Abstract
Background
In this study, using a meta-analysis approach, we examined the correlation between serum levels of lysophosphastidic acid (LPA) and ovarian cancer (OC).
Methods
Relevant published studies were identified from multiple scientific literature databases by using a pre-determined electronic and manual search strategy. The search results were screened through a multi-step process to select high-quality case–control studies suitable for the present meta-analysis. Mean values and standardized mean differences (SMD) were calculated for plasma LPA levels. Two investigators independently extracted the data from the studies and performed data analysis using STATA software version 12.0 (Stata Corp, College Station, TX, USA).
Results
Nineteen case–control studies met our selection criteria and contained a total of 980 OC patients, 872 benign controls and 668 healthy controls. Our meta-analysis results revealed that the plasma levels of LPA in OC patients were significantly higher than benign controls (SMD = 2.36, 95 % CI: 1.61–3.10, P <0.001) and healthy controls (SMD = 2.32, 95 % CI: 1.77–2.87, P <0.001). Subgroup analysis by ethnicity showed that the plasma LPA levels in OC patients were significantly higher than the benign controls only in Asian populations (SMD = 2.52, 95 % CI: 1.79–3.25, P <0.001). However, a comparison between healthy controls and OC patients revealed that, in both Asians and Caucasians, the OC patients displayed significantly higher plasma LPA levels compared to healthy controls (all P <0.05).
Conclusion
Our meta-analysis showed strong evidence that a significantly higher plasma LPA levels are present in OC patients, compared to benign controls and healthy controls, and plasma LPA levels may be used as a biomarker or target of OC.
Keywords: Lysophosphatidic acid, Ovarian cancer, Bioactive phospholipid, Meta-analysis
Introduction
Ovarian cancer (OC) is the deadliest among gynecological cancers, with 5-year survival rates ranging between 30-50 %. OC is not a single disease but is a collection of diverse tumors with distinct morphologies and genetic deficiencies. The pathogenesis of OC is not well characterized, but most OCs occurs spontaneously, with only 5-10 % of the cases linked to a genetic predisposition. According to the latest estimates, OC is the 7th most common cancer worldwide, and the age-standardized incidence rates range from more than 11 per 100,000 women in central and eastern Europe to less than 5 per 100,000 in parts of Africa, and is the eighth most common cause of cancer death in women globally [1]. OC often has no overt symptoms at early stages and the disease is usually advanced at diagnosis, with metastatic spread, which is the main reason for the high death rates associated with OC [2]. The late diagnosis and advanced metastatic stage severely limits treatment options and severely impacts the quality of life in OC patients [3]. Although most OC patients with an advanced disease initially respond to first-line therapy, only 10-15 % will maintain a complete response. Therefore, discovery of underlying mechanisms and disease factors promoting tumor growth and metastasis is of urgent need for developing novel tools for OC diagnosis and treatment [4].
LPA is a small bioactive phospholipid present in ascetic fluid and blood of OC patients [4]. The G protein-coupled receptors of the endothelial differentiation gene (Edg) family are stimulated by LPA and LPA mediated signaling effects cell proliferation, invasion, smooth muscle cell contraction, platelet aggregation, cell migration, cell survival, wound healing and alteration in morphology and differentiation of cells [5–7]. LPA mediated pathways are prominently linked to tumor growth and metastasis in various cancers and thus significant efforts are underway to understand the precise role of LPA and design effective intervention strategies. LPA is converted from lysophospholipids in the serum and plasma, and from phosphatidic acid in platelets and cancer cells [8]. LPA production from lysophospholipids requires the action of phospholipase A1 (PLA1)/PLA2 plus lysophospholipase D (lysoPLD), while the production of LPA from phosphatidic acid requires phospholipase D (PLD) plus PLA1/PLA2 activities [8, 9]. Previous studies showed that ovarian tumor cells are a major source of LPA and autotaxin (ATX)/lysophospholipase D (PLD) pathway is the primary LPA producing pathway in ovarian tumor cells [10]. Consistent with this, plasma levels of LPA are strongly associated with presence of ovarian tumors and plasma levels of LPA are significantly higher in OC patients compared to benign ovarian lesions [4, 11]. Furthermore, previous studies showed that increased LPA levels are closely associated with the elevated expression levels of other prominent metastasis promoters critical for OC progression [12, 13]. Nevertheless, perhaps due to the complexity of the LPA pathway and the diversity of its receptors, several other studies reported results contradicting the links between LPA and ovarian cancer [14, 15]. Therefore, we performed a meta-analysis to closely examine this issue and obtain a correlation between plasma LPA level and OC.
Materials and methods
Literature search
The following computerized databases were searched electronically (last updated search in May 30st, 2014) for published studies reporting a correlation between plasma LPA levels and OC: China BioMedicine (CBM), Cochrane Library, PubMed and China National Knowledge Infrastructure (CNKI). The following keywords and search terms were used: (“lysophosphatidic acid” or “MOPA” or “LPA” or “1-oleoyl-lysophosphatidic acid” or “monooleylphosphatidate” or “1-O-oleyllysophosphatidic acid”) and (“ovarian neoplasms” or “ovary neoplasms” or “ovary cancers” or “ovarian cancer” or “cancer of ovary” or “ovarian carcinoma” or “ovarian adenocarcinoma” or “ovarian tumor” or “EOC”). The language of publication was not a restriction in our search criteria. Bibliographies of closely related studies were further examined manually to identify additional studies relevant to this meta-analysis.
Study selection
The inclusion criteria for selection of published studies for this meta-analysis were: (1) OC patients must be confirmed by pathological diagnosis; (2) study design must be case–control studies reporting the correlation between plasma LPA levels and OC; (3) the plasma LPA levels and sample size must be supplied; (4) published studies with full text. If a 50 % identity in study subjects were identified between two extracted studies, only the study with the largest sample size was enrolled. The latest and most complete study was chosen from studies published by same authors.
Data extraction
Two investigators independently extracted data from the selected studies, based on the pre-determined selection criteria, and any disagreements were resolved by discussion and reexamination. The following information was extracted: first author, publication date, country and ethnicity, study type and design, sample size, sex and age of subjects, detection method for plasma LPA levels, and plasma levels of LPA in OC patients, benign controls and healthy controls.
Quality assessment
To assess the quality of the selected studies, the two investigators used the criteria outlined in the Critical Appraisal Skill Program (CASP) (http://www.casp-uk.net/#!casp-tools-checklists/c18f8). The CASP criteria for case–control studies include Section A (CASP01 ~ CASP07), Section B (CASP08 ~ CASP09) and Section C (CASP10 ~ CASP11): clear focus in the study (CASP01); appropriate research questions and pertinent answers to the questions (CASP02); propriety in the case enrollment (CASP03); propriety in the control selection (CASP04); accuracy in the measurement of exposure factors for the least bias (CASP05); controls with other important confounding factors (CASP06); completeness of research results (CASP07); precision research results (CASP08); reliability of research results (CASP09); applicability of research results to the local population (CASP10); coherence of research results to other available evidence (CASP11).
Statistical analysis
The summary standard mean differences (SMDs) and their 95 % confidence interval (CI) were calculated and Z test was used to estimate the effect size. The SMDs for plasma LPA levels were aggregated utilizing STATA software, version 12.0 (Stata Corp, College Station, TX, USA) independently by two investigators. If heterogeneity was detected, random-effects model was employed for meta-analysis; otherwise fixed-effects model was adopted. Cochran’s Q-statistic was used to evaluate the heterogeneity across the enrolled studies, P <0.05 referring to statistical significance. I2 test was used to provide further evidence of heterogeneity, with 0 % as no heterogeneity and 100 % as maximal heterogeneity [16, 17]. If heterogeneity was detected, meta-regression and subgroup analysis based on ethnicity and detection methods were conducted to explore potential influencing factors. The influence of any single study on the overall results was verified by sensitivity analysis. To ensure the accuracy of the results, publication bias was evaluated by constructing a funnel plot, with the symmetry of the funnel plot as evidence of no publication bias and vice versa. Classic fail-safe N and Egger’s linear regression test was used for verifying the results displayed by the funnel plot [18].
Results
Included studies
Figure 1 presents the study inclusion process. A total of 381 studies were initially retrieved from database searches. From the retrieved studies, 169 studies were excluded for being duplicates (n = 2), letters, reviews or meta-analyses (n = 51), non-human studies (n = 55), or studies unrelated to the present topic (n = 61) and 190 were not for being non-case-control studies (n = 53), studies irrelevant to LPA (n = 65), or studies irrelevant to OC (n = 72), and another 3 others studies were removed because they failed to provide did not have the complete data. Finally, 19 case–control studies met the inclusion criteria [11–15, 19–32]. Fig. 2 presents the methodological quality assessment for these 19 studies. All 19 studies reported the correlation between plasma LPA levels and OC and were published between 1996 and 2013. Table 1 lists the demographic information on the OC patients and the baseline characteristics of the studies. Among the 19 studies, 16 studies were performed in Asian populations [China (n =15) and Turkey (n = 1)], and 3 were performed in Caucasians [Slovenia (n = 1) and USA (n = 2)]. Collectively, the 19 studies contained a total of 2,520 subjects, with 980 OC patients, 872 benign controls and 668 healthy controls. The plasma LPA levels in patients and controls were detected by ELISA (n = 13), bioassay (n = 4), Inorganic phosphorus (n = 1) and phosphorus determination (n = 1).
Fig. 1.
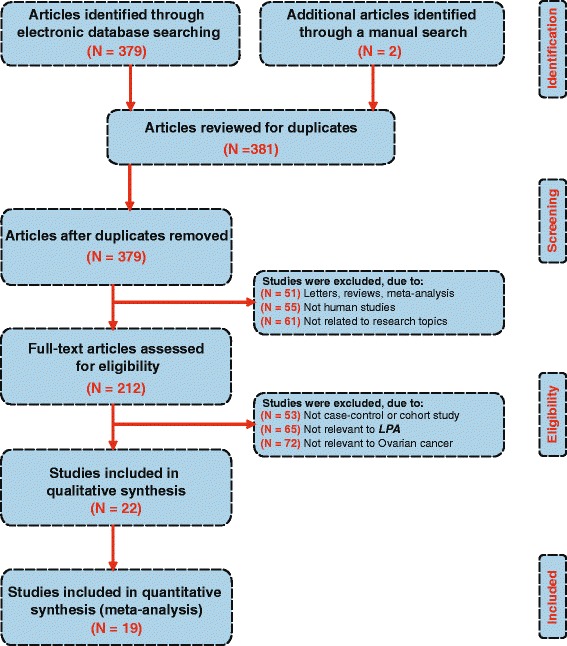
Flow chart shows study selection procedure. Nineteen studies were included in this meta-analysis
Fig. 2.
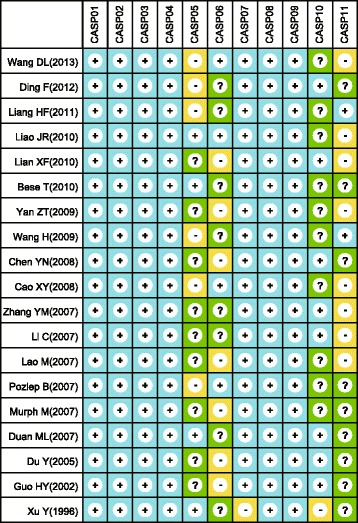
The methodological quality of eligible studies using critical appraisal skill program criteria (+: Yes; −: No; ? : Unclear)
Table 1.
Main characteristics and methodological quality of eligibly studies
| First author | Year | Country | Ethnicity | Number | Age (years) | Methods | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor | Benign | Normal | Tumor | Benign | Normal | |||||
| Wang DL [12] | 2013 | China | Asians | 80 | 40 | 30 | - | - | 34 ~ 67 | ELISA |
| Ding F [13] | 2013 | China | Asians | 36 | 26 | 20 | 46.7 | 40.5 | 44.5 | Bioassay |
| Liang HF [25] | 2011 | China | Asians | 42 | 408 | 0 | 25 ~ 75 | Bioassay | ||
| Liao JR-a [24] | 2010 | China | Asians | 8 | 30 | 30 | - | - | - | ELISA |
| Liao JR-b [24] | 2010 | China | Asians | 40 | 30 | 30 | - | - | - | ELISA |
| Lian XF [26] | 2010 | China | Asians | 31 | 40 | 50 | 22 ~ 76 | 19 ~ 71 | 20 ~ 71 | Inorganic phosphorus measurement |
| Bese T [11] | 2010 | Turkey | Asians | 87 | 74 | 50 | - | - | - | Bioassay |
| Yan ZT [21] | 2009 | China | Asians | 76 | 35 | 29 | - | - | - | ELISA |
| Wang H [23] | 2009 | China | Asians | 30 | 32 | 36 | 28 ~ 68 | ELISA | ||
| Chen YN [32] | 2008 | China | Asians | 24 | 10 | 10 | 52 | 49 | 50 | Bioassay |
| Cao XY [15] | 2008 | China | Asians | 36 | 36 | 36 | 52.8 | 52.3 | 50.4 | Phosphorus determination method |
| Zhang YM [20] | 2007 | China | Asians | 50 | 44 | 20 | 29 ~ 73 | ELISA | ||
| Li C-a [27] | 2007 | China | Asians | 60 | 0 | 60 | 27 ~ 65 | ELISA | ||
| Li C-b [27] | 2007 | China | Asians | 60 | 0 | 60 | 27 ~ 65 | ELISA | ||
| Lao M [28] | 2007 | China | Asians | 80 | 40 | 40 | 19 ~ 68 | 19 ~ 65 | 18 ~ 55 | ELISA |
| Pozlep B [19] | 2007 | Slovenia | Caucasians | 142 | 0 | 78 | 48 ± 16.07 | 42.2 | ELISA | |
| Murph M [14] | 2007 | USA | Caucasians | 26 | 27 | 25 | - | - | - | ELISA |
| Duan ML-a [30] | 2007 | China | Asians | 30 | 30 | 30 | - | - | - | ELISA |
| Duan ML-a [30] | 2007 | China | Asians | 30 | 30 | 30 | - | - | - | ELISA |
| Du Y [31] | 2005 | China | Asians | 37 | 0 | 30 | 53.5 ± 7.65 | 51.6 ± 6.57 | ELISA | |
| Guo HY-a [29] | 2002 | China | Asians | 16 | 0 | 46 | 56.5 ± 8.64 | - | - | ELISA |
| Guo HY-b [29] | 2002 | China | Asians | 15 | 0 | 46 | 56.2 ± 10.9 | - | - | ELISA |
| Xu Y [22] | 1996 | USA | Caucasians | 34 | 0 | 48 | - | - | - | ELISA |
ELISA, enzyme linked immunosorbent assay
Meta-analysis of findings
As shown in Fig. 3, the pooled SMDs revealed that the plasma levels of LPA in OC patients were significantly higher than the benign controls (SMD = 2.36, 95 % CI: 1.61–3.10, P <0.001) and healthy controls (SMD = 2.32, 95 % CI: 1.77–2.87, P <0.001). The results of subgroup analysis based on ethnicity and detection methods are as follows: plasma LPA levels in OC patients are significantly higher compared to benign controls only among the Asian subjects (SMD = 2.52, 95 % CI: 1.79–3.25, P <0.001), as quantified by both ELISA (SMD = 2.42, 95 % CI: 1.62–3.23, P <0.001) and non-ELISA methods (SMD = 2.15, 95 % CI: 0.56–3.75, P = 0.008). Such a significant difference in plasma LPA levels between OC patients and benign controls was not found in the Caucasian population studied (P >0.05) (as shown in Fig. 4a-b). However, significantly higher plasma LPA levels were found in OC patients, compared to healthy controls, in both Asian (SMD = 2.53, 95 % CI: 1.91–3.16, P <0.001) and Caucasian populations (SMD = 1.09, 95 % CI: 0.20–1.98, P = 0.017) and in the ELISA subgroup (SMD = 2.47, 95 % CI: 1.95–2.98, P <0.001), but not in the non-ELISA subgroup (P >0.05) (Fig. 4c-d).
Fig. 3.
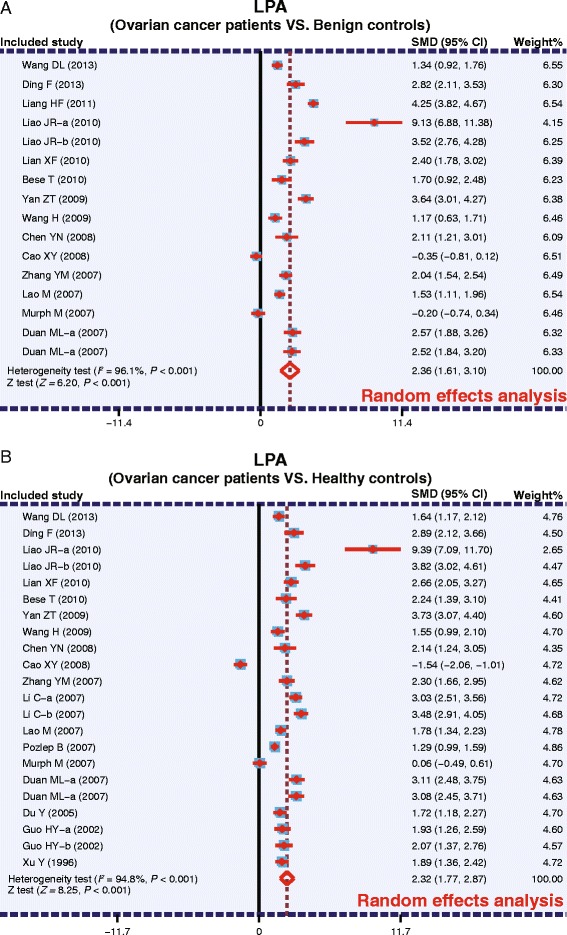
Forest plots for the relationship between plasma lysophosphatidic acid levels and ovarian cancer (a: Ovarian cancer patients VS. Benign controls; b: Ovarian cancer patients VS. Healthy controls)
Fig. 4.
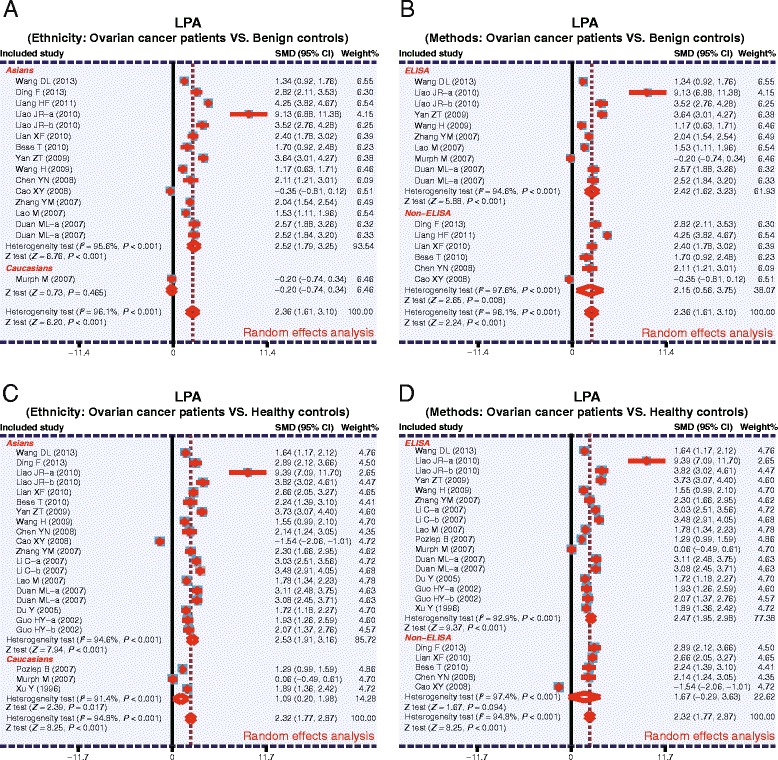
Subgroup analysis for the relationships between plasma lysophosphatidic acid levels and ovarian cancer (a: Ethnicity: Ovarian cancer patients VS. Benign controls; b: Methods: Ovarian cancer patients VS. Benign controls; c: Ethnicity: Ovarian cancer patients VS. Healthy controls; d: Methods: Ovarian cancer patients VS. Healthy controls)
Sensitivity analysis suggested that no single study had an impact on the overall statistical significance. For comparison between OC patients and benign controls, the constructed funnel plot was symmetrical, suggesting no publication bias, while it was asymmetrical for the comparison between OC patients and healthy controls (Fig. 5).
Fig. 5.

Funnel plot of publication biases on the relationship between plasma lysophosphatidic acid levels and ovarian cancer (a: Ovarian cancer patients VS. Benign controls; b: Ovarian cancer patients VS. Healthy controls)
Discussion
Based on the analysis presented in this study and the data from previous studies, LPA and OC appear to be strongly linked. For example, Hu et al. showed that LPA stimulates ovarian cancer cells by a dual mechanism: (1) LPA indirectly promotes tumor growth by acting as a chemoattractant to endothelial cells, resulting in increased angiogenesis and (2) directly increases the cell proliferation through cyclin D1 activation [33]. Autotaxin (ATX) is the predominant enzyme that produces LPA and ATX transgene expression in mouse mammary glands is sufficient to induce tumors. Conversely, ATX or LPA receptor knockdowns prevent bone metastasis and decrease tumor incidence and progression, respectively, in mouse models of chemically induced carcinogenesis. As such, ATX-LPA signaling in cancer is one of the prominent pathways for tumorigenesis and is a prime target for current therapeutic drug development [9]. Interestingly, contradictory results were obtained by several studies which could not find a clear correlation between LPA and OC. In order to examine this issue closer, we pooled the data from several high-quality published studies and performed a meta-analysis to obtain a better correlation between LPA and OC.
Our meta-analysis clearly showed that the plasma LPA levels in OC patients are significantly higher compared to the benign and healthy controls. Angiogenesis is a key factor for tumor growth and involves VEGF and the activation of VEGF receptors, Flt1 and KDR [19]. OC patients exhibit elevated serum levels of both VEGF and LPA [34]. ATX is an autocrine motility factor and a member of the ectonucleotide pyrophosphatase and phosphodiesterase family of enzymes, but also possesses lysophospholipase D activity. This enzymatic activity hydrolyzes lysophosphatidylcholine to generate the potent tumor growth factor and mitogen, LPA [8, 35]. ATX is highly overexpressed in OC, thus, tumor cells are the major source of LPA production in OC patients [8]. Notably, VEGF signaling was shown to further elevate ATX expression and, thus, OC patients have increased PLD activity and higher LPA plasma levels, compared to healthy controls.
A subgroup analysis was performed to identify factors influencing our study results. The potential factors tested were ethnicity, country and experimental method. Based on the limitations of the data available to us from the selected studies, we focused on ethnicity and detection methods for subgroup analysis. Our subgroup analysis based on ethnicity showed that OC patients from Asian populations exhibited higher plasma LPA levels compared to their benign and healthy counterparts, and the difference was statistically significant. Caucasian OC patients showed significantly higher plasma LPA levels compared to the healthy controls but not the benign counterparts. However, these results may be influenced by the fact that majority of the studies were performed in Asian populations (16 in Asians and 3 in Caucasians). Based on the detection methods, higher plasma LPA levels were observed in OC patients, compared to both benign and healthy controls, using ELISA based methods. The results from the analysis of non-ELISA based methods were largely in agreement with ELISA based results, but a significant correlation between LPA and OC was not observed between the OC patients and healthy controls using non-ELISA methods. This negative result may be due to the small sample size of non-ELISA methods. Collectively, our data provide strong evidence that LPA may be involved in OC development or is produced by the tumor and thus may be a tumor marker or target of treatment.
Our study has several significant limitations. Firstly, the dataset used in this study was limited by nineteen prospective trials, which might not be ideal for statistical analysis. Secondly, due to the heterogeneity of the disease, a uniform histopathological diagnosis would have ensured consistent diagnostic standards, which was not the case in the selected studies. Thirdly, some of the studies did not provide complete data and therefore plasma LPA levels at different stages of OC progression could not be compared. Fourthly, sample sizes of OC cases, controls and healthy controls varied widely. Therefore, for a more accurate statistical analysis, a comprehensive dataset and proportionate sample size may be needed. Future studies also must attempt to compare plasma LPA levels at different stages of OC progression to obtain more thorough disease correlations.
Conclusion
In summary, a significantly higher plasma LPA levels were observed in OC patients compared to benign controls and healthy controls. Based on our results, we conclude that plasma LPA level is closely correlated to OC and may involve in the development of OC. However, further studies are needed to confirm our findings and explore therapeutic targets within the identified pathway.
Acknowledgments
This study was funded by the Postdoctoral Science Foundation of China (nos. 2014T70298, 2013M541316, 2013M540256),the training program of Jilin universiy oustanding young teacher project (no. 41908050037). We owe sincere thanks to the excellent reviewers for the great suggestion for my paper.
Abbreviations
- OC
Ovarian carcinoma
- LPA
Lysophosphatidic acid
- Edg
Endothelial differentiation gene
- NOS
Newcastle-Ottawa scale
- SMDs
Standard mean differences
- CI
Confidence interval
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CZ carried out the experiments, HZ prepared the tables and figures, JZ performed the statistical analysis, YL and WZ wrote the manuscript. HY and LF participated in the interpretation of the data and writing the manuscript. The manuscript received final confirmation from all the authors. All authors read and approved the final manuscript.
Contributor Information
Yi-Yang Li, Email: liyiyang85@126.com.
Wen-Chao Zhang, Email: zhangwenchao0830@126.com.
Jia-Ling Zhang, Email: zhangjialingzjl85@126.com.
Chang-Jun Zheng, Email: zhengchangjun85@126.com.
He Zhu, Email: zhuhezh85@126.com.
Hui-Mei Yu, Email: yuhuimei0413@163.com.
Li-Mei Fan, Phone: +86-0431-88796624, Email: fanlimeiflm85@126.com.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi E, Ueda Y, Matsuzaki S, Yokoyama T, Kimura T, Yoshino K, et al. Biomarkers for screening, diagnosis, and monitoring of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:1902–12. doi: 10.1158/1055-9965.EPI-12-0646. [DOI] [PubMed] [Google Scholar]
- 3.Lowe KA, Chia VM, Taylor A, O’Malley C, Kelsh M, Mohamed M, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol. 2013;130:107–14. doi: 10.1016/j.ygyno.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Sedlakova I, Vavrova J, Tosner J, Hanousek L. Lysophosphatidic acid (LPA)-a perspective marker in ovarian cancer. Tumour Biol. 2011;32:311–6. doi: 10.1007/s13277-010-0123-8. [DOI] [PubMed] [Google Scholar]
- 5.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–64. doi: 10.1016/S1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 6.Smicun Y, Reierstad S, Wang FQ, Lee C, Fishman DA. S1P regulation of ovarian carcinoma invasiveness. Gynecol Oncol. 2006;103:952–9. doi: 10.1016/j.ygyno.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Wang FQ, Ariztia EV, Boyd LR, Horton FR, Smicun Y, Hetherington JA, et al. Lysophosphatidic acid (LPA) effects on endometrial carcinoma in vitro proliferation, invasion, and matrix metalloproteinase activity. Gynecol Oncol. 2010;117:88–95. doi: 10.1016/j.ygyno.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477–89. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 1781;2008:513–8. doi: 10.1016/j.bbalip.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Luquain C, Singh A, Wang L, Natarajan V, Morris AJ. Role of phospholipase D in agonist-stimulated lysophosphatidic acid synthesis by ovarian cancer cells. J Lipid Res. 2003;44:1963–75. doi: 10.1194/jlr.M300188-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Bese T, Barbaros M, Baykara E, Guralp O, Cengiz S, Demirkiran F, et al. Comparison of total plasma lysophosphatidic acid and serum CA-125 as a tumor marker in the diagnosis and follow-up of patients with epithelial ovarian cancer. J Gynecol Oncol. 2010;21:248–54. doi: 10.3802/jgo.2010.21.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang DL, Chen LX, Liao XL, Liao XJ, Wu QH. The relationship of lysophosphatidic acid and MMP-2 in diagnosing epithelial ovarian carcinoma. Proc Clin Med. 2013;22:403–5. [Google Scholar]
- 13.Ding F, Niu B. Clinical significance of detecting lysophosphatidic acid and vascular endothelial growth factor in patients with epithelial ovarian carcinoma. Practical J Med Pharmacy. 2013;30:775–7. [Google Scholar]
- 14.Murph M, Tanaka T, Pang J, Felix E, Liu S, Trost R, et al. Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: potential biomarkers for cancer diagnosis. Methods Enzymol. 2007;433:1–25. doi: 10.1016/S0076-6879(07)33001-2. [DOI] [PubMed] [Google Scholar]
- 15.Cao XY. The applicable value of combined detection of LPA, CA1 25 and AFP in the early diagnosis of ovarian cancer. Laboratory Med Clinic. 2008;5:1430–1. [Google Scholar]
- 16.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31:3805–20. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 18.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–3. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 19.Pozlep B, Meleh M, Kobal B, Verdenik I, Osredkar J, Kralj LZ, et al. Use of lysophosphatidic acid in the management of benign and malignant ovarian tumors. Eur J Gynaecol Oncol. 2007;28:394–9. [PubMed] [Google Scholar]
- 20.Zhang YM, Zhang XN. Expression of XIAP, Survivin, LPA and CA12 5 in human epithelial ovarian carcinoma. Journal of Shandong University (Health Science) 2007;45:317–21. [Google Scholar]
- 21.Yan ZT, Huang N, Zhao ML, Pang AP. Clinical Values of serum CA125 and LPA determination in early diagnosis of ovarian cancer. Chinese Journal of Primary Medicine and Pharmacy. 2009;16:1993–4. [Google Scholar]
- 22.Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–23. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Chen DZ. Study on the diagnostic Value of Plasm a Lysopho sphatidic Acid (LPA) Level Determination in Patients with Ovarian Carcinoma. Journal of Radioimmunology. 2008;21:355–7. [Google Scholar]
- 24.Liao JR, Zong JH, Lv YY. Detection of Plasma Iysophosphatidic Acid (LPA) in Epithelial Ovarian Tumor Patients. Inner Mongolia Medical Journal. 2010;42:1295–6. [Google Scholar]
- 25.Liang HF, Zhang H, Ma XY. Comparative analysis of the value of lysophosphatidic acid and CA125 in the diagnosis of ovarian cancer. Clinical Medicine of China. 2011;27:348–50. [Google Scholar]
- 26.Lian XF, Li XL, Zhan HL, Sun F. The diagnostic value when lysophospholipids acid, epididymis protein 4 used in early ovarian cancer’ s detection. International Medicine and Health Guidance News. 2010;16:2778–81. [Google Scholar]
- 27.Li C, Fang SH, Chen W. The diagnostic value of sol uble P185 in ovarian cancer. Chinese Journal of Laboratory Diagnosis. 2007;11:1238–9. [Google Scholar]
- 28.Lao M, Pan ZM, Huang WC, Huang LS, Zhu B, Zhao HL, et al. The value of plasma lysophosphatidic acid measurement in the diagnosis of gynecology tumor. The Practical Journal of Cancer. 2007;22:347–9. [Google Scholar]
- 29.Guo HY, Han JS, Wu QZ, Yang CS. A study of diagnostic value of plasma lysophosphatidic acid for ovarian epithelial cancer. Chinese Journal of Clinical Obstetrics and Gynecology. 2002;03:36–8. [Google Scholar]
- 30.Duan ML, Wu F. The diagnostic value of secretory phospholipase A2 and lysophosphatic acid for ovarian cancer. Chinese Journal of Laboratory Medicine. 2005;28:622–4. [Google Scholar]
- 31.Du Y. Study on correlation between diagnosis of epithlial ovarian cancer and plasma lysophosphatidic acid. Zhejiang Medical Journal. 2005;27:827–8. [Google Scholar]
- 32.Chen YN, Tao M, Tu FP, Zuo Y, Lu WD. LPA, IL-6 and IL-8 levels in the plasma of patients with epithelial ovarian carcinoma. The Practical Journal of Cancer. 2008;23:580–2. [Google Scholar]
- 33.Hu YL, Albanese C, Pestell RG, Jaffe RB. Dual mechanisms for lysophosphatidic acid stimulation of human ovarian carcinoma cells. J Natl Cancer Inst. 2003;95:733–40. doi: 10.1093/jnci/95.10.733. [DOI] [PubMed] [Google Scholar]
- 34.Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93:762–8. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K, Igarashi K, Ohkawa R, Yokota H, Masuda A, Nakagawa S, et al. Serum autotaxin is not a useful biomarker for ovarian cancer. Lipids. 2012;47:927–30. doi: 10.1007/s11745-012-3691-0. [DOI] [PubMed] [Google Scholar]


