in some cases the higher drug doses of SAHA and/or prostratin actually reduced overall activation, due to significant cell toxicity (data not shown). When we calculated prostratin-SAHA synergy, we found that it varied significantly across donors, with almost no synergy observed for Donor 1, significant synergy observed for Donor 2, and an intermediate level of synergy observed for Donor 3 (Fig. 5C, closed circles). Thus, the advantage of co-drugging varies for different primary cultures of latent TCM-like cells.
Figure 5. Latent infections in cultured primary memory CD4+ TCM cells indicated patient-specific requirements for co-drugging.
A-B) Latent HIV activation induced by A) SAHA alone and B) prostratin in the presence of increasing doses of SAHA (0, 0.25, and 1 uM) in primary cultured TCM cells derived from PBMCs from 3 healthy donors. Percent of infected cells showing activation was measured by intracellular staining for p24-Gag antigen and analyzed by flow cytometry. Results were normalized to the maximal activation measured following CD3/CD28 stimulation and are presented as the mean ± standard deviation for 3 biological replicates (Donors 1 and 2) or a single replicate (Donor 3). C) Calculation of prostratin-SAHA synergy across 3 donors. Results from two independent latent infections are shown for Donors 1 and 2 (compare closed and open circles). Dotted line indicates synergy = 1 (i.e., no drug interactions).
Sensitivity to prostratin and thus the requirement for co-drugging could be a characteristic of the donor or of the infection. To test this, we repeated CD4+ T cell isolation from Donors 1 and 2 and generated new latent infections. Interestingly, we again observed sensitivity to prostratin and thus no significant prostratin–SAHA synergy for Donor 1, but a requirement for SAHA and significant drug synergy for Donor 2 (Fig. 5C, open circles). These data suggest that the molecular mechanisms establishing and maintaining latency in a particular patient–such as the average heterochromatin level at latent HIV promoters–vary by individual and may result in drug effects that are at least in part patient specific. Thus, tailored strategies for anti-latency therapy may be required.
Optimizing drug synergy rather than maximizing drug response is a viable strategy for reducing off-target toxicity during latency activation
We have demonstrated that co-drugging is not always required to activate latent HIV in both in vitro cell line models and in cultured primary TCM-like cells. In many cases co-drugging increases the absolute level of latent HIV activation, however in primary latency models, significant toxicity can lower overall activation (Fig. 5B). Furthermore, because LRAs are not targeted specifically to infected cells, using multiple drugs to maximize activation of the latent population could exacerbate off-target toxicity in healthy, uninfected cells. We therefore considered whether it would be possible to reduce overall drug dose (either by using a single drug or by taking advantage of synergistic drug interactions) in order to lower toxicity while maintaining viral activation via repeated stimulations.
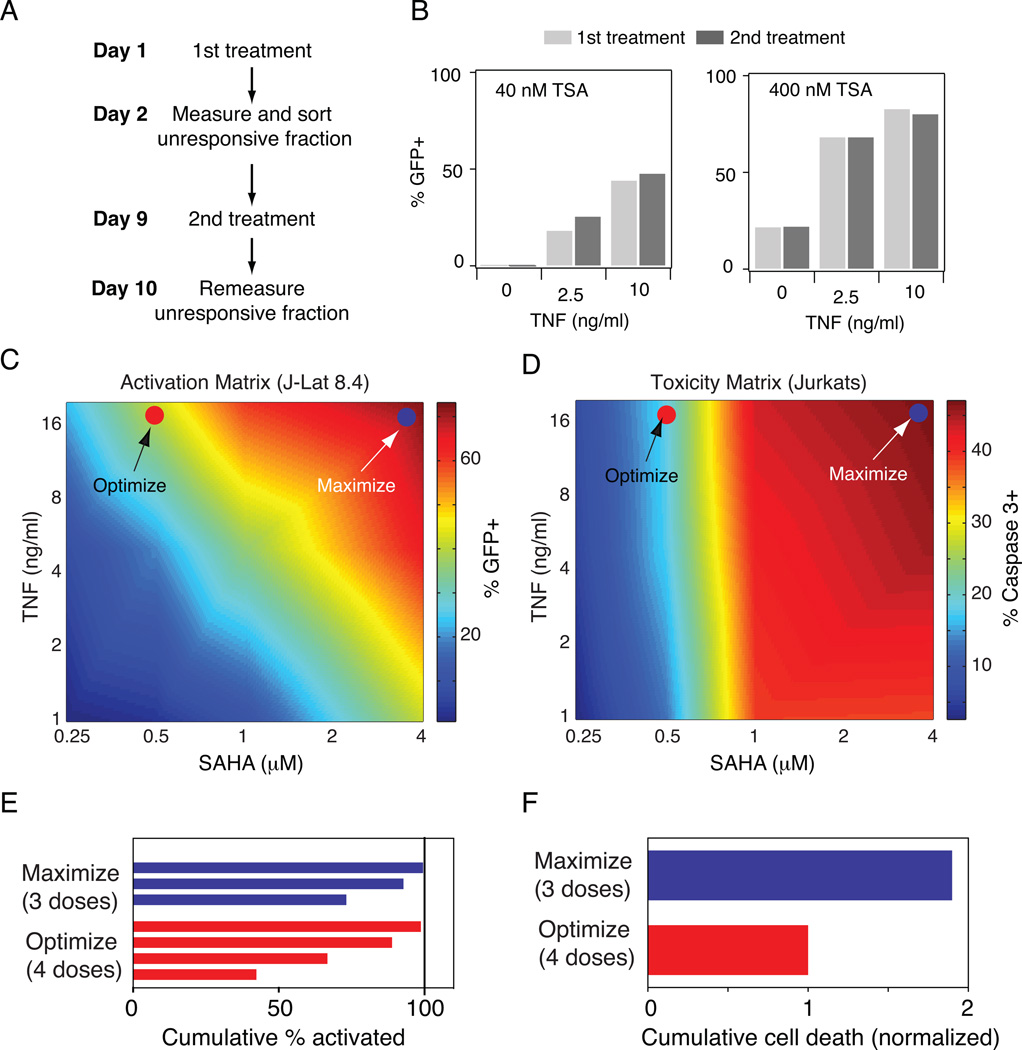
We first considered if multiple stimulations with the latency-reducing agents (LRAs) is a viable strategy to fully purge the latent population. This therapy design assumes that activation of latent viruses is in part stochastic [36,39,40], such that a different subset of the latent population will be purged by each treatment. A recent study demonstrated the validity of this assumption in HIV-infected patients undergoing ART therapy [41]. To confirm the probabilistic nature of latent viral activation in the J-Lat model, we treated J-Lat 8.4 with a range of doses of TNF and TSA for 24 hours, sorted the unresponsive cells, and then cultured those cells for one week (Fig. 6A). Upon restimulation with the same dose of TNF–TSA, we observed that activation was nearly identical in both cases (Fig. 6B), confirming that activation of latent HIV is in part stochastic, as previously demonstrated. This suggests that repeated doses of sub-maximal drug concentrations would continually activate a new fraction of the latent pool.
Figure 6. Optimization of drug synergy to reduce off-target toxicity during latency activation.
A) Schematic of experimental protocol to mimic multiple stimulations of latent infections. B) Activation of J-Lat 8.4 by TNF and TSA before sorting the unresponsive fraction (1st treatment; light gray bars) and after sorting the unresponsive fraction (2nd treatment; dark gray bars). C-D) Heat maps of (C) activation of J-Lat 8.4 and (D) toxicity in uninfected Jurkat cells for a matrix of TNF and SAHA doses. GFP expression was measured by flow cytometry and toxicity was measured by staining for anti-active caspase 3. Activation and toxicity values were measured for a range of doses and were linearly interpolated to produce a continuous plot (see Supp. Tables S1-2 for data matrix values). E-F) Cumulative activation and F) cumulative toxicity for repeated dosing of a combination TNF+SAHA that maximizes total activation (blue) or optimizes between activation and toxicity (red).
To see if a sub-maximal drug concentration could reduce toxicity, we compared the TNF-SAHA activation matrix of J-Lat 8.4 (Fig. 6C) with a toxicity matrix for uninfected Jurkat cells using the same range of drug concentrations (Fig. 6D). Similar to our previous results, we found that co-drugging with TNF and SAHA synergistically enhanced activation of J-Lat 8.4, and the largest increase in activation occurred by increasing both TNF and TSA concentrations (Fig. 6C). In contrast, toxicity was primarily determined by SAHA concentration, with little dependence on TNF (Fig. 6D). Therefore, by choosing a high TNF concentration in the presence of a low SAHA concentration, it may be possible to reduce toxicity while maintaining activation with repeated dosing strategies. We note that in the case of J-Lat 10.6, a single dose of TNF would almost fully activate the latent viruses without requiring the more toxic addition of SAHA (Fig. 1).
As a proof-of-concept, we compared two co-drugging strategies: one chosen to maximize activation and the second chosen to optimize between activation and toxicity (Fig. 6C-D). We then calculated the number of doses required to activate 99% of the latent viruses at each drug-dose combination. We found that 3 drug doses would be required for the Maximize strategy, while 4 drug doses would be required for the Optimize strategy (Fig. 6E). We then calculated overall cell death in these two scenarios and demonstrated that in the Optimize strategy, cell death would be decreased by nearly half, despite requiring an extra drug dose. Although TNF is not a viable clinical drug due to its inflammatory activity in vivo, our results provide proof-of-concept data that drug combinations and dosing strategies can be optimized to achieve multiple therapeutic goals (i.e., high virus activation and reduced toxicity).
Discussion
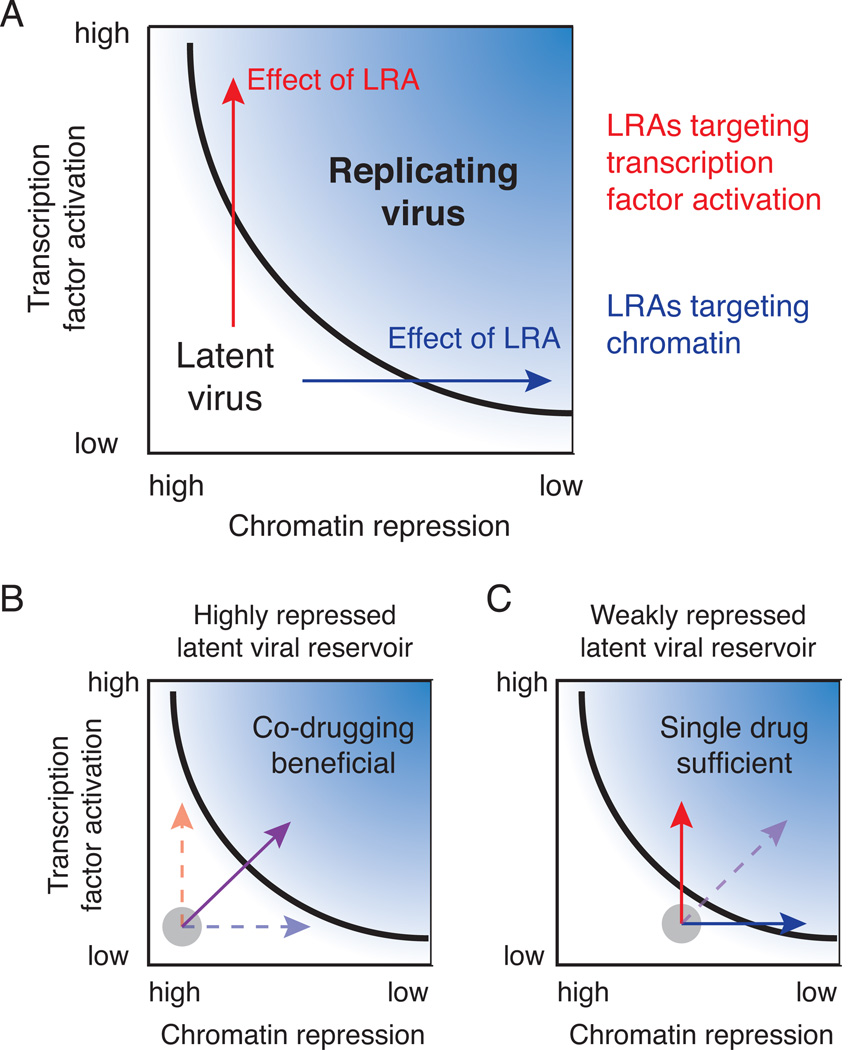
Latency-reversing agents (LRAs) show significant promise as a strategy to purge cellular reservoirs harboring latent viruses that could ultimately cure HIV infection in patients. In this translational research study, we applied a quantitative engineering approach to evaluate the efficacy of clinically relevant LRAs, including the HDAC inhibitor vorinostat (aka, SAHA), which has recently been tested in patients [29]. Pharmacological reversal of latency can be depicted as driving viruses over a threshold from latency to a replicative state [23] by increasing transcription factor activation and/or reversing chromatin repression (Fig. 7A). Multiple studies in the HIV latency field have explored synergistic drug interactions between SAHA and other LRAs that target chromatin or transcription factors with mixed results. Here, we systematically evaluated drug interactions between NF-κB activators and HDAC inhibitors across a range of in vitro cell lines and primary cell models of HIV latency. We demonstrated that the benefit of co-drugging with these two drug classes depends on the level of chromatin repression at the latent viral integrations (Fig. 1–2). For one subset of latent viral integrations, promoter accessibility due to chromatin repression is much lower than the threshold level needed for viral activation and therefore significant synergistic drug interactions were observed (Fig. 7B). For a different subset of integrations, promoter accessibility is close to the level required for promoter activation (i.e., there is low chromatin repression), and thus these latent viruses were efficiently activated by a single drug (e.g., either an HDAC inhibitor or an NF-κB activator; Fig. 7C).
Figure 7. Schematic representations of how different classes of latent HIV integrations are affected by LRA treatment.
A) Pharmacological reversal of latency moves virus from latency to a replicative state by increasing transcription factor activation (red) and/or reversing chromatin repression (blue). B-C) Viral integrations can be divided into two classes based on level of chromatin repression at the promoter. B) Viral promoter accessibility due to chromatin repression is much lower than the threshold level needed for viral activation and therefore co-drugging is beneficial (purple) or C) Promoter accessibility is close to the level required for promoter activation due to low chromatin repression and only one drug is required for activation.
We explored the clinical implications of this discovery by demonstrating that evidence of synergistic drug interactions varies by the donor patient (Fig. 5), motivating a potential need for patient-specific HIV-activating strategies. The observation that heterogeneous latent HIV infections established in primary cultured TCM-like cells are a property of the donor patient and not of the infection suggests that biological mechanisms affecting the establishment of latency, such as variation in chromatin states, may be patient dependent. A recent study of chromatin states across 19 individuals indeed demonstrated a wide degree of variation [42]. An important follow-up experiment would be to determine if heterochomatin levels of latent infections in primary cultured TCM-like cells from different donors correlated with LRA responsiveness, similar to in vitro cell line models (Fig. 3). If so, then it may be possible to discover a biomarker–such as global HDAC levels or histone modifications for a set of reference gene promoters–that is predictive of patient-specific combination drug synergy.
An important reason to quantify the requirements for co-drugging is because off-target toxicity in uninfected cells may increase as a result of using multiple drugs. Therefore, if a single drug can efficiently activate the latent reservoir, it may be better to increase the dose of the less toxic drug than to combine drugs. If co-drugging is required, we provide proof-of-concept data demonstrating that it is possible to limit off-target toxicity by taking advantage of synergistic interactions between two LRAs. Specifically we calculate that more cycles of co-drugging administered at a lower drug dose could maintain robust latent virus activation (99%) while minimizing toxicity as compared to co-drugging treatments administered at higher doses (Fig. 6).
Despite significant excitement about co-drugging anti-latency strategies, our results suggest additional reasons why co-drugging might not always be advantageous in the clinic. First, data from our study and others [20] suggest that the majority of integrations harbored in a polyclonal population of latent HIV-infected Jurkat cells do not require co-drugging for activation (Fig. 5C). This result is consistent with the observation that resting CD4+ T cells from HIV-infected patients have HIV genomes integrated within actively transcribed host genes that are unlikely to display significant chromatin repression and thus may not require multiple drugs for activation [43]. However, a very recent study demonstrated that most single LRAs do not induce substantial activation of the latent reservoir from patient cells on ART, suggesting that mechanisms regulating HIV latency in vivo may be absent in in vitro latency models [44].
Finally, although the goal of “shock and kill” HIV eradication strategies is to completely purge HIV viruses from a patient, a more likely scenario is that the latent reservoir will be sufficiently reduced such that the host-mediated immune response will be able to control ongoing reactivation in the absence of ART [3]. In this case, clearing the latent infections that are “easily” reactivated would be the primary goal and latent infections that require co-drugging to activate may be less important because they are unlikely to reactivate in the patient. Overall, our study provides a quantitative explanation for the mixed conclusions about the benefits of co-drugging with SAHA in the literature and motivates consideration of patient-specific anti-latency strategies.
Methods
HIV latency models in leukemic T cell lines
J-Lat [27] and ACH-2 latent HIV cell lines were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH and Jurkat cells clone E6-1 were obtained from ATCC. Jurkat, J-Lat, and ACH-2 cell lines were cultured in RPMI media supplemented with 10% FBS and penicillin and streptomycin at a concentration of 2×105-2×106 cells/ml at 37°C and 5% CO2. Polyclonal latent infections in Jurkat cells were established as previously described [36].
Generation of latent HIV infections in primary cultured memory T cells
Cultured memory CD4+ T cells harboring latent HIV infections were cultured as previously described [37]. Briefly, naïve CD4+ T cells were isolated from peripheral blood mononuclear cells using an isolation kit (Miltenyi, Order no:130-091-894) according to manufacturer’s instructions with a manual MACS LC column. To activate naïve cells, 106 cells were cultured in complete media supplemented with 2µg/ml anti-IL-12 (Peprotech 500-P154G), 1 µg/ml anti-IL-4 (Peprotech 500-P24) and 10 ng/ml TGF-β in the presence of 25 µl Dynabeads CD3/CD28 T cell Expander (Invitrogen, 111.31D) for 3 days. Cells were then switched to complete media supplemented with 30 IU/ml IL-2 for two more days. On day 5, cells were infected with DHIV virus corresponding to 100 ng p24 (measured by ELISA) per 106 cells/ml. Cells were centrifuged at 2900 RPM for 2 hours at 37C, at which point media was removed and replaced with complete media plus IL-2. Cells are cultured to a quiescent state for 9 days before reactivation experiments.
Pharmacological treatments
For in vitro latent cell line models, 500,000 cells/ml were incubated with drugs for 24 hours prior to flow cytometry analysis, except for 5-aza-deoxycytidine which was added for 48 h. For primary cultured memory CD4+ T cells, 150,000 cells were incubated with drugs for 72 hours prior to flow cytometry analysis. The following drugs and concentrations were tested (with sources): 0.05–20 ng/ml tumor necrosis factor alpha (TNF-α) (Peprotech); 10 nM phorbol myristate acetate (PMA), 4–400 nM trichostatin A (TSA), 5 mM hexamethylene bisacetamide (HMBA) and 1 µM 5-aza-deoxycyidine (Aza) (Sigma-Aldrich); 0.04–4 µM prostratin and 0.25–4 µM SAHA (Santa Cruz Biotechology).
Latency activation assay
For J-Lat and polyclonal Jurkat latent infections, activation was quantified by analyzing green fluorescent protein (GFP) level in 10,000 cells. For the ACH-2 cell line and for primary cultured memory T cells, activation was assayed by fixing cells and staining for intracellular p24 according to the manufacturer’s protocol (BD Cytofix/Cytoperm). Antibody sources include: FITC-conjugated KC57 anti-HIV-1 Core Antigen (Beckman Coulter) for ACH-2 cell staining; and anti-p24 (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) with Alexa-488 secondary conjugation (Life Technologies). All cells were analyzed on an Accuri™ C6 Flow Cytometer (BD Biosciences).
Nuclease sensitivity assay
The nuclease sensitivity assay was performed using the EpiQ™ Chromatin Analysis Kit (Bio-Rad) as previously described [23].
Toxicity assays
For general toxicity, cells were resuspended in 5 µg/ml propidium iodide (PI), after which cells were immediately analyzed by flow cytometry. To measure apoptosis, cells were fixed as described for p24 analysis and then co-stained with antiactive caspase 3 (BD Biosciences) with Alexa 647 secondary conjugation (Life Technologies) prior to flow cytometry analysis.
Analysis of drug synergy and statistics
Drug interactions were predicted according to the Bliss independence model [28]: BlissA+B = 1 – (1-mA)*(1-mB) where mA and mB are the mean GFP+ fraction activated by co-drugging with drug A and drug B. Synergy between drug A and drug B was then calculated as follows: synergy = ObservedA+B/BlissA+B, where ObservedA+B is the measured GFP+ fraction activated by co-drugging with drug A and drug B. We used Student’s t-test to compare two means.
Supplementary Material
Acknowledgements
This work was supported by the Bill and Melinda Gates Foundation’s Grand Challenges Explorations (Round 7 to K.M.J.) and the National Science Foundation (NSF CBET-1264246 to K.M.J.). V.C.W. was supported by a National Institutes of Health Predoctoral Training Grant in Genetics (2T32GM007499-36, 5T32GM007499-34, 5T32GM007499-35).
Biography
Kathryn Miller-Jensen is an Assistant Professor of Biomedical Engineering and Molecular, Cellular, and Developmental Biology at Yale University. Her lab uses experimental and computational approaches to study signaling and transcriptional regulation in response to pathogens, with a focus on activation of latent HIV, as well as innate immune signaling. Her work is funded by the National Institutes of Health, the National Science Foundation, and the Bill and Melinda Gates Foundation. Kathryn was an NIH NSRA Postdoctoral Fellow at the University of California at Berkeley and she holds a Ph.D. in Chemical Engineering from the Massachusetts Institute of Technology and A.B. and B.E. degrees in Engineering Sciences from Dartmouth College. She is a member of the Biomedical Engineering Society and a former Christine Mirzayan Science and Technology Policy Fellow at the National Academies in Washington, DC.
Footnotes
Conflicts of Interest
V.C.W., L.E.F., N.M.A., Q.X., S.S.D., and K.M.J. declare that they have no conflicts of interest.
Ethical Standards
No human studies or animal studies were carried out by the authors for this article.
References
- 1.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 3.Durand CM, Blankson JN, Siliciano RF. Developing strategies for HIV-1 eradication. Trends Immunol. 2012;33:554–562. doi: 10.1016/j.it.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, et al. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 5.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 6.Böhnlein E, Lowenthal JW, Siekevitz M, Ballard DW, Franza BR, et al. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988;53:827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- 7.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, et al. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 9.Ganesh L, Burstein E, Guha-Niyogi A, Louder MK, Mascola JR, et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426:853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- 10.Kim YK, Bourgeois CF, Pearson R, Tyagi M, West MJ, et al. Recruitment of TFIIH to the HIV LTR is a rate-limiting step in the emergence of HIV from latency. EMBO J. 2006;25:1–9. doi: 10.1038/sj.emboj.7601248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barboric M, Yik JHN, Czudnochowski N, Yang Z, Chen R, et al. Tat competes with HEXIM1 to increase the active pool of P-TEFb for HIV-1 transcription. Nucleic Acids Research. 2007;35:2003–2012. doi: 10.1093/nar/gkm063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao SY, Calman AF, Luciw PA, Peterlin BM. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 13.Selby MJ, Peterlin BM. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990;62:769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coull JJ, Romerio F, Sun JM, Volker JL, Galvin KM, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams SA, Chen L-F, Kwon H, Ruiz-Jarabo CM, Verdin E, et al. NF-κB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang G, Espeseth A, Hazuda DJ, Margolis DM. c-Myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. J Virol. 2007;81:10914–10923. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyagi M, Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO J. 2007;26:4985–4995. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blazkova J, Trejbalova K, Gondois-Rey F, Halfon P, Philibert P, et al. CpG Methylation Controls Reactivation of HIV from Latency. PLoS Pathog. 2009;5:e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5:e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quivy V, Adam E, Collette Y, Demonte D, Chariot A, et al. Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: potential perspectives for the development of therapeutic strategies. J Virol. 2002;76:11091–11103. doi: 10.1128/JVI.76.21.11091-11103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett JC, Lim K-I, Calafi A, Rossi JJ, Schaffer DV, et al. Combinatorial latency reactivation for HIV-1 subtypes and variants. Journal of Virology. 2010;84:5958–5974. doi: 10.1128/JVI.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller-Jensen K, Dey SS, Pham N, Foley JE, Arkin AP, et al. Chromatin accessibility at the HIV LTR promoter sets a threshold for NF-kappaB mediated viral gene expression. Integr Biol (Camb) 2012;4:661–671. doi: 10.1039/c2ib20009k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuse S, Calao M, Kabeya K, Guiguen A, Gatot J-S, et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS ONE. 2009;4:e6093. doi: 10.1371/journal.pone.0006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez G, Zeichner SL. Cell line-dependent variability in HIV activation employing DNMT inhibitors. Virol J. 2010;7:266. doi: 10.1186/1743-422X-7-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol. 2006;2:458–466. doi: 10.1038/nchembio817. [DOI] [PubMed] [Google Scholar]
- 27.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BLISS CI. The calculation of microbial assays. Bacteriol Rev. 1956;20:243–258. doi: 10.1128/br.20.4.243-258.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emiliani S, Van Lint C, Fischle W, Paras P, Jr, Ott M, et al. A point mutation in the HIV-1 Tat responsive element is associated with postintegration latency. Proc Natl Acad Sci U S A. 1996;93:6377–6381. doi: 10.1073/pnas.93.13.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott M, Geyer M, Zhou Q. The control of HIV transcription: keeping RNA polymerase II on track. Cell Host Microbe. 2011;10:426–435. doi: 10.1016/j.chom.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowinsky EK, Ettinger DS, Grochow LB, Brundrett RB, Cates AE, et al. Phase I and pharmacologic study of hexamethylene bisacetamide in patients with advanced cancer. J Clin Oncol. 1986;4:1835–1844. doi: 10.1200/JCO.1986.4.12.1835. [DOI] [PubMed] [Google Scholar]
- 33.Choudhary SK, Archin NM, Margolis DM. Hexamethylbisacetamide and disruption of human immunodeficiency virus type 1 latency in CD4(+) T cells. J Infect Dis. 2008;197:1162–1170. doi: 10.1086/529525. [DOI] [PubMed] [Google Scholar]
- 34.Contreras X, Barboric M, Lenasi T, Peterlin BM. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog. 2007;3:1459–1469. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller-Jensen K, Skupsky R, Shah PS, Arkin AP, Schaffer DV. Genetic selection for context-dependent stochastic phenotypes: Sp1 and TATA mutations increase phenotypic noise in HIV-1 gene expression. PLoS Comput Biol. 2013;9:e1003135. doi: 10.1371/journal.pcbi.1003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosque A, Planelles V. Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells. Methods. 2011;53:54–61. doi: 10.1016/j.ymeth.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell. 2005;122:169–182. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Burnett JC, Miller-Jensen K, Shah PS, Arkin AP, Schaffer DV. Control of stochastic gene expression by host factors at the HIV promoter. PLoS Pathog. 2009;5:e1000260. doi: 10.1371/journal.ppat.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasowski M, Kyriazopoulou-Panagiotopoulou S, Grubert F, Zaugg JB, Kundaje A, et al. Extensive variation in chromatin states across humans. Science. 2013;342:750–752. doi: 10.1126/science.1242510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han Y, Lassen K, Monie D, Sedaghat AR, Shimoji S, et al. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78:6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20:425–429. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.