Abstract
Optical coherence tomography (OCT) is a noninvasive imaging modality providing high-resolution images of the central retina that has completely transformed the field of ophthalmology. While traditional OCT has produced longitudinal cross-sectional images, advancements in data processing have led to the development of en-face OCT, which produces transverse images of retinal and choroidal layers at any specified depth. This offers additional benefit on top of longitudinal cross-sections because it provides an extensive overview of pathological structures in a single image. The aim of this review was to discuss the utility of en-face OCT in the diagnosis and management of age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV). En-face imaging of the inner segment/outer segment junction of retinal photoreceptors has been shown to be a useful indicator of visual acuity and a predictor of the extent of progression of geographic atrophy. En-face OCT has also enabled high-resolution analysis and quantification of pathological structures such as reticular pseudodrusen (RPD) and choroidal neovascularization, which have the potential to become useful markers for disease monitoring. En-face Doppler OCT enables subtle changes in the choroidal vasculature to be detected in eyes with RPD and AMD, which has significantly advanced our understanding of their pathogenesis. En-face Doppler OCT has also been shown to be useful for detecting the polypoid lesions and branching vascular networks diagnostic of PCV. It may therefore serve as a noninvasive alternative to fluorescein and indocyanine green angiography for the diagnosis of PCV and other forms of the exudative macular disease.
Keywords: Age-related macular degeneration, en-face optical coherence tomography, polypoidal choroidal vasculopathy
Optical coherence tomography (OCT) is a noninvasive imaging modality providing high-resolution images of the central retina and optic nerve head that has completely transformed the field of ophthalmology. Utilizing light waves emitted from a broadband infra-red light source, it detects light reflected from the retina at various depths to create cross-sectional images of the retina.
Time-domain OCT (TD-OCT) was the first technique employed, using a moving reference mirror to collect back-scattered light from the retina. TD-OCT had the ability to obtain images at a rate of 400 A-scans per second and a resolution of 8–10 μm.[1]
The development of spectral-domain OCT (SD-OCT), which utilized an interferometer and high speed spectrometer to simultaneously detect light of different frequencies, without the need for a moving reference mirror, allowed faster acquisition of images of up to 20,000–52,000 A-scans per second, as well as higher resolution of 5–7 μm.[1]
Conventional OCT methods were unable to visualize the choroid well due to scattering of light by the retinal pigment epithelium (RPE). Nevertheless, new techniques have been developed with the aim of improving visualization of the choroid. Examples include image averaging and enhanced depth imaging (EDI). Image averaging obtains the average of multiple B-scans of the retina at a given location, to increase the signal-to-noise ratio. EDI-OCT utilizes the sensitivity roll-off phenomenon of SD-OCT and places the choroid-sclera interface at the zero line, to enhance visualization of the choroid.
Ultra high-resolution OCT (UHR-OCT) and swept source OCT (SS-OCT) are newer prototype systems. UHR-OCT uses broadband light sources to achieve a resolution of 3 μm. SS-OCT is a variation of SD-OCT, in which the swept laser light source sequentially emits light of varying frequencies, of which the reflections are detected by photodetectors rather than a spectrometer. SS-OCT uses light with a wavelength of 1060 nm that increases depth penetration, thus improving visualization of the choroid. SS-OCT has acquisition rates of up to 100,000–236,000 A-scans per second and a resolution of up to 11 μm.[2]
Doppler OCT is a technique that utilizes ultra-high-speed image acquisition with SD-OCT, using blood flow as a contrast medium, to evaluate retinal and choroidal vasculature with precise depth resolution.
Advancements in software and data processing have led to the development of en-face OCT. En-face imaging is an emerging technique that combines SD-OCT with transverse confocal analysis, producing transverse images of retinal and choroidal layers at a specified depth. In other words, it provides an en-face view of any given layer within the retina and choroid. En-ace imaging offers many additional advantages over conventional longitudinal cross-sections because it can delineate microstructural and morphological changes in coronal view, allows precise measurements to be made, and provides a more extensive overview of pathological structures in a single image. It also provides a familiar view of the fundus similar to that seen on fundus photography and direct ophthalmoscopy.
Nevertheless, there are some drawbacks of OCT compared with other imaging modalities such as fluorescein angiography (FA), indocyanine green (ICG), angiography and fundus autofluorescence (FAF). FA and ICG are both dynamic studies that can demonstrate leakage of dye from abnormal blood vessels and pooling of dye within pigment epithelial detachments (PEDs) or neurosensory retinal detachments. This is in contrast to OCT, which is static and does not give direct information about the integrity of the vasculature. Furthermore, the difficulty in differentiating between various causes of PEDs based on their morphology on en-face OCT makes it less specific than FA for the diagnosis of exudative age-related macular degeneration (AMD). Similarly, ICG studies the choroidal circulation and is more specific than OCT for the diagnosis of polypoidal choroidal vasculopathy (PCV) and retinal angiomatous proliferation, as it can demonstrate the polyps and branching vascular networks in PCV[3] as well as the retinal-retinal anastomoses and retinal-choroidal anastomoses in retinal angiomatous proliferation.[4] FAF is useful in monitoring the progression of geographic atrophy (GA) because it gives information concerning the metabolic activity of the RPE cells, something, which cannot be demonstrated by en-face OCT.
The aim of this review was to discuss the utility of en-face OCT in the diagnosis and management of AMD and PCV.
Methods
A literature search was performed using the PubMed database up to October 2014. Keywords including “en-face,” “OCT,” “AMD,” “PCV,” “retinal angiomatous proliferation,” and “RAP” were used in various combinations. Articles are reporting the use of en-face OCT for in AMD, PCV, and RAP were reviewed. A search through the references of the retrieved articles was also performed. This review included all the clinical studies published. Only articles published in English were included.
Age-Related Macular Degeneration
Good correlation with other imaging modalities
Superimposing fluorescein angiography images with en-face optical coherence tomography images
As early as 2005, van Velthoven et al. demonstrated the value of overlaying fluorescein angiograms onto en-face OCT images in retinal disease. By combining functional and structural information into one image, zones of vascular leakage could be correlated with changes in the retina and RPE. In a case of retinal angiomatous proliferation, the overlay image demonstrated the potential for en-face OCT to reveal the extent of retinal PED where fluorescein leakage was blocked.[5]
Correlating pathological features in dry and wet age-related macular degeneration with other imaging modalities
A pilot study on en-face OCT in AMD was done in 2008. A total of 7 eyes with dry AMD and 5 eyes with wet AMD were studied, and en-face SD-OCT images were superimposed onto conventional imaging modalities for correlation of pathological features such as drusen, GA, choroidal neovascularization (CNV), macular edema, and subretinal fluid. Findings were promising: In wet AMD patients, the location and extent of CNV, macular edema, and subretinal fluid were visualized on en-face OCT and in some cases even identified in areas not seen with conventional imaging. In dry AMD patients, the location of drusen and GA corresponded to autofluorescence. In 4 of 12 eyes, en-face OCT identified subretinal fluid, which was not seen on FA.[6]
Simultaneous functional and structural imaging with combined optical coherence tomography/indocyanine green angiography
Rosen et al. studied 30 eyes with macular disease, the majority of which had neovascular AMD, and performed simultaneous imaging with OCT, confocal scanning laser ophthalmoscopy (SLO), and ICG. A mixer channel was used to superimpose information from the en-face OCT and the ICG into a single transverse image. They demonstrated how simultaneous OCT/ICG imaging allowed better correlation between structural and functional abnormalities. The two imaging modalities complemented each other, with OCT providing greater depth resolution, allowing identification of occult neovascularization in areas not well visualized on ICG, such as beneath regions of scarring and PEDs.[7]
Imaging of the inner segment/outer segment junction
Imaging of photoreceptor integrity
The brightness or intensity of the inner segment/outer segment (IS/OS) junction on OCT was found by Hayashi et al. to be correlated with visual acuity in retinal diseases including AMD.[8] Wanek et al. used en-face SD-OCT imaging of the IS/OS junction to visualize the integrity of the photoreceptor cells in various retinal diseases.[9] In a patient with neovascular AMD, en-face OCT of the IS/OS junction showed nonuniform intensity levels and focal areas of higher intensities, corresponding to topographic variations in the inner and OSs as a result of the underlying drusen deposition. En-face OCT of the IS/OS junction revealed focal areas of reduced intensity in patients with other retinal disease including macular hole, central serous retinopathy, cystoid macular edema and diabetic retinopathy, clearly delineating the areas of pathology. Fig. 1 is an example of en-face OCT scan delineating a localized area of damaged photoreceptor IS/OS junction. En-face imaging may be a useful tool for monitoring the integrity of photoreceptor cells in retinal disease.
Figure 1.
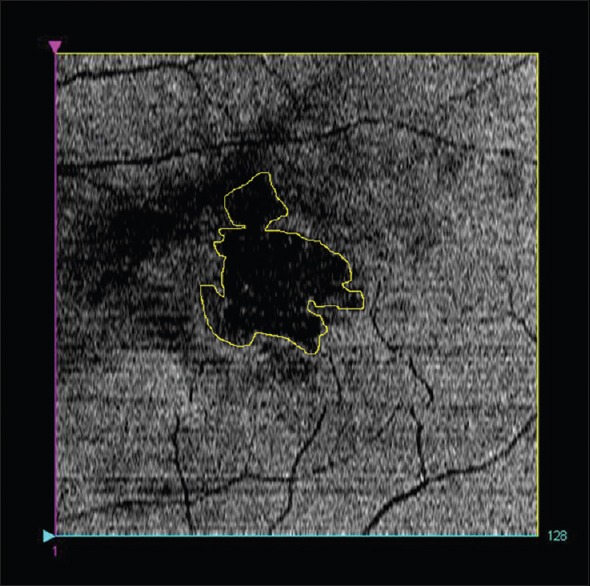
En-face image at the level of inner segment/outer segment junction (IS/OS) showing IS/OS junction damaged area (yellow boundary)
Correlation of inner segment/outer segment junction areas with visual acuity
Kiernan et al. used en-face OCT to calculate the area of intact IS/OS junction in the outer retinal photoreceptor layer. Totally, 74 eyes were examined, of which 30 were healthy, 19 had intermediate to advanced dry AMD, and the rest had other retinal pathology. They found that there was a significant correlation between central en-face IS/OS junction area and visual acuity in all eyes except those with dry AMD. The correlation was greater than that shown between visual acuity and central subfield thickness, macular volume, and average macular thickness. However, there was poor correlation between IS/OS junction area and visual acuity in patients with dry AMD. This may be explained by the disruption of the IS/OS and RPE planes on en-face sections due to drusen, RPE changes and PEDs, causing loss of the hyper-reflective band at the IS/OS junction.[10]
Predicting the progression of geographic atrophy
Holz et al. demonstrated the utility of en-face OCT in predicting the area of progression of GA in dry AMD. Similar to the hyperautofluorescent border of progressing GA in FAF imaging,[11] en-face OCT of the IS/OS junction showed a dark area extending beyond the borders of GA that accurately predicted the extent of progression of GA over the following year in 13 of 30 eyes. In other words, en-face OCT can identify the area of IS/OS disruption corresponding to the area of photoreceptor loss, which is at high risk of progression of GA.[12]
Imaging of the outer retina
Characterization of outer retinal tubulations
Outer retinal tubulations (ORTs) are lesions located in the outer nuclear layer, often overlying areas of subretinal fibrosis or pigment epithelial alterations in both neovascular and atrophic AMD. Wolff et al. used en-face SD-OCT to differentiate between ORTs. They found that ORTs in neovascular AMD formed a branching “pseudo-dendritic” pattern emanating from a fibrovascular (FV) scar. On the other hand, ORTs in atrophic AMD followed a circular pattern with invaginations around the periphery of the atrophic zone.[13]
Imaging of the subretinal space, retinal pigment epithelium, pigment epithelial detachments, drusen, and choroidal neovascularization
Visualization of sub-retinal pigment epithelium changes in exudative age-related macular degeneration - detection of occult choroidal neovascularization
De Bruin et al. were the first to show that ultra-high-speed SS-OCT using a wavelength of 1050 nm provided a better penetration below the RPE compared with conventional SD-OCT at 850 nm. En-face images were reconstructed from 120 cross-sections. In patients with exudative AMD, the en-face reconstructions were able to detect areas of reduced reflectivity between the RPE and Bruch's membrane, which correlated well with areas of type 1 CNV on FA. After treatment with ranibizumab, this area showed a marked reduction in size. However, the authors acknowledged the difficulty in differentiating between CNV and sub-RPE fluid.[14]
Characterization of pigment epithelial detachments
En-face OCT was used to compare the morphology of PED in 9 eyes with central serous chorioretinopathy (CSC) and 21 eyes with neovascular AMD. In CSC, PEDs were frequently circular in shape, had a smooth inner silhouette, contained clear content, and had a uniform or slightly irregular wall aspect. In neovascular AMD, PEDs were often irregular or multilobular in shape had a granular inner silhouette, and an irregular wall aspect. PED wall thickness and dimensions were larger in AMD compared with CSC. The authors suggested that en-face sections were able to provide additional information to conventional longitudinal sections, as they gave a better overview of the area of PED and allowed more accurate measurements of PED wall thickness and shape. En-face OCT may, therefore, be useful in distinguishing between PEDs in CSC and AMD.[15]
Detection of intraretinal retinal pigment epithelium migration in dry age-related macular degeneration
Intra-retinal RPE migration can be detected on en-face OCT as small discrete hyper-reflective, highly-back-scattering lesions within the neurosensory retina. In a study of 55 eyes with early to intermediate dry AMD, en-face OCT demonstrated intra-retinal RPE migration in 54.5%. In all of these eyes, the areas of intraretinal RPE migration corresponded to RPE pigment clumping on fundus photographs. RPE pigment migration was seen most frequently in the outer nuclear layer and occurred above areas of drusen in 74%. This suggests that drusen may play a role in the stimulation of intraretinal RPE migration.[16]
Visualization of choroidal neovascularization within fibrovascular pigment epithelial detachment - a potential alternative to indocyanine green angiography
En-face imaging can also visualize CNV in FV-PEDs due to exudative AMD. Coscas et al. showed that en-face EDI SD-OCT was able to demonstrate the CNV as a hyper-reflective network within the FV-PED in 30 out of 38 eyes with AMD, with an underlying area of homogeneous hypo-reflectivity consistent with serous exudation. The course of the hyper-reflectivity on OCT corresponded well with the hyper-fluorescent network on ICG. In the other 8 eyes, en-face imaging showed dense, homogenous hyper-reflectivity within the FV-PED, suggestive of fibrous tissue obscuring the CNV. Fig. 2 is an en-face OCT scan revealing the total extent of occult CNV. With the ability to detect CNV on en-face OCT, it may be possible in future to diagnose type 1 exudative AMD noninvasively, without the need for FA and ICG, both of which require intravenous contrast injection.[17]
Figure 2.
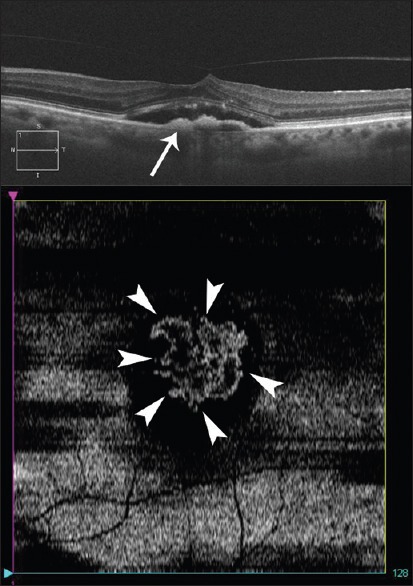
Spectral domain optical coherence tomography B-scan showing a type 1 membrane in an eye with occult choroidal neovascularization (top image-arrow). En-face image (bottom image) at the level of retinal pigment epithelium showing the total extent of the occult membrane (arrowheads)
Quantitative drusen measurements – potential biomarkers for age-related macular degeneration progression
Quantitative drusen measurements calculated from en-face projection images of the RPE layer could be used to monitor the progression of dry AMD. Chen et al. developed a novel algorithm for automated drusen segmentation using SD-OCT. By superimposing the observed and expected RPE contours, the differences in between the two layers can be marked as drusen. An en-face projection image of the drusen and RPE layer is then generated, which not only helps eliminate false positive drusen, but also provides a useful method of visualization of drusen, and enables the computation of potential biomarkers such as drusen area and shape, which could be used to monitor progression of dry AMD.[18]
Microstructure of reticular pseudodrusen
Subretinal drusenoid deposits (SDD) or reticular pseudodrusen (RPD) is a lesion found in AMD that is highly associated with progression to CNV, GA, and outer retinal atrophy. Meadway et al. used adaptive optics (AO)-SLO, correlated with AO-OCT to analyze the en-face microstructure of SDD. On AO-SLO areas of SDD appeared as a hypo-reflective annulus surrounding a reflective core of regularly spaced hyper-reflective dots in a mosaic pattern. It was uncertain whether the reflective core was attributed to changes in the overlying photoreceptors. When correlated with en-face AO-OCT, the authors concluded that the reflective core represented SDD material itself, rather than the overlying photoreceptors. AO-OCT also revealed that the SDD consisting of discrete hyper-reflective granules were deposited above the RPE and encroached upon the IS/OS junction with little effect on layers of retina above the external limiting membrane.[19]
Imaging of the choroidal vasculature
Correlation of reticular pseudodrusen with choroidal stroma
En-face OCT sections of the choroid demonstrated the close relationship between RPD and the intervascular choroidal stroma. Sohrab et al. examined 97 eyes with dry AMD, who had RPD. Point-to-point comparison of infrared reflectance, autofluorescence, and red-free images with en-face OCT sections of the choroid revealed that the RPD lesions aligned with the choroidal stroma in areas between choroidal vessels. The RPD closely abutted and often aligned along the course of the large choroidal vessels. Interestingly, disruptions of the IS/OS junction was found immediately adjacent to the RPD lesions rather than overlying them. This suggests that RPD may be associated with inflammatory or atrophic changes in the intervascular choroidal stroma, whereas the IS/OS disruptions may be secondary changes induced by the RPD.[20]
Quantification of choroidal vasculature
To further elucidate the role of choroidal vessels in the pathogenesis of AMD and RPD, Sohrab et al. performed a case–control study of patients with early dry AMD and RPD, in which en-face volumetric SD-OCT was used to quantify choroidal vascular density, vessel size, and choroidal thickness. Choroidal vascular density was defined as the area occupied by choroidal vessels, expressed as a percentage of the total area of choroid visualized on a 6 mm × 6 mm en-face image. The patients were divided into three groups: Controls (14 patients), early AMD (11 patients), and RPD (33 patients). Eyes that fit the criteria for both early AMD and RPD were excluded. En-face OCT choroidal sections showed that the choriocapillaris was absent in 100% of RPD patients, 55% of early AMD patients and 0% of controls. Choroidal vascular density was lowest in early AMD and highest in RPD patients. Although RPD patients had a complete loss of choriocapillaris, choroidal vascular density remained the highest because of the presence of larger choroidal vessels. Choroidal vessel diameter was comparable among the three groups, as was the choroidal thickness. These findings suggest a role for larger choroidal vessels in the pathogenesis of RPD. They are also consistent with the histopathology of choriocapillaris atrophy and vascular dropout in dry AMD.[21]
Imaging of the choriocapillaris– potential to detect early, subtle choroidal vascular abnormalities in geographic atrophy
Kim et al. developed phase-variance OCT (pvOCT), which uses the motion observed over time to calculate contrast and produce capillary perfusion maps of the retina and en-face images of vasculature in different layers of the retina and choroid. These layers include the choriocapillaris underneath Bruch's membrane, Sattler's layer containing medium-sized choroidal vessels, and Haller's layer containing large choroidal vessels and pigmented cells. En-face pvOCT of a patient with GA due to dry AMD revealed small focal areas of choriocapillaris dropout within the region of GA. Such early morphological changes in the choriocapillaris in GA are poorly visualized on fundal examination and other imaging modalities, such as autofluorescence, FA, and ICG. Therefore, en-face pvOCT, with its ability to provide high-resolution, depth-resolved microvascular imaging, particularly of the anterior choroidal vasculature, and choriocapillaris has the potential to detect subtle choroidal vascular abnormalities and permit the early diagnosis of GA. Moreover, pvOCT is not affected by regional vascular leakage, unlike contrast imaging modalities. Another advantage of pvOCT is that it can be integrated into existing OCT systems by means of software, without requiring any hardware modifications.[22]
Investigation of abnormal choroidal vasculature in exudative macular disease
Hong et al. developed Doppler optical coherence angiography (OCA) to investigate choroidal vasculature in patients with myopic CNV, AMD, and PCV. SS-OCT using 1050 nm wavelength of light for high penetration (HP) was modified to measure chorioretinal blood flow, and en-face angiogram images were reconstructed. En-face angiograms showed pathological choroidal vessels as areas of hyper-scattering, whereas choroidal vessels in normal eyes showed hypo-scattering. Bidirectional OCA visualized choroidal feeder vessels close to the PED in 3 of 5 eyes with PCV, which correlated with feeder vessels appearing in the early phase of ICG. High-sensitive OCA visualized the morphology of the branching vascular networks and polyps in eyes with PCV. There was a high correlation between high-sensitive OCA en-face angiograms and the mid-phase of ICG. With the ability to provide noninvasive three-dimensional choroidal vascular imaging with good correlation with ICG, en-face OCA may in future be an alternative to FFA and ICG for imaging of exudative macular disease.[23]
Quantifying choroidal neovascularization flow
Structural OCT cannot identify CNV because of its similar reflectivity to drusen and hemorrhage. Therefore, a flow-imaging OCT technology is needed to visualize CNV. Jia et al. used SS-OCT angiography to identify and quantify CNV flow. En-face projections of three distinct vascular layers were generated: Inner retina (showing retinal vasculature), outer retina (showing CNV), and choroid. CNV area and flow were also calculated from the en-face angiograms. Results were promising, with the en-face composite images combining angiographic information (CNV size, location, and flow index) with structural information (sub-retinal fluid and intraretinal cysts). In patients with neovascular AMD, OCT angiograms of the choroidal layer showed patchy loss of choriocapillaris, which led to the exposure of the underlying large, deep choroidal vessels, which were not visualized in normal controls. They also showed focal areas of decreased choroidal flow and loss of deeper choroidal vessels adjacent to the CNV, which may play a role in the pathogenesis of CNV. The en-face angiograms were also able to show vascular patterns, which were obscured by subretinal hemorrhage on FA.[24]
Calculation of choroidal vascular area
Ueda-Arakawa et al. used en-face HP SS-OCT to evaluate the choroidal vasculature in patients with RPD, of which some had early AMD, some had GA, and some had neovascular AMD. The en-face image at the level mid-way between Bruch's membrane and the chorioscleral interface was used to calculate the area of the choroidal vasculature. The choroidal vascular area was significantly lower in eyes with RPD compared to controls. En-face images showed narrow and sparse choroidal vessels in eyes with RPD.[25]
Polypoidal Choroidal Vasculopathy
Detection of branching vascular networks and polypoidal lesions using en-face optical coherence tomography - ophthalmoscopy
As early as 2007, Kameda demonstrated the utility of en-face OCT as a screening examination for PCV. Totally, 57 eyes with PCV were examined with en-face OCT at sequential depths, which identified polypoidal lesions in 84% and branching vascular networks in 53%. Polypoidal lesions appeared as characteristic small and round protrusions of the RPE, whereas branching vascular networks appeared as slight elevations of the RPE. Based on the RPE abnormalities detected on the en-face sections, further longitudinal scans could be performed to characterize further the lesions.[26]
Features of polypoidal lesions on en-face optical coherence tomography - ophthalmoscopy
Saito further characterized features of PCV using OCT-ophthalmoscope, which combined en-face OCT with SLO. A total of 50 eyes with PCV were examined. In each of the 22 eyes with serous or hemorrhagic PED, en-face images demonstrated the polypoidal lesions as irregularities or protrusions in the reflective line corresponding to the PED. In the remaining, 28 eyes without a PED, the polypoidal lesions appeared on en-face images as distinctive bright rings of a highly reflective RPE line in 26 of 28 eyes. Branching vascular network was revealed as a highly reflective geographic area on en-face imaging in 32 of 41 eyes. En-face OCT-ophthalmoscopy imaging can detect polypoidal lesions and branching vascular networks, which may be useful for noninvasive diagnostic imaging of PCV.[27]
Visualization of polypoidal lesions and dilated choroidal vascular networks on high penetration-optical coherence tomography
In a study of 19 eyes with PCV, HP SS OCT using 1060 nm wavelength for better penetration of the choroidal layer was able to detect polypoidal lesions and dilated choroidal vascular networks. En-face HP-OCT images showed 41 of 47 of the polypoidal lesions seen on ICG, appearing as RPE rings with inner reflectivity, with hyporeflective dots, which were presumed to be the sites at which the polyps entered Bruch's membrane. In addition, six polypoidal lesions detected on en-face HP-OCT were not visualized on ICG, which was postulated to be due to reduction in blood flow in polypoidal lesions after treatment with intravitreal injection of an anti-vascular endothelial growth factor and/or verteporfin photodynamic therapy. En-face HP-OCT images showed dilated choroidal vessels beneath 47% of the polypoidal lesions compared with 33% on ICG. The branching choroidal vascular network was visualized on HP-OCT as tortuous lines within hyper-reflective mesh-like configuration in 79% of eyes. The advantage of using en-face OCT in screening for PCV lies in its ability to view the extent of the entire lesion in a single image in a noninvasive manner.[28] Fig. 3 shows an en-face OCT image revealing a hyperreflective area of elevated RPE by the underlying branching vascular network, with abnormal choroidal vessels appearing as hyporeflective lines in-between.
Figure 3.
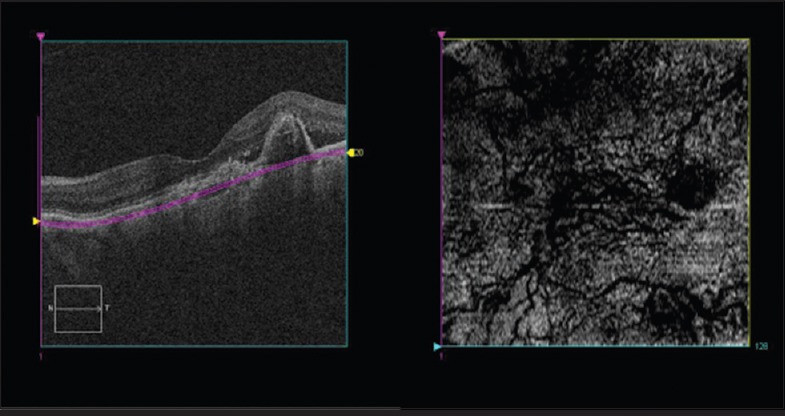
En-face image (right panel) of an eye with polypoidal choroidal vasculopathy showing abnormal vascular network just below the level of retinal pigment epithelium as shown on cross-sectional scan (left panel)
Conclusion
En-face OCT has opened up a whole spectrum of imaging capabilities for the diagnosis and management of the exudative macular disease. This review identified a number of publications that described the utility of en-face OCT in the management of AMD and PCV.
En-face OCT allows noninvasive, depth-resolved imaging of any layer within the retina and the choroid, without the need for intravenous contrast injection. Potential applications of en-face OCT include prediction of the extent of progression of GA and quantification of pathological structures such as RPD and CNV for disease monitoring.
Finally, the development of en-face Doppler OCT has demonstrated subtle changes in the choroidal vasculature in AMD, which has significantly advanced our understanding of its pathogenesis. En-face Doppler OCT can also detect the polypoidal lesions and branching vascular networks diagnostic of PCV. It may, therefore, give additional information for the diagnosis of PCV and other forms of exudative macular disease on top of those revealed by fluorescein and ICG.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Adhi M, Duker JS. Optical coherence tomography – Current and future applications. Curr Opin Ophthalmol. 2013;24:213–21. doi: 10.1097/ICU.0b013e32835f8bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grulkowski I, Liu JJ, Potsaid B, Jayaraman V, Lu CD, Jiang J, et al. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed Opt Express. 2012;3:2733–51. doi: 10.1364/BOE.3.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa RA, Navajas EV, Farah ME, Calucci D, Cardillo JA, Scott IU. Polypoidal choroidal vasculopathy: Angiographic characterization of the network vascular elements and a new treatment paradigm. Prog Retin Eye Res. 2005;24:560–86. doi: 10.1016/j.preteyeres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Yannuzzi LA, Negrão S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–34. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 5.van Velthoven ME, de Vos K, Verbraak FD, Pool CW, de Smet MD. Overlay of conventional angiographic and en-face OCT images enhances their interpretation. BMC Ophthalmol. 2005;5:12. doi: 10.1186/1471-2415-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stopa M, Bower BA, Davies E, Izatt JA, Toth CA. Correlation of pathologic features in spectral domain optical coherence tomography with conventional retinal studies. Retina. 2008;28:298–308. doi: 10.1097/IAE.0b013e3181567798. [DOI] [PubMed] [Google Scholar]
- 7.Rosen RB, Hathaway M, Rogers J, Pedro J, Garcia P, Dobre GM, et al. Simultaneous OCT/SLO/ICG imaging. Invest Ophthalmol Vis Sci. 2009;50:851–60. doi: 10.1167/iovs.08-1855. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi H, Yamashiro K, Tsujikawa A, Ota M, Otani A, Yoshimura N. Association between foveal photoreceptor integrity and visual outcome in neovascular age-related macular degeneration. Am J Ophthalmol. 2009;148:83–9.e1. doi: 10.1016/j.ajo.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Wanek J, Zelkha R, Lim JI, Shahidi M. Feasibility of a method for en face imaging of photoreceptor cell integrity. Am J Ophthalmol. 2011;152:807–14.e1. doi: 10.1016/j.ajo.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiernan DF, Zelkha R, Hariprasad SM, Lim JI, Blair MP, Mieler WF. En face spectral-domain optical coherence tomography outer retinal analysis and relation to visual acuity. Retina. 2012;32:1077–86. doi: 10.1097/IAE.0b013e31823c23bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holz FG, Bellman C, Staudt S, Schütt F, Völcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1051–6. [PubMed] [Google Scholar]
- 12.Nunes RP, Gregori G, Yehoshua Z, Stetson PF, Feuer W, Moshfeghi AA, et al. Predicting the progression of geographic atrophy in age-related macular degeneration with SD-OCT en face imaging of the outer retina. Ophthalmic Surg Lasers Imaging Retina. 2013;44:344–59. doi: 10.3928/23258160-20130715-06. [DOI] [PubMed] [Google Scholar]
- 13.Wolff B, Matet A, Vasseur V, Sahel JA, Mauget-Faÿsse M. En face OCT imaging for the diagnosis of outer retinal tubulations in age-related macular degeneration. J Ophthalmol 2012. 2012 doi: 10.1155/2012/542417. 542417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bruin DM, Burnes DL, Loewenstein J, Chen Y, Chang S, Chen TC, et al. In vivo three-dimensional imaging of neovascular age-related macular degeneration using optical frequency domain imaging at 1050 nm. Invest Ophthalmol Vis Sci. 2008;49:4545–52. doi: 10.1167/iovs.07-1553. [DOI] [PubMed] [Google Scholar]
- 15.Lumbroso B, Savastano MC, Rispoli M, Balestrazzi A, Savastano A, Balestrazzi E. Morphologic differences, according to etiology, in pigment epithelial detachments by means of en face optical coherence tomography. Retina. 2011;31:553–8. doi: 10.1097/IAE.0b013e3181eef3eb. [DOI] [PubMed] [Google Scholar]
- 16.Ho J, Witkin AJ, Liu J, Chen Y, Fujimoto JG, Schuman JS, et al. Documentation of intraretinal retinal pigment epithelium migration via high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2011;118:687–93. doi: 10.1016/j.ophtha.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coscas F, Coscas G, Querques G, Massamba N, Querques L, Bandello F, et al. En face enhanced depth imaging optical coherence tomography of fibrovascular pigment epithelium detachment. Invest Ophthalmol Vis Sci. 2012;53:4147–51. doi: 10.1167/iovs.12-9878. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Leng T, Zheng L, Kutzscher L, Ma J, de Sisternes L, et al. Automated drusen segmentation and quantification in SD-OCT images. Med Image Anal. 2013;17:1058–72. doi: 10.1016/j.media.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meadway A, Wang X, Curcio CA, Zhang Y. Microstructure of subretinal drusenoid deposits revealed by adaptive optics imaging. Biomed Opt Express. 2014;5:713–27. doi: 10.1364/BOE.5.000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohrab MA, Smith RT, Salehi-Had H, Sadda SR, Fawzi AA. Image registration and multimodal imaging of reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2011;52:5743–8. doi: 10.1167/iovs.10-6942. [DOI] [PubMed] [Google Scholar]
- 21.Sohrab M, Wu K, Fawzi AA. A pilot study of morphometric analysis of choroidal vasculature in vivo, using en face optical coherence tomography. PLoS One. 2012;7:e48631. doi: 10.1371/journal.pone.0048631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DY, Fingler J, Zawadzki RJ, Park SS, Morse LS, Schwartz DM, et al. Optical imaging of the chorioretinal vasculature in the living human eye. Proc Natl Acad Sci U S A. 2013;110:14354–9. doi: 10.1073/pnas.1307315110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong YJ, Miura M, Makita S, Ju MJ, Lee BH, Iwasaki T, et al. Noninvasive investigation of deep vascular pathologies of exudative macular diseases by high-penetration optical coherence angiography. Invest Ophthalmol Vis Sci. 2013;54:3621–31. doi: 10.1167/iovs.12-11184. [DOI] [PubMed] [Google Scholar]
- 24.Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121:1435–44. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueda-Arakawa N, Ooto S, Ellabban AA, Takahashi A, Oishi A, Tamura H, et al. Macular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomography. Am J Ophthalmol. 2014;157:994–1004. doi: 10.1016/j.ajo.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Kameda T, Tsujikawa A, Otani A, Sasahara M, Gotoh N, Tamura H, et al. Polypoidal choroidal vasculopathy examined with en face optical coherence tomography. Clin Experiment Ophthalmol. 2007;35:596–601. doi: 10.1111/j.1442-9071.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 27.Saito M, Iida T, Nagayama D. Cross-sectional and en face optical coherence tomographic features of polypoidal choroidal vasculopathy. Retina. 2008;28:459–64. doi: 10.1097/IAE.0b013e318156db60. [DOI] [PubMed] [Google Scholar]
- 28.Sayanagi K, Gomi F, Akiba M, Sawa M, Hara C, Nishida K. En-face high-penetration optical coherence tomography imaging in polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99:29–35. doi: 10.1136/bjophthalmol-2013-304658. [DOI] [PubMed] [Google Scholar]


