Abstract
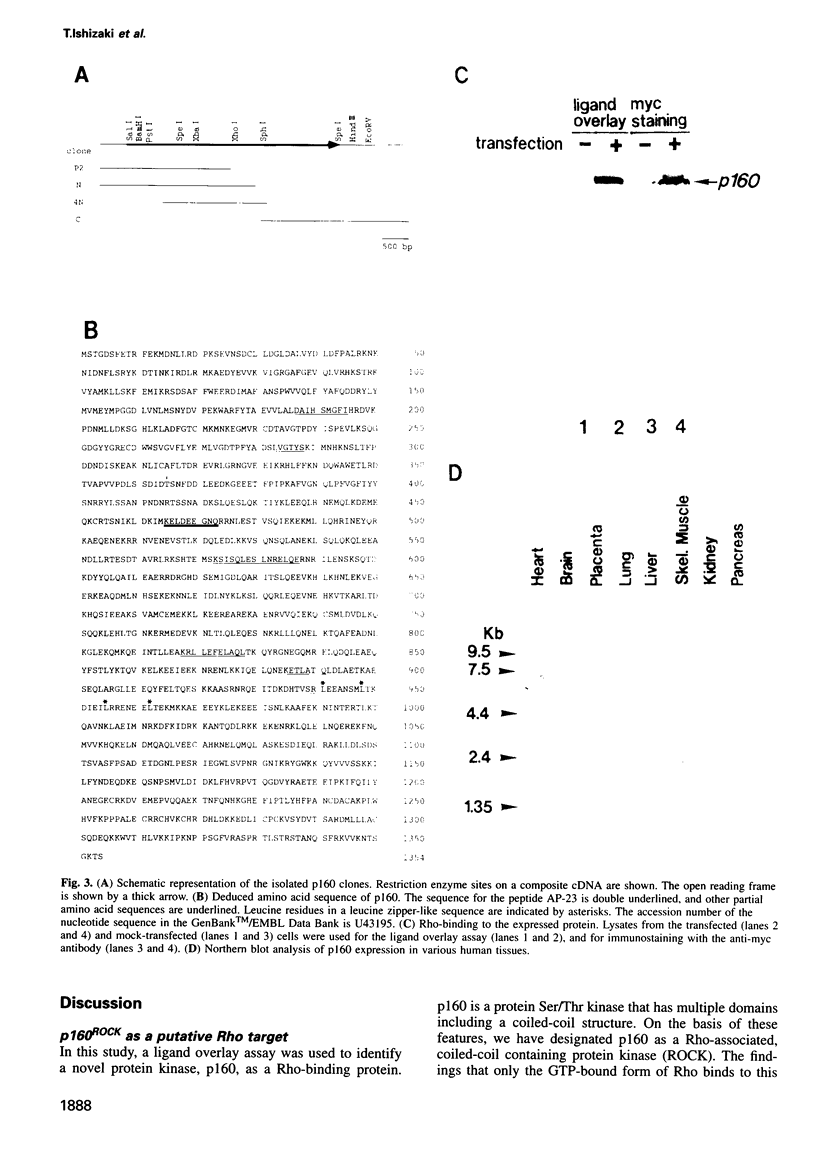
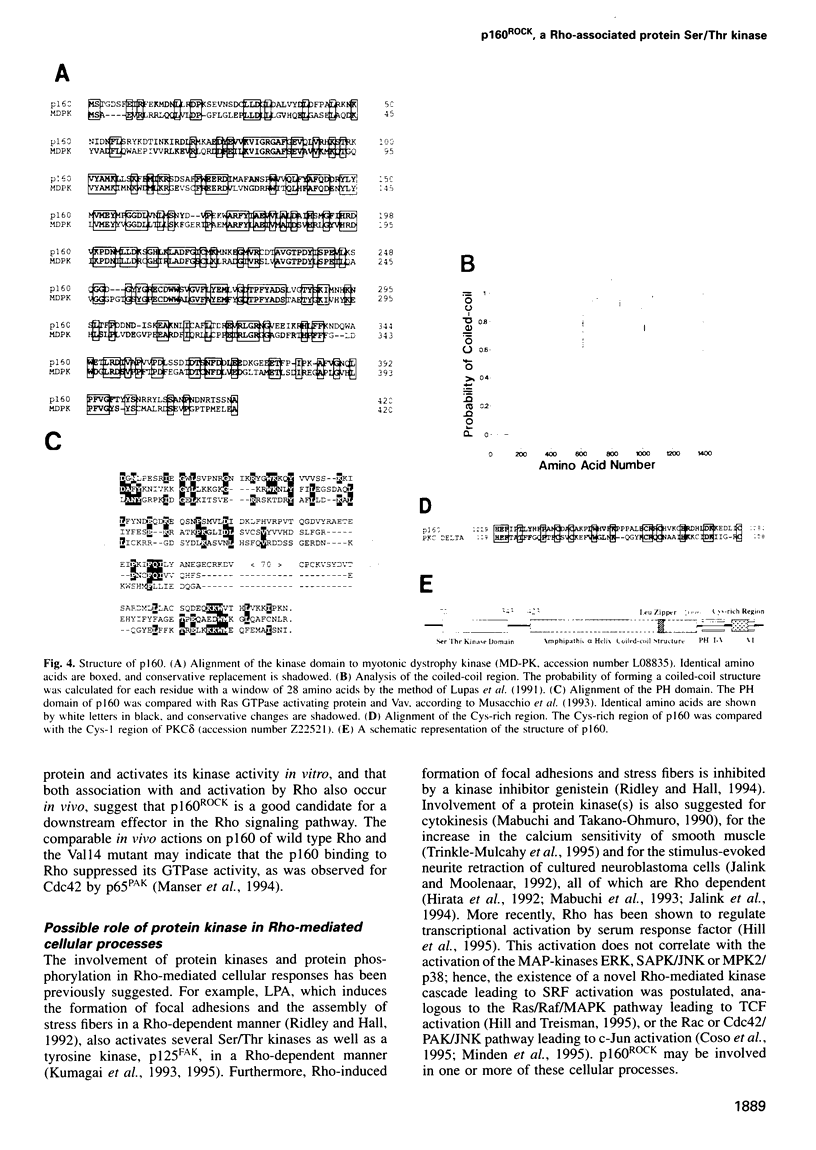
The small GTP-binding protein Rho functions as a molecular switch in the formation of focal adhesions and stress fibers, cytokinesis and transcriptional activation. The biochemical mechanism underlying these actions remains unknown. Using a ligand overlay assay, we purified a 160 kDa platelet protein that bound specifically to GTP-bound Rho. This protein, p160, underwent autophosphorylation at its serine and threonine residues and showed the kinase activity to exogenous substrates. Both activities were enhanced by the addition of GTP-bound Rho. A cDNA encoding p160 coded for a 1354 amino acid protein. This protein has a Ser/Thr kinase domain in its N-terminus, followed by a coiled-coil structure approximately 600 amino acids long, and a cysteine-rich zinc finger-like motif and a pleckstrin homology region in the C-terminus. The N-terminus region including a kinase domain and a part of coiled-coil structure showed strong homology to myotonic dystrophy kinase over 500 residues. When co-expressed with RhoA in COS cells, p160 was co-precipitated with the expressed Rho and its kinase activity was activated, indicating that p160 can associate physically and functionally with Rho both in vitro and in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Chong L. D., Traynor-Kaplan A., Bokoch G. M., Schwartz M. A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994 Nov 4;79(3):507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Coso O. A., Chiariello M., Yu J. C., Teramoto H., Crespo P., Xu N., Miki T., Gutkind J. S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995 Jun 30;81(7):1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Diekmann D., Abo A., Johnston C., Segal A. W., Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science. 1994 Jul 22;265(5171):531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- Dyck J. A., Maul G. G., Miller W. H., Jr, Chen J. D., Kakizuka A., Evans R. M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994 Jan 28;76(2):333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Harlan J. E., Hajduk P. J., Yoon H. S., Fesik S. W. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994 Sep 8;371(6493):168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995 Jan 27;80(2):199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Wynne J., Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995 Jun 30;81(7):1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hirata K., Kikuchi A., Sasaki T., Kuroda S., Kaibuchi K., Matsuura Y., Seki H., Saida K., Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992 May 5;267(13):8719–8722. [PubMed] [Google Scholar]
- Hirata M., Hayashi Y., Ushikubi F., Yokota Y., Kageyama R., Nakanishi S., Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991 Feb 14;349(6310):617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Iwamatsu A. S-carboxymethylation of proteins transferred onto polyvinylidene difluoride membranes followed by in situ protease digestion and amino acid microsequencing. Electrophoresis. 1992 Mar;13(3):142–147. doi: 10.1002/elps.1150130129. [DOI] [PubMed] [Google Scholar]
- Jalink K., Moolenaar W. H. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992 Jul;118(2):411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Hengeveld T., Morii N., Narumiya S., Moolenaar W. H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994 Aug;126(3):801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R., Solski P. A., Clark G. J., Kinch M. S., Der C. J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995 Nov;15(11):6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi K., Sasaki T., Kuroda S., Itoh T., Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J Cell Biol. 1993 Mar;120(5):1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R., Ahmed S., Best A., Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995 Apr;15(4):1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai N., Morii N., Fujisawa K., Nemoto Y., Narumiya S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J Biol Chem. 1993 Nov 25;268(33):24535–24538. [PubMed] [Google Scholar]
- Kumagai N., Morii N., Ishizaki T., Watanabe N., Fujisawa K., Saito Y., Narumiya S. Lysophosphatidic acid-induced activation of protein Ser/Thr kinases in cultured rat 3Y1 fibroblasts. Possible involvement in rho p21-mediated signalling. FEBS Lett. 1995 Jun 5;366(1):11–16. doi: 10.1016/0014-5793(95)00478-r. [DOI] [PubMed] [Google Scholar]
- Leevers S. J., Paterson H. F., Marshall C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994 Jun 2;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Hamaguchi Y., Fujimoto H., Morii N., Mishima M., Narumiya S. A rho-like protein is involved in the organisation of the contractile ring in dividing sand dollar eggs. Zygote. 1993 Nov;1(4):325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- Madaule P., Furuyashiki T., Reid T., Ishizaki T., Watanabe G., Morii N., Narumiya S. A novel partner for the GTP-bound forms of rho and rac. FEBS Lett. 1995 Dec 18;377(2):243–248. doi: 10.1016/0014-5793(95)01351-2. [DOI] [PubMed] [Google Scholar]
- Maekawa M., Li S., Iwamatsu A., Morishita T., Yokota K., Imai Y., Kohsaka S., Nakamura S., Hattori S. A novel mammalian Ras GTPase-activating protein which has phospholipid-binding and Btk homology regions. Mol Cell Biol. 1994 Oct;14(10):6879–6885. doi: 10.1128/mcb.14.10.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan M. S., Amemiya C., Jansen G., Sabourin L., Baird S., Neville C. E., Wormskamp N., Segers B., Batzer M., Lamerdin J. Structure and genomic sequence of the myotonic dystrophy (DM kinase) gene. Hum Mol Genet. 1993 Mar;2(3):299–304. doi: 10.1093/hmg/2.3.299. [DOI] [PubMed] [Google Scholar]
- Malcolm K. C., Ross A. H., Qiu R. G., Symons M., Exton J. H. Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J Biol Chem. 1994 Oct 21;269(42):25951–25954. [PubMed] [Google Scholar]
- Manser E., Leung T., Monfries C., Teo M., Hall C., Lim L. Diversity and versatility of GTPase activating proteins for the p21rho subfamily of ras G proteins detected by a novel overlay assay. J Biol Chem. 1992 Aug 15;267(23):16025–16028. [PubMed] [Google Scholar]
- Manser E., Leung T., Salihuddin H., Tan L., Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature. 1993 May 27;363(6427):364–367. doi: 10.1038/363364a0. [DOI] [PubMed] [Google Scholar]
- Marais R., Light Y., Paterson H. F., Marshall C. J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995 Jul 3;14(13):3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. A., Bollag G., McCormick F., Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995 May 1;14(9):1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A., Lin A., Claret F. X., Abo A., Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995 Jun 30;81(7):1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Morii N., Kumagai N., Nur-E-Kamal M. S., Narumiya S., Maruta H. rho GAP of 28 kDa (GAP2), but not of 190 kDa (p190), requires Asp65 and Asp67 of rho GTPase for its activation. J Biol Chem. 1993 Dec 25;268(36):27160–27163. [PubMed] [Google Scholar]
- Morii N., Sekine A., Ohashi Y., Nakao K., Imura H., Fujiwara M., Narumiya S. Purification and properties of the cytosolic substrate for botulinum ADP-ribosyltransferase. Identification as an Mr 22,000 guanine nucleotide-binding protein. J Biol Chem. 1988 Sep 5;263(25):12420–12426. [PubMed] [Google Scholar]
- Morii N., Teru-uchi T., Tominaga T., Kumagai N., Kozaki S., Ushikubi F., Narumiya S. A rho gene product in human blood platelets. II. Effects of the ADP-ribosylation by botulinum C3 ADP-ribosyltransferase on platelet aggregation. J Biol Chem. 1992 Oct 15;267(29):20921–20926. [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Rice P., Thompson J., Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993 Sep;18(9):343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Quest A. F., Bardes E. S., Bell R. M. A phorbol ester binding domain of protein kinase C gamma. Deletion analysis of the Cys2 domain defines a minimal 43-amino acid peptide. J Biol Chem. 1994 Jan 28;269(4):2961–2970. [PubMed] [Google Scholar]
- Ridley A. J., Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994 Jun 1;13(11):2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P., Warne P. H., Dhand R., Vanhaesebroeck B., Gout I., Fry M. J., Waterfield M. D., Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994 Aug 18;370(6490):527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Seckl M. J., Morii N., Narumiya S., Rozengurt E. Guanosine 5'-3-O-(thio)triphosphate stimulates tyrosine phosphorylation of p125FAK and paxillin in permeabilized Swiss 3T3 cells. Role of p21rho. J Biol Chem. 1995 Mar 24;270(12):6984–6990. doi: 10.1074/jbc.270.12.6984. [DOI] [PubMed] [Google Scholar]
- Self A. J., Paterson H. F., Hall A. Different structural organization of Ras and Rho effector domains. Oncogene. 1993 Mar;8(3):655–661. [PubMed] [Google Scholar]
- Siddiqi A. R., Smith J. L., Ross A. H., Qiu R. G., Symons M., Exton J. H. Regulation of phospholipase D in HL60 cells. Evidence for a cytosolic phospholipase D. J Biol Chem. 1995 Apr 14;270(15):8466–8473. doi: 10.1074/jbc.270.15.8466. [DOI] [PubMed] [Google Scholar]
- Singer W. D., Brown H. A., Bokoch G. M., Sternweis P. C. Resolved phospholipase D activity is modulated by cytosolic factors other than Arf. J Biol Chem. 1995 Jun 23;270(25):14944–14950. doi: 10.1074/jbc.270.25.14944. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994 Jun 3;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Tolias K. F., Cantley L. C., Carpenter C. L. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995 Jul 28;270(30):17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Sugie K., Hirata M., Morii N., Fukata J., Uchida A., Imura H., Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993 Mar;120(6):1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L., Ichikawa K., Hartshorne D. J., Siegman M. J., Butler T. M. Thiophosphorylation of the 130-kDa subunit is associated with a decreased activity of myosin light chain phosphatase in alpha-toxin-permeabilized smooth muscle. J Biol Chem. 1995 Aug 4;270(31):18191–18194. doi: 10.1074/jbc.270.31.18191. [DOI] [PubMed] [Google Scholar]
- Watanabe G., Saito Y., Madaule P., Ishizaki T., Fujisawa K., Morii N., Mukai H., Ono Y., Kakizuka A., Narumiya S. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science. 1996 Feb 2;271(5249):645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- Yamamori B., Kuroda S., Shimizu K., Fukui K., Ohtsuka T., Takai Y. Purification of a Ras-dependent mitogen-activated protein kinase kinase kinase from bovine brain cytosol and its identification as a complex of B-Raf and 14-3-3 proteins. J Biol Chem. 1995 May 19;270(20):11723–11726. doi: 10.1074/jbc.270.20.11723. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Marui N., Sakai T., Morii N., Kozaki S., Ikai K., Imamura S., Narumiya S. ADP-ribosylation of the rhoA gene product by botulinum C3 exoenzyme causes Swiss 3T3 cells to accumulate in the G1 phase of the cell cycle. Oncogene. 1993 Jun;8(6):1449–1455. [PubMed] [Google Scholar]
- Zhang G., Kazanietz M. G., Blumberg P. M., Hurley J. H. Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell. 1995 Jun 16;81(6):917–924. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Zhang J., King W. G., Dillon S., Hall A., Feig L., Rittenhouse S. E. Activation of platelet phosphatidylinositide 3-kinase requires the small GTP-binding protein Rho. J Biol Chem. 1993 Oct 25;268(30):22251–22254. [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Bagrodia S., Cerione R. A. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994 Jul 22;269(29):18727–18730. [PubMed] [Google Scholar]
- van der Ven P. F., Jansen G., van Kuppevelt T. H., Perryman M. B., Lupa M., Dunne P. W., ter Laak H. J., Jap P. H., Veerkamp J. H., Epstein H. F. Myotonic dystrophy kinase is a component of neuromuscular junctions. Hum Mol Genet. 1993 Nov;2(11):1889–1894. doi: 10.1093/hmg/2.11.1889. [DOI] [PubMed] [Google Scholar]