Abstract
Inhibitors against the p110δ isoform of PI3K have shown remarkable therapeutic efficacy in some human leukaemias1,2. Since p110δ is primarily expressed in leukocytes3, drugs against p110δ have not been considered for the treatment of solid tumours4. We report here that p110δ inactivation in mice protects against a broad range of cancers, including non-haematological solid tumours. We demonstrate that p110δ inactivation in regulatory T cells (Treg) unleashes CD8+ cytotoxic T cells and induces tumour regression. Thus, p110δ inhibitors can break tumour-induced immune tolerance and should be considered for wider use in oncology.
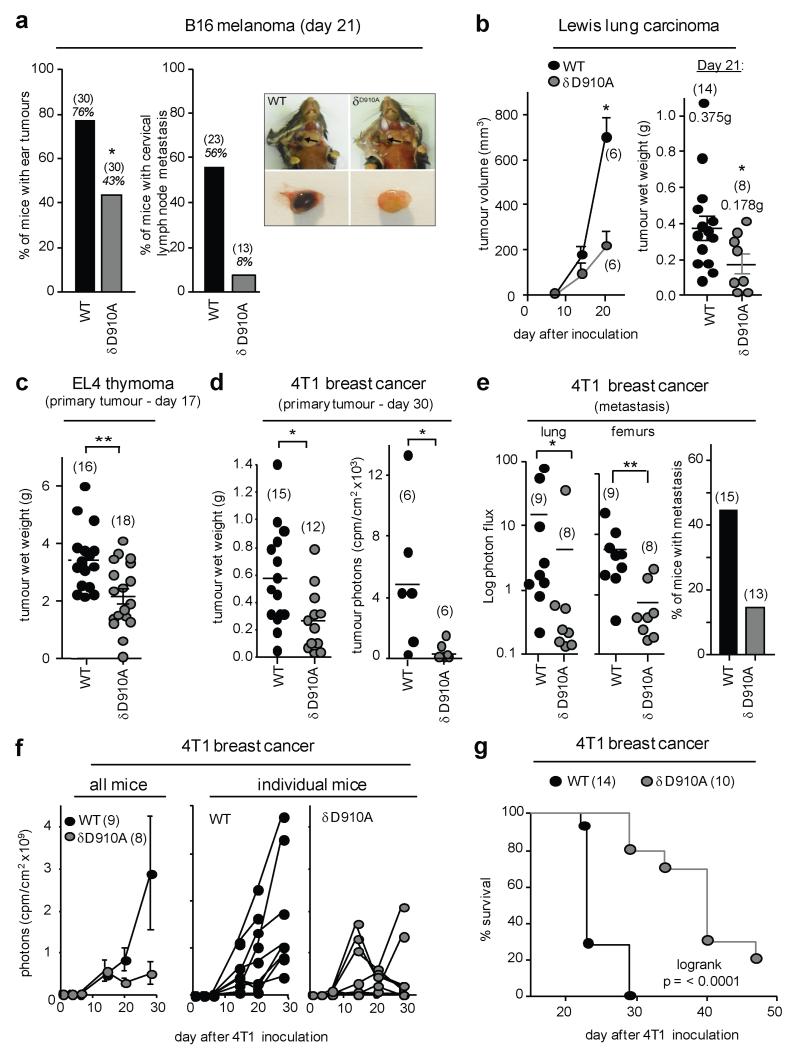
PI3K p110δD910A (δD910A) mice in which endogenous p110δ kinase is inactive, present specific immune deficiencies5,6 but are not predisposed to cancer. To test if host p110δ activity affects tumour growth, we inoculated weakly immunogenic syngeneic cancer cell lines into δD910A mice. Compared to wild-type (WT) mice, δD910A mice were more resistant to B16 melanoma, with reduced tumour incidence and almost abrogated lymph node metastasis in those mice that developed tumours (Fig. 1a). Growth of Lewis lung carcinoma (LLC) and EL4 thymoma cells was also suppressed in δD910A mice (Fig. 1b,c). Similar observations were made with luciferase-labelled 4T1 breast cancer cells injected into the mammary fat pad. At sacrifice, δD910A mice showed reduced mass and luciferase activity of the primary 4T1 tumour (Fig. 1d) and lower metastasis (Fig. 1e). In WT mice, 4T1 tumours were detected by day 10 and grew progressively until day 30, at which point the mice became moribund (Fig. 1f). In some δD910A mice, 4T1 tumours grew initially, but then started to regress from day 15-20 onwards (Fig. 1f). Across 10 independent experiments, 97% (71/73) of WT mice had an observable cancer mass at the end of study, compared to 65% (43/66) of δD910A mice, with a median survival time of 23 and 40 days in WT and δD910A mice, respectively (Fig. 1g).
Figure 1. Impact of genetic inactivation of p110δ on tumour growth and metastasis.
a, percentage of mice with visible B16 ear tumours (left) or lymph nodes metastasis (right). Photographs show B16 metastases in cervical lymph nodes and representative excised lymph nodes. b-d, primary tumour burden of the indicated tumour lines. e, 4T1 metastasis as detected by luciferase activity (left and middle panel) or histology (right panel), expressed as a percentage of the total number of tumour-bearing animals/group. f, Growth of primary 4T1 tumours. g, Survival of 4T1 tumour-bearing mice. a-f, Statistically significant differences are indicated by * (P < 0.05) or ** (P < 0.01), as determined by the non-parametric Mann-Whitney t test. Between brackets: number of mice used per experiment. Each dot represents an individual mouse.
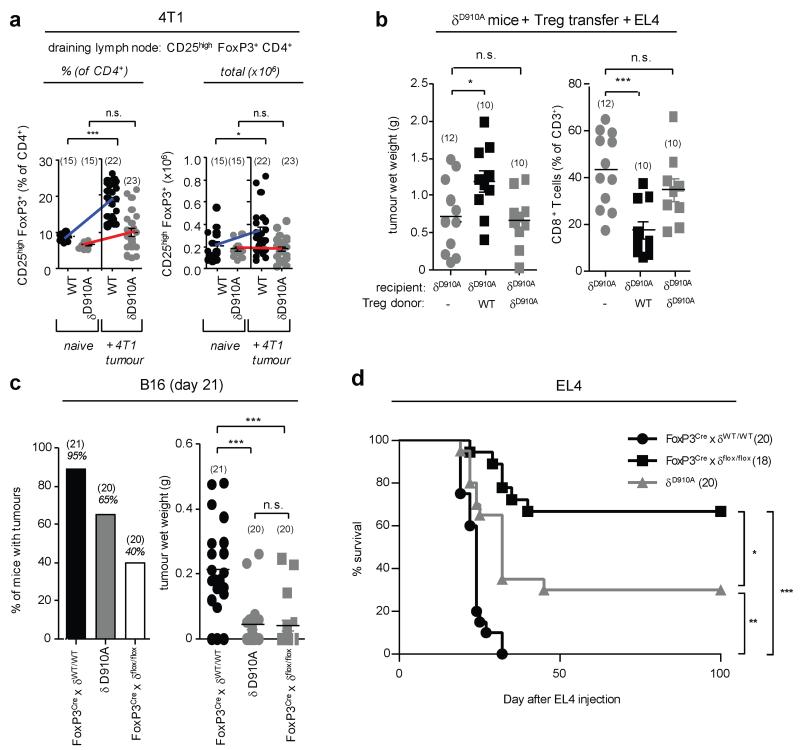
Effective tumour immunity is limited by Treg-mediated immune suppression7. δD910A mice show enhanced FoxP3+CD4+ Treg in the thymus but impaired subsequent Treg maintenance and functionality in the periphery8. δD910A Treg also produce less IL-10 and express lower levels of CD38, but show normal expression of most ‘Treg-signature’ genes, including FoxP3, CD25, CTLA4 and ICOS8,9. We therefore considered that reduced Treg function in δD910A mice might lead to enhanced tumour resistance. FoxP3+CD4+ Treg in the draining lymph nodes of 4T1 tumour-bearing δD910A mice did not expand as robustly as in WT mice (Fig. 2a), however no consistent differences in Treg expansion were observed in the B16 or EL4 tumour models between naive and tumour-bearing mice of either genotype (not shown). To assess Treg function, we carried out adoptive Treg transfer experiments in EL4 tumour-bearing mice. Transfer of WT Treg into δD910A mice restored EL4 tumour growth and suppressed the relative abundance of tumour-infiltrating CD8+ T cells (Fig. 2b). By contrast, the transfer of the same number of δD910A Treg into δD910A mice did not affect EL4 tumour growth (Fig. 2b), indicating a functional defect in δD910A Treg. FoxP3YFP-Crexδflox/flox mice in which p110δ was selectively deleted in Treg (by a Cre transgene expressed from the Foxp3 locus) did not display spontaneous autoimmune or inflammatory responses (not shown) but showed reduced growth of B16 cells (Fig. 2c) and extended survival time upon inoculation of EL4 cells, to an even greater extent than in δD910A mice (Fig. 2d). These data demonstrate that p110δ inactivation in Treg is both necessary and sufficient to confer tumour resistance. However, these data also revealed a potential negative impact of p110δ inhibition on effector T cells, since FoxP3YFP-Crexδflox/flox mice were more cancer-resistant than δD910A mice (Fig. 2d). We therefore investigated the effect of p110δ inactivation on CD4 and CD8 effector T cells in the context of an ongoing tumour response.
Figure 2. Inactivation of p110δ in Treg is sufficient to confer cancer resistance.
a, Relative and total numbers of Treg in the draining lymph nodes of naive and 4T1 tumour-bearing mice. b, Impact of adoptive transfer of Treg into δD910A mice on EL4 tumour wet weight and tumour-infiltrating CD8+ T cells. c, Number of mice with visible B16 tumours and B16 tumour weight in mice of the indicated genotype. d, Survival of EL4 tumour-bearing mice of the indicated genotype. a-c, Statistically significant differences are indicated by * (P<0.05) or ** (P<0.01), as determined by the non-parametric Mann-Whitney t test or Anova. Between brackets: number of mice used per experiment. Each dot represents an individual mouse.
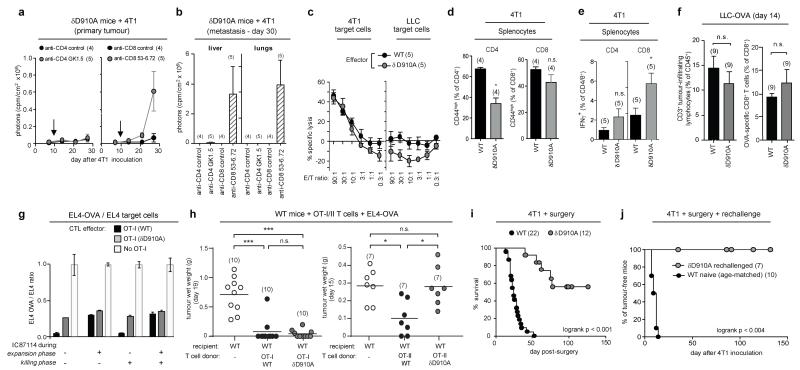
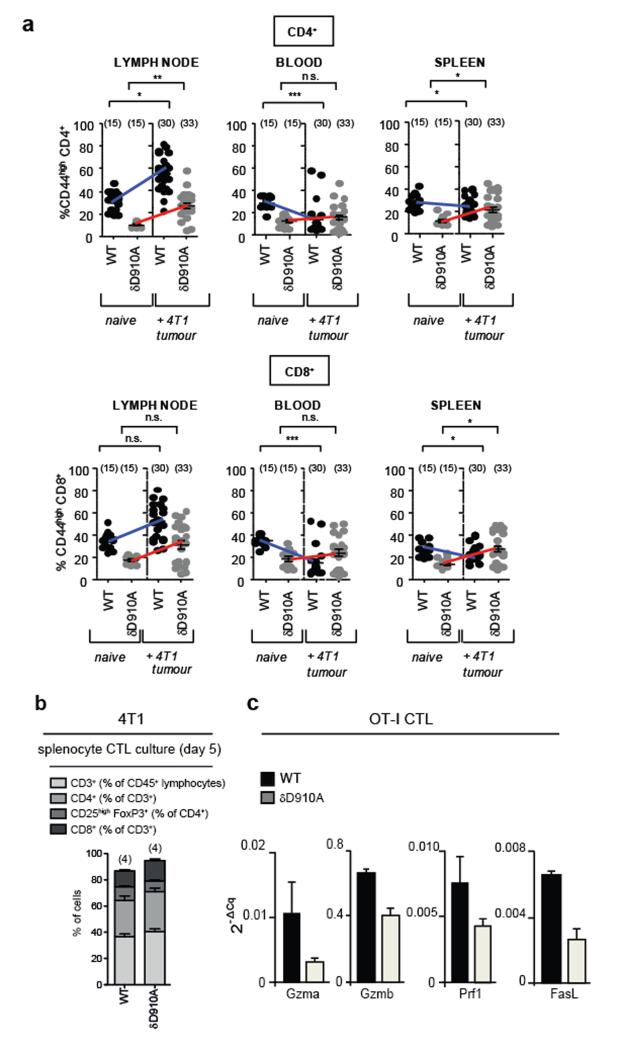
Depletion of CD8+ T cells but not of CD4+ T cells on day 10 after 4T1 inoculation in δD910A mice eliminated cancer protection (Fig. 3a,b). These data show that CD8+ T cells are responsible for restricting tumour growth in δD910A mice, but do not exclude an accessory role for CD4+ T cells. In line with published data5, naive WT mice had higher relative numbers of activated/memory CD44highCD4+ and CD44highCD8+ T cells than δD910A mice (Extended Data Fig. 1a). Upon 4T1 inoculation in WT mice, the relative numbers of these cells were either enhanced (tumour-draining lymph nodes) or reduced (blood and spleen), but in δD910A mice showed a trend towards expansion (Extended Data Fig. 1a), indicating that δD910A mice are capable of mounting both CD4+ and CD8+ T cell responses against 4T1 tumours. WT and δD910A splenocytes from tumour-bearing mice, incubated in vitro with mitomycin C-treated 4T1 cells, generated equivalent cytotoxic activity against 4T1, with no specific lysis of LLC (Fig. 3c). Compared to WT cultures, δD910A cultures contained similar proportions of CD4 and CD8 T cell subsets (Extended Data Fig. 1b), with a reduced frequency of activated/memory CD44highCD4+ cells (Fig. 3d) and unaffected frequency of CD44high CD8+ cells (Fig. 3d). Interestingly, despite this reduced proportion of δD910A CD44highCD4+ cells, the frequency of IFNγ+ CD4+ cells in PMA/ionomycin-stimulated cultures of splenocytes from 4T1 tumour-bearing mice was unaffected by p110δ inactivation (Fig. 3e), with the frequency of IFNγ+ CD8+ cells even enhanced upon p110δ inactivation (Fig. 3e). Upon inoculation with LLC-OVA, WT and δD910A mice generated similar levels of tumour-infiltrating OVA-specific CD8+ T cells (Fig. 3f), showing that systemic in vivo inactivation of p110δ does not impede the development or recruitment of antigen-specific anti-tumour CD8+ cells.
Figure 3. Impact of p110δ inactivation on T cell-mediated anti-tumour immunity.
a, Growth of 4T1 in δD910A mice injected with antibodies to CD4 or CD8. Arrow indicates the time of antibody injection. b, Metastasis in CD4 or CD8 T cell-depleted 4T1 tumour-bearing δD910A mice. c, In vitro cytotoxic activity of splenocytes, isolated from 4T1 tumour-bearing WT and δD910A mice 21 days after inoculation and cultured for 4 days with mitomycin-treated 4T1 cells. E/T, effector to target (4T1 or LLC) ratio. d, Frequency of CD44high CD4+ and CD8+ T cells in splenocytes from 4T1 tumour-bearing mice cultured for 5 days with mitomycin-treated 4T1 cells. e, Frequency of IFNγ+ T cells after 16h PMA+ionomycin stimulation of splenocytes from WT and δD910A 4T1 tumour-bearing mice. f, Relative levels of tumour-infiltrating CD3+ lymphocytes and OVA-specific CD8+ T cells in LLC-OVA tumours in WT or δD910A mice. g, In vitro cell killing of a 1:1 EL4-OVA:EL4 mix following 24h incubation with CTL from WT or a δD910A OT-I mice (at an E/T ratio of 10:1), incubated with or without the p110δ inhibitor IC87114 during the 8 day expansion phase, the 24h killing phase, or both. Cell killing efficiency is expressed as the ratio of EL4-OVA cells over EL4 cells remaining after incubation with effector cells. h, Effect of adoptive transfer in WT mice of OT-I CD8+ or OT-II CD4+ cells on growth of subsequently inoculated EL4-OVA. i, Survival of post-surgical 4T1 tumour-bearing mice. j, Survival of δD910A mice which had remained tumour-free >200 days after surgery, and of naïve WT mice, following injection of 10,000 4T1 cells. Statistics are as described in the legend to Figure 1.
To test the intrinsic ability of δD910A CD8 T cells to eliminate tumours, we crossed δD910A mice to OT-I transgenic mice which carry an OVA-specific MHC class I-restricted TCR transgene. In vitro generated δD910A OT-I CTLs were less efficient than WT OT-I CTLs at EL4-OVA killing (Fig. 3g) and produced lower levels of cytotoxic mediators (Extended Data Fig. 1c). Pharmacological inactivation of p110δ during the in vitro CTL expansion of WT OT-I cells partially suppressed CTL function, in a manner indistinguishable from genetic inactivation of p110δ (Fig. 3g), whereas p110δ blockade during the killing phase itself did not affect CTL function (Fig. 3g). Despite these in vitro defects in δD910A OT-I CTLs, adoptive transfer of these cells in WT mice prior to challenge with EL4-OVA, provided equal cancer protection to inoculation of WT OT-I T cells (Fig. 3h), showing that in vivo CTL responses can remain competent in the absence of CD8 T cell-intrinsic p110δ activity. Taken together, these data indicate that p110δ inhibition impairs differentiation of CD8 T cells to become fully competent CTL; however, fully differentiated CTL do not seem to require p110δ activity to kill target cells and on balance, in the context of reduced Treg function in δD910A mice, can mediate effective anti-tumour activity.
CD4 T cells can also contribute to tumour elimination by promoting the activation of macrophages and NK cells or by direct lysis of MHC class II+ tumour cells10. Indeed, CD4+ T cells with enhanced PI3K activity are superior in their capacity to reject tumour growth, probably as a consequence of their increased production of IFNγ11. Conversely, δD910A OT-II CD4 cells were less effective than WT OT-II cells in preventing EL4-OVA tumour growth (Fig. 3h), consistent with our previous finding that δD910A OT-II T cells produce less IFNγ in vitro and in vivo12,13. Therefore, in the context of an otherwise normal immune system, δD910A CD4+ cells show inferior anti-tumour immunity. However, the production of IFNγ by CD4 and CD8 T cells from 4T1 tumour-bearing δD910A mice, where Treg are also defective, appeared to be intact (Fig. 3e), suggesting that p110δ inhibition can affect the balance between regulatory and effector CD4+ T cells such that the effector cells prevail in the context of anti-tumour responses.
A salient feature of CD4 and CD8 T cells is the ability to raise a more potent and rapid immune response to subsequent exposure to cognate antigen. Upon surgical removal of 4T1 primary tumours when they had reached 9 mm diameter and established metastatic foci14, WT mice all succumbed to re-growth of the primary tumour and metastatic disease. In contrast, >50% of post-surgical δD910A mice showed survival extension beyond 100 days (Fig. 3i), demonstrating that p110δ inhibition can suppress cancer relapse and presumably metastatic cancer after surgery. δD910A mice which had remained tumour-free >200 days after surgery were cancer-resistant upon rechallenge with a higher 4T1 dose (Fig. 3j), suggesting that surgical intervention in δD910A mice supports the development of an effective memory anti-tumour response.
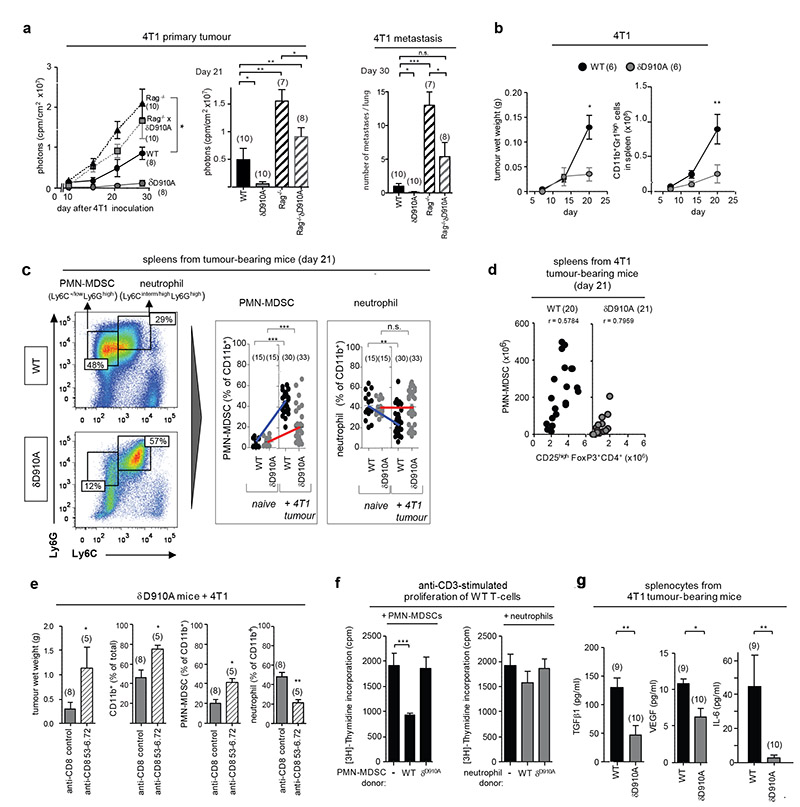
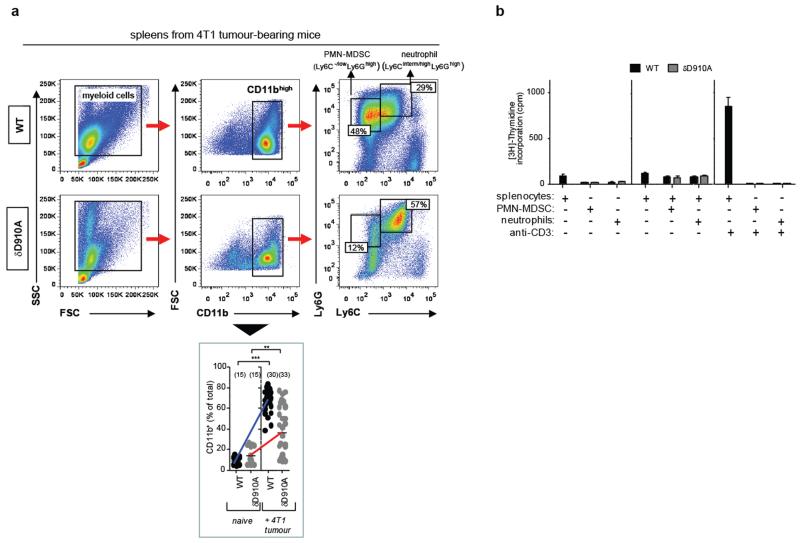
To assess the potential importance of p110δ in myeloid cells in cancer, we tested the impact of p110δ inactivation in Rag−/− mice. Rag−/− mice, which lack mature B and T cells, showed enhanced primary 4T1 tumour size and metastasis (Fig. 4a) compared to WT mice. Rag−/−xδD910A mice showed similar 4T1 primary tumour burden as Rag−/− mice (Fig. 4a) but had fewer metastatic lesions in lung (Fig. 4a) and liver (not shown), indicating that p110δ inactivation in a non-B/T cell lineage delays 4T1 tumour progression but is not sufficient to instigate tumour rejection. We next assessed the impact of p110δ inactivation on myeloid-derived suppressor cells (MDSCs), a heterogeneous population of bone marrow-derived myeloid cells that co-express the CD11b and Gr1 surface markers and which play a prominent role in immune suppression in cancer15,16. Neutrophils are also CD11b+Gr1+ but are thought not to be immune-suppressive17. Upon inoculation with 4T1 cells, known to be potent MDSC inducers16,17, CD11b+Gr1high cells accumulated in the spleens of both WT and δD910A mice, even before tumours were palpable, and continued to differentially accumulate in both genotypes as tumours grew, correlating with tumour size (Fig. 4b). The Ly6C and Ly6G surface markers, which are both recognized by the Gr1 antibody, have been used to subdivide MDSCs into two CD11b+ subpopulations, namely monocytic (M)-MDSCs (Ly6ChighLy6Glow) and polymorphonuclear (PMN)-MDSCs (Ly6C-/lowLy6Ghigh)17. Although neutrophils are difficult to differentiate from PMN-MDSCs, here we designated the neutrophil population as Ly6Ghigh cells with intermediate/high Ly6C expression (Fig. 4c, Extended Data Fig. 2a). PMN-MDSCs, predominant in 4T1 tumour-bearing WT mice (Fig. 4c), were substantially reduced in δD910A mice, correlating with a relative increase in neutrophils in the latter (Fig. 4c). Interestingly, the number of PMN-MDSCs in spleens from 4T1 tumour-bearing mice correlated with the number of Tregs (Fig. 4d). Depletion of CD8+ cells in 4T1 tumour-bearing δD910A mice, which led to enhanced tumour growth (Fig. 3a,b), also led to increased PMN-MDSC numbers and reduced neutrophil numbers (Fig. 4e). It was therefore difficult to ascertain whether the reduced PMN-MDSC numbers in δD910A mice are a consequence of an intrinsic role for p110δ in these cells or an indirect consequence of a reduced tumour burden in δD910A mice (Fig. 4b). In support of the former, WT PMN-MDSCs suppressed T cell proliferation in vitro, whereas MDSCs from δD910A mice with regressing tumours did not (Fig. 4f; Extended Data Fig. 2b). Neutrophils from both genotypes did not suppress T cell responses (Fig. 4f). Moreover, splenocytes from tumour-bearing δD910A mice showed reduced in vitro production of TGFβ, VEGF and IL-6 (Fig. 4g), each of which can contribute to immune suppression and/or tumour growth15,16.
Figure 4. Impact of p110δ inactivation on myeloid cells in 4T1 tumour-bearing mice.
a, 4T1 primary tumour growth and lung metastasis in WT, δD910A, Rag−/− and Rag−/− xδD910A mice. b, 4T1 tumour growth and total numbers of splenic CD11b+Gr1high myeloid cells in WT and δD910A mice. c, Gating strategy used to identify myeloid cell subsets and frequency of splenic PMN-MDSCs and neutrophils of naïve and 4T1 tumour-bearing WT and δD910A mice. d, Spearman correlation between accumulation of splenic PMN-MDSCs and Treg in WT or δD910A mice. e, Impact of depleting CD8+ T cells in δD910A mice on 4T1 tumour burden and presence of splenic myeloid cell populations. f, Impact of purified splenic myeloid cells on proliferation of anti-CD3-stimulated WT T cells. g, Cytokine production by splenocytes from 4T1 tumour-bearing (30 days after inoculation) from WT or δD910A mice, individually cultured for 4 days. Statistics are as described in the legend to Figure 1.
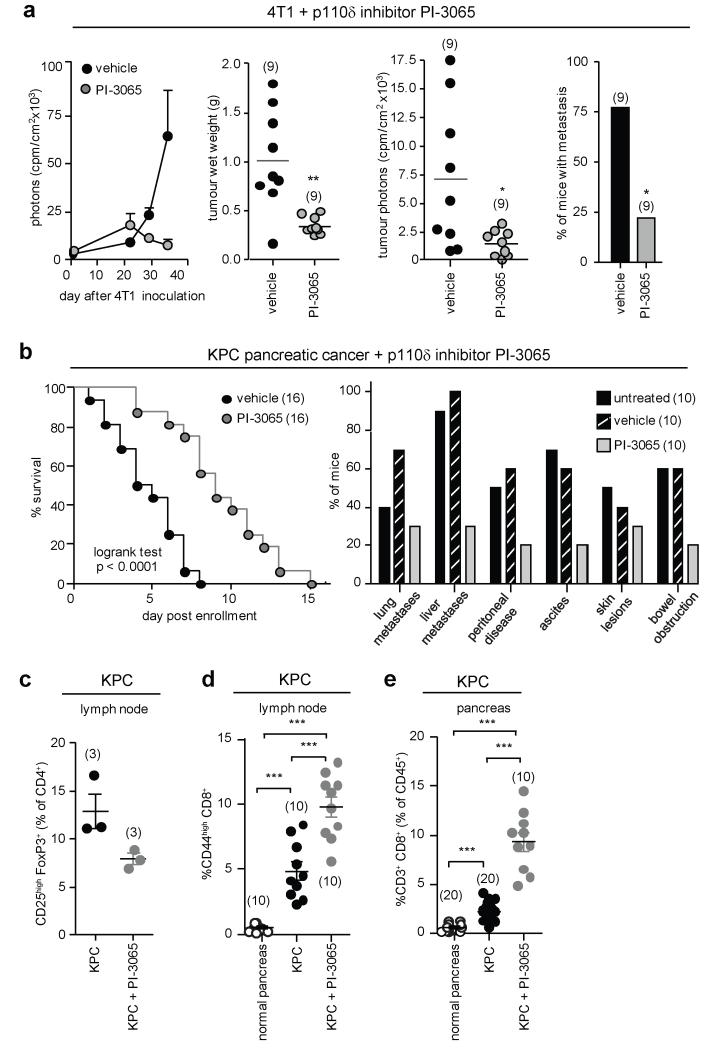
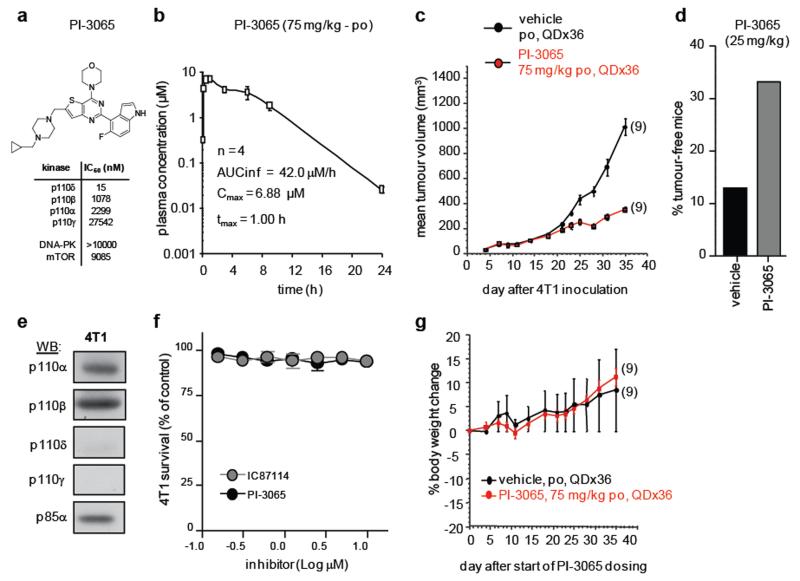
Administration of PI-3065, a small molecule inhibitor with selectivity for p110δ (Extended Data Fig. 3a,b and Extended Data Table 1), also suppressed 4T1 tumour growth and metastasis, to a similar extent as genetic inactivation of p110δ, marked by initial tumour progression, followed by tumour regression (Fig. 5a; Extended Data Fig. 3c,d). Of interest, 4T1 do not express detectable levels of p110δ (Extended Data Fig. 3e) and are not growth-inhibited in vitro by PI-3065 (Extended Data Fig. 3f). Long-term administration of PI-3065 to mice was well-tolerated and did not induce weight loss (Extended Data Fig. 3g).
Figure 5. Impact of pharmacological inactivation of p110δ on tumour growth and T cell responses.
a, Mice, dosed with vehicle or PI-3065 (75 mg/kg, daily) for 36 days and inoculated with 105 4T1 cells 12h post first dosing, were assessed for tumour growth by luciferase imaging (first panel), tumour weight (second panel) or luciferase activity in tumours excised 35 days after inoculation (third panel). Incidence of 4T1 metastasis (fourth panel), as detected by H&E staining and histology, expressed as percentage of the total number of tumour-bearing animals per group. b, Impact of PI-3065 (75 mg/kg) on KPC mouse survival (left) and macrometastases (as detected by H&E staining) and cancer-associated pathology (right). c, Proportion of Treg (% of CD4+) in the draining lymph nodes of KPC mice administered vehicle or PI-3065. d, Proportion of CD44high T cells (% of CD8+) in the draining lymph nodes of KPC mice administered vehicle or PI-3065. e, Relative numbers of CD8+ T cells (% of CD45+) in normal pancreas and PDAC lesions of KPC mice treated or not with PI-3065. Statistics are as described in the legend to Figure 1.
We next tested the impact of PI-3056 in the KPC model of pancreatic ductal adenocarcinoma (PDAC), which expresses endogenous mutant KrasG12D and p53R172H genes in Pdx1+ pancreatic cells. KPC mice were left to develop palpable disease before treatment with vehicle or PI-3065 was commenced. Under these therapeutic conditions, PI-3065 prolonged survival and reduced the incidence of metastases and other disease-associated pathologies (Fig. 5b). The relative abundance of peripheral Treg in lymph nodes after 7 days of treatment was reduced (Fig. 5c), correlating with higher levels of CD44highCD8+ lymphocytes in the draining lymph nodes (Fig. 5d) and relatively more infiltrating CD8+ T cells in pancreatic lesions 14 days after treatment (Fig. 5e). These data indicate that therapeutic targeting of p110δ can promote immune-mediated elimination of cancer.
Concerns have been raised about inhibiting p110δ in cancer as this might impair CTLs and negatively impact on cancer immune surveillance4,18. Our data show that although p110δ blockade reduces the effectiveness of CTLs, it also overrides Treg- and probably also MDSC-mediated suppression of anti-tumour immune responses, enabling even weakened CTLs to successfully attack tumours. Thus, p110δ is apparently more essential for regulatory rather than effector T cell responses against cancer cells. In addition, inhibition of the PI3K pathway in CD8 T cells may help maintain them in a stem-cell like state19 with enhanced potential for generating durable anti-tumour responses. Consistent with this notion, δD910A mice resisted tumour rechallenge following surgical removal of the first tumour. The p110δ inhibitor Idelalisib has shown impressive therapeutic impact in chronic lymphocytic leukaemia (CLL) and non-Hodgkin’s lymphoma1,2. In CLL, p110δ blockade interferes with stroma-derived survival and adhesion signals supporting the tumour cells4, but it is unclear if this fully explains the effectiveness of p110δ inhibition. Our finding that p110δ inhibition can unlock adaptive anti-tumour responses provides a potential additional mechanism for the efficacy of p110δ blockade in CLL, and add to the emerging rationale for targeting PI3K in the tumour stroma4, to dampen inflammation (p110γ)20 and angiogenesis (p110α)21.
Tumour-induced immune suppression constitutes an important barrier for effective anti-tumour immunity and immunotherapy in cancer. Our work suggests that p110δ inhibitors, by disrupting the function of Treg and possibly of MDSCs, have the potential to shift the balance from immune tolerance towards effective anti-tumour immunity. This provides a rationale for p110δ inhibition both in solid and haematological cancer, possibly as an adjuvant to cancer vaccines, adoptive cell therapy, or other strategies that promote tumour-specific immune responses.
METHODS SUMMARY
All animal procedures were in compliance with institutional animal care and use committee guidelines. Details of procedures and reagents are described in Methods.
Extended Data
Extended Data Figure 1. Impact of p110δ inactivation on CD4 and CD8 T cells in mice with 4T1 or EL4 tumours.
a, Levels of CD44highCD4+ and CD44highCD8+ T cells in the indicated immune compartments of naive and 4T1 tumour-bearing on day 26 after inoculation in WT or δD910A mice. b, Distribution of cells on day 5 of culture of splenocytes, isolated from 4T1 tumour-bearing WT and δD910A mice 21 days after inoculation, in the presence of mitomycin-treated 4T1 cells. c, Gene expression in CTLs derived from splenocytes from WT and δD910A OT-I mice, cultured in the presence of SIINFEKL OVA peptide and IL2. GzmA, granzyme A; GzmB, granzyme B, Prf1, perforin and (FasL or CD95L) Fas ligand. Expression levels are presented relative to β2-microglobulin. a-b, Statistically significant differences are indicated by * (P < 0.05) or ** (P < 0.01), as determined by the non-parametric Mann-Whitney t test. Between brackets: number of mice used per experiment. Each dot represents an individual mouse.
Extended Data Figure 2. Impact of p110δ inactivation on myeloid cells in 4T1 tumours.
a, Gating strategy used to identify myeloid cell subsets. Splenic cells were gated on CD11bhigh cells followed by Ly6C and Ly6G gating. FSC, forward scatter; SSC, side scatter (top panel). Frequency of CD11b+ cells in the spleen of WT and δD910A naïve mice and in 4T1 tumour-bearing mice on day 21 after inoculation (bottom panel). b, [3H]-Thymidine incorporation in co-cultures of splenocytes and purified myeloid cells, in combinations as indicated, with or without stimulation with anti-CD3 antibodies. Cultures were made using cells derived from individual mice. Error bars represent standard deviation from the mean of biological replicates. Statistically significant differences are indicated by * (P < 0.05) or ** (P < 0.01), as determined by the non-parametric Mann-Whitney t test. Between brackets: number of mice used per experiment. Each dot represents an individual mouse.
Extended Data Figure 3. Characterisation of the p110δ-selective inhibitor PI-3065.
a, PI-3065 structure and in vitro IC50 on selected PI3K family members. No significant activity against 72 protein kinases was observed at ≤ 10 μM in a KinaseProfiler assay (Millipore). b, Pharmacokinetic parameters of PI-3065. Mean (±SD) plasma concentration profile of PI-3065 following a single oral dose (75 mg/kg) administred per os (po) to female BALB/c mice. AUCinf, area under the curve, extrapolated to infinity; Cmax, highest observed plasma concentration; tmax, time at which Cmax occurred, QD; quaque die, every day. c, Growth of primary 4T1 tumours, inoculated in the breast fat pad, measured by calipers and expressed as tumour volume. Mice were dosed per os with vehicle or PI-3065 (75 mg/kg, daily) for 36 days. 105 tumour cells were inoculated 12 h post first dosing. d, Percentage of tumour-free mice upon continuous per os treatment of mice with vehicle or PI-3065 (25 mg/kg, twice daily) for 37 days, with tumour cells inoculated on day 7 of PI-3065 dosing. 15 mice were used for each genotype. e, Class I PI3K isoform expression in 4T1 cells. f, Proliferation of 4T1 cells following a 4 h treatment with the indicated p110δ inhibitors, washing and MTS staining after 48 h culture. g, Percentage body weight change (from day 0) of 4T1 tumour-bearing mice upon daily per os administration of PI-3065 (75 mg/kg) or vehicle for 36 consecutive days. Statistically significant differences are indicated by * (P < 0.05) or ** (P < 0.01), as determined by the non-parametric Mann-Whitney t test. Between brackets: number of mice used per experiment.
Extended Data Table 1. Comparison of PI-3065 with Idelalisib (formerly called GS-1101 or CAL-101) and IC87114.
Human whole blood was stimulated with anti-IgM followed by FACS for CD69 as described1,2. Human B cell lymphoma Ri-1 cells were preincubated for 30 min with vehicle or compound prior to stimulation with anti-IgM for 1 h at 37°C, followed by determination of Akt-Ser473 phosphorylation, as described1,2.
| Compound | Ki(nM) | IC50 (nM) | anti-IgM stimulated whole blood | anti-IgM-stimulated human R1 B cell lymphoma | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p110δ | p110α | p110β | P110γ | p110δ | p110α | p110β | p110γ | CD69 expression IC50 (n.M) | pAkt IC50 (nM) | |
| PI-3065 | 1.5 | 110 | 130 | 940 | 5 | 910 | 600 | >10000 | 38 | 36 |
| Idelalisib | 1.1 | 270 | 121 | 16 | 2.5 | 670 | 260 | 22 | 60 | 41 |
| IC87114 | 34 | >2100 | >2100 | 370 | 46 | >10000 | >10000 | 1300 | 3500 | >7800 |
- 1.Murray JM, et al. Potent and highly selective benzimidazole inhibitors of PI3-kinase delta. Journal of medicinal chemistry. 2012;55:7686–7695. doi: 10.1021/jm300717c. [DOI] [PubMed] [Google Scholar]
- 2.Safina BS, et al. Discovery of novel PI3-kinase delta specific inhibitors for the treatment of rheumatoid arthritis: taming CYP3A4 time-dependent inhibition. Journal of medicinal chemistry. 2012;55:5887–5900. doi: 10.1021/jm3003747. [DOI] [PubMed] [Google Scholar]
Supplementary Material
Acknowledgements
This research was supported by Cancer Research UK (C23338/A10200; C23338/A15965 to B.V. and C18270/A12888 to T. Hagemann), BBSRC (BB/E009867/1 to K.O.) and Wellcome Trust (095691/Z/11/Z) (to K.O.). We thank Dan Sutherlin, Jim Nonomiya, John Lesnick, Karin Reif (Genentech) for technical assistance, Maria Whitehead, Delphine Dubuisson, Dan Patton, Elizabeth Slack, Norelene Harrington, Guglielmo Rosignoli, William P. Day, Anthony Brown, Paul Depledge, Frauke Leenders, Jodie Pang, Laurent Salphati and Xiaolin Zhang for experimental help, Doug Fearon and James Arnold for LLC-OVA cells, Alexander Rudensky for FoxP3YFP-Cre mice.
Footnotes
Online content Any additional Methods and Extended Data display items are available in the online version of the paper; references unique to these sections appear only in the online paper.
Supplementary Information is available in the online version of the paper.
References
- 1.Furman RR, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopal AK, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. The New England journal of medicine. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews. Drug discovery. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 6.Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annual review of immunology. 2013;31:675–704. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curiel TJ. Regulatory T cells and treatment of cancer. Current opinion in immunology. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patton DT, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 9.Patton DT, Wilson MD, Rowan WC, Soond DR, Okkenhaug K. The PI3K p110delta regulates expression of CD38 on regulatory T cells. PloS one. 2011;6:e17359. doi: 10.1371/journal.pone.0017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Current opinion in immunology. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soond DR, et al. Pten loss in CD4 T cells enhances their helper function but does not lead to autoimmunity or lymphoma. J Immunol. 2012;188:5935–5943. doi: 10.4049/jimmunol.1102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okkenhaug K, et al. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 13.Soond DR, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115:2203–2213. doi: 10.1182/blood-2009-07-232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulaski BA, Smyth MJ, Ostrand-Rosenberg S. Interferon-gamma-dependent phagocytic cells are a critical component of innate immunity against metastatic mammary carcinoma. Cancer research. 2002;62:4406–4412. [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews. Immunology. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 17.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putz EM, et al. PI3Kdelta Is Essential for Tumor Clearance Mediated by Cytotoxic T Lymphocytes. PloS one. 2012;7:e40852. doi: 10.1371/journal.pone.0040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nature reviews. Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. doi:10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid MC, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer cell. 2011;19:715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soler A, et al. Inhibition of the p110alpha isoform of PI 3-kinase stimulates nonfunctional tumor angiogenesis. The Journal of experimental medicine. 2013;210:1937–1945. doi: 10.1084/jem.20121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.