Abstract
Optical coherence tomography (OCT) has revolutionized imaging of ocular structures and various disease conditions. Though it has been used in the clinic for some decades, the OCT has only recently found its way into the operating theater. Early attempts at intraoperative OCT, hand-held and microscope mounted, have already improved our understanding of the surgical pathology and the role it might play in surgical decision-making. The microscope-integrated OCT now allows seamless, high-resolution, real-time imaging of surgical maneuvers from the incision to wound closure. Visualization of instruments and intraoperative tissue manipulation are possible with this in vivo modality and, therefore, help improve the outcome of surgery. In this article, we describe the advantages it offers during various vitreoretinal procedures.
Keywords: Microscope-integrated optical coherence tomography, RESCAN, spectral domain optical coherence tomography, vitreoretinal surgery
The inclusion of the optical coherence tomography (OCT) to ocular imaging techniques has dramatically changed the way we diagnose, manage, and follow-up retinal conditions. Although OCT as an imaging modality has been in use for long, its use during surgery was limited to intraoperative hand-held, externally mounted or microscope-mounted devices. The conventional OCT unit requires a compliant patient who can sit upright and hence difficult to use in patients who need to be examined in a supine position like infants or during surgery. Attempts have been made by modifying the Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) to convert the table top system into a hand-held device to obtain high resolution images of infants with aggressive posterior retinopathy of prematurity (ROP) without the need for general anesthesia.[1] A portable spectral domain OCT (SDOCT, Bioptigen Inc., Research Triangle Park, NC) unit with a hand-held probe is also available which allows imaging in the supine position.[2] It gives the clinician the option of using it during retinal surgeries but requires a steady operator. Obtaining reproducible images of similar areas of the macula can also be time-consuming and difficult. The surgical maneuvers need to be paused to acquire images. Therefore, to ensure sterility, image quality and reproducibility, and reduce image capture time, microscope mounted SDOCT devices have been described.[3,4] This gave the surgeon or assistant, albeit with a learning curve for assembly and imaging, the ability to move the device above the patient's eye using the microscope foot pedal.
Though these systems provide good visualization of the structural changes that occur during surgery, they are limited by the need to bring them into alignment with the microscope, and hence real-time visualization of surgical steps is not feasible with these systems. In addition, as these devices lack true co-axiality, it is difficult to focus on specific regions of interest. To overcome these limitations, several groups have been developing OCT technology capable of intrasurgical imaging of live maneuvers with some integrating an OCT scanner into an intraocular probe to image intraocular structures in model eyes.[5,6,7] A prototype microscope-integrated (MIOCT) device designed to enable simultaneous surgical viewing and high-resolution SDOCT imaging is based on folding of the optical OCT path into the full beam path of the operating microscope to enable high-resolution imaging.[8] This device has demonstrated high-resolution SDOCT images and videos during cadaveric porcine vitreoretinal surgical maneuvers, providing the first OCT characterization of vitreoretinal surgical instruments interacting with the retina.[9,10,11] The system shares a common focus with the surgical microscope, which allows for simple multimodal imaging of supine patients. In clinical use, there was a relatively rapid learning curve to familiarize the imaging team with the nuances of human intraoperative imaging, particularly the alignment and coordination required. Horizontal translations of the microscope along with external manipulations of the orientation of the eye were necessary to obtain images through the fovea of adequate resolution and intensity. The shared optical path of the MIOCT and surgical microscope enabled OCT imaging simultaneous with surgical manipulations.[12]
The RESCAN 700 (Carl Zeiss Meditec, Germany) is another MIOCT system that has been used clinically to visualize steps of surgeries “real-time” that could not be documented before.[13,14] It is an SDOCT that provides in vivo imaging and offers the surgeon a real-time cross-sectional view of the surgical field to help in instant decision-making. The system is based on the Lumera 700 (Carl Zeiss Meditec, Germany) platform and the RESIGHT lens system is used for posterior segment imaging. Salient features include Z-tracking and focus control for image stabilization with quality control. The OCT images are projected into the surgeon's operating field as a “heads up display” without interfering with the surgical procedure. The surgeon can select the scan length, angle, and location either using the foot pedal control or through the video monitor display system.
Clinical Applications of a Microscope- integrated Optical Coherence Tomography
Below, we describe some surgical steps that can be imaged using the MIOCT and its advantages:
Scleral wound
Unlike the hand-held OCT, the MIOCT has the capability to image the anatomical configuration of the scleral entry wound - uniplanar with a 25G trocar and biplanar with a 23G trocar. Wound apposition after removal of the trocar can also be confirmed [Fig. 1].
Figure 1.
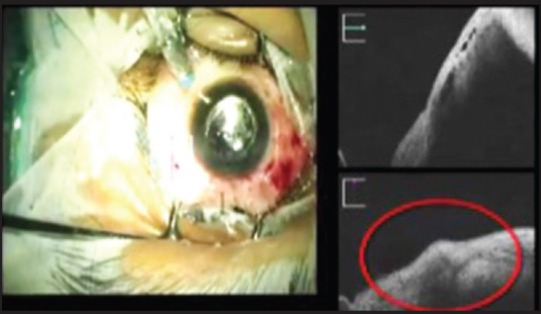
Sclerotomy wound closure with edematous edges (red circle) after removal of 23G trocar
Proliferative retinopathy
The MIOCT helps in initiating delamination or segmentation of membranes with improved precision, thereby reducing the risk of damage to the underlying tissues. In cases of diffuse and plastered tractional retinal detachment, the most crucial step is in identifying a safe plane to initiate membrane peeling which the MIOCT aids in [Fig. 2]. At the end of surgery, it helps determine if any residual traction exists in the form of secondary or tertiary membranes, as well as in determining the adequacy of membrane peeling [Fig. 3]. MIOCT is particularly useful in cases of vitreous hemorrhage to assess the macular status intra-operatively for the presence of membranes or macular edema since preoperative evaluation may not have been possible.
Figure 2.
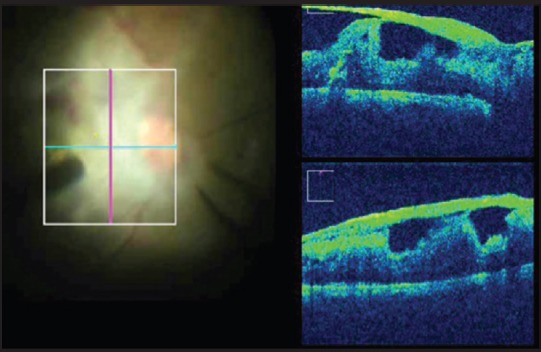
Broad based adhesions with bridging retinal tissue
Figure 3.
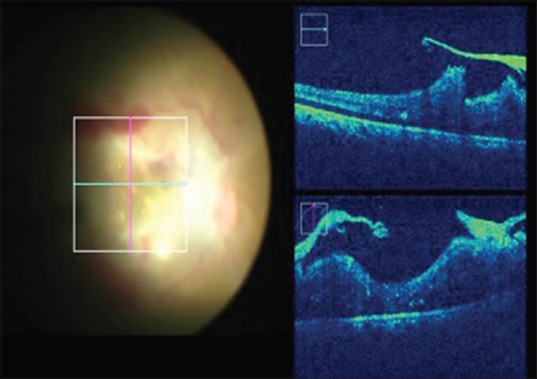
Assessing the adequacy of membrane peeling
Vitreomacular traction
The MIOCT helps the surgeon to assess the strength of vitreomacular adhesions, and this may, therefore, decrease the risk of de-roofing of the foveal cyst and inadvertent creation of a macular hole [Fig. 4]. It helps in the complete removal of the epiretinal membrane/internal limiting membrane (ILM) and prevents inadvertent grasping of the retina thereby avoiding iatrogenic retinal tears or breaks.
Figure 4.
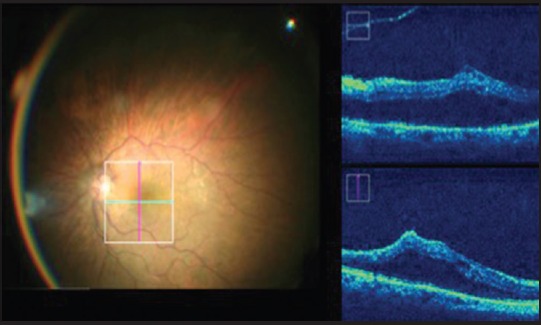
Vitreomacular traction extent can be assessed intraoperatively
Myopic foveoschisis
In myopic foveoschisis, there are multiple layers of vitreoschisis [Fig. 5] requiring vital dye staining to ensure that all the cortical vitreous has been removed. Multiple staining processes can be avoided by confirming intraoperatively that no posterior hyaloid has been left behind. Furthermore, in myopic eyes, due to a longer axial length and poor contrast of the stain, the MIOCT can help better assess the ILM. Since myopic retinae are thinner, iatrogenic breaks may be prevented by better visualization of the planes intraoperatively.
Figure 5.
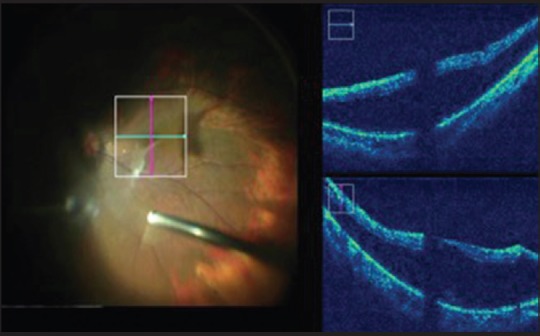
Myopic foveoschisis is imaged despite the longer axial length
Macular hole
In cases of the macular hole, while initiating the ILM peeling, surgeons are often concerned about inadvertent nerve fiber layer touch or breaks, which may lead to positive scotomas. This can be avoided with the MIOCT as it provides real-time imaging of the ILM peel. It is possible to do away with staining using vital dyes as the peeled ILM is well visualized as a scrolled membrane [Fig. 6]. In cases of failed macular hole surgeries, the edge of previously peeled ILM can be visualized. It is also possible to observe the opposition of edges of the macular hole after peeling the ILM. Cystoid macular edema with a lamellar macular hole can be well-demonstrated with the MIOCT [Fig. 7].
Figure 6.
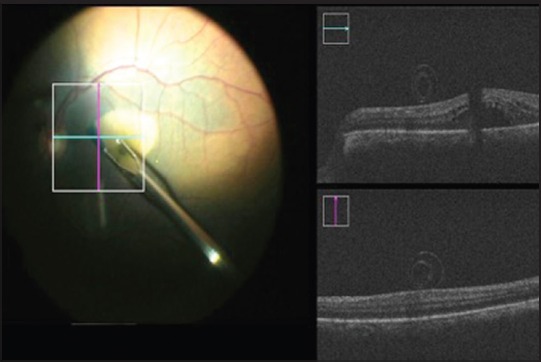
Peeled internal limiting membrane seen as a scroll providing a visual cue of completion
Figure 7.
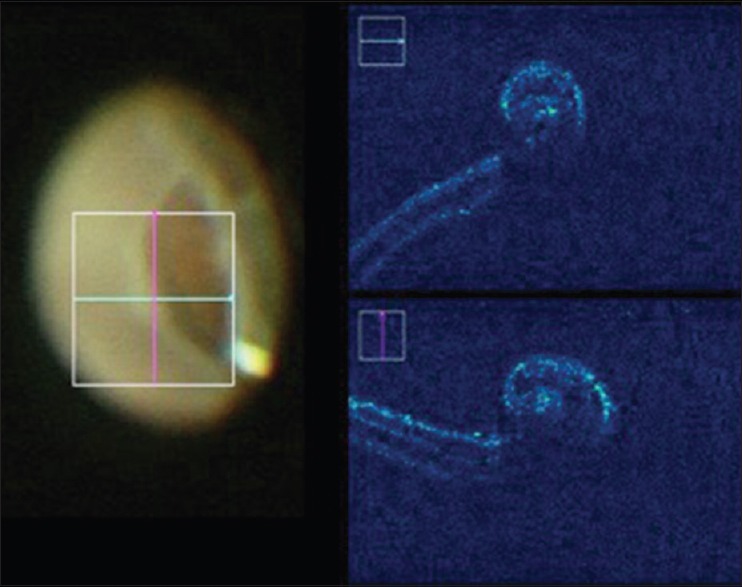
Rolled edges of the retinal break helps localization of the lesion during surgery
Retinal detachment
In cases of retinal detachment, MIOCT can assist during induction of posterior vitreous detachment, detection of any residual subretinal fluid at the posterior pole and completion of fluid air exchange. It can also detect the presence of proliferative vitreoretinopathy changes and rolled edges of retinal breaks [Fig. 8]. During pars plana lensectomy, since the anterior segments details are well-delineated on MIOCT, it is possible to monitor an intact anterior capsule [Fig. 9].
Figure 8.
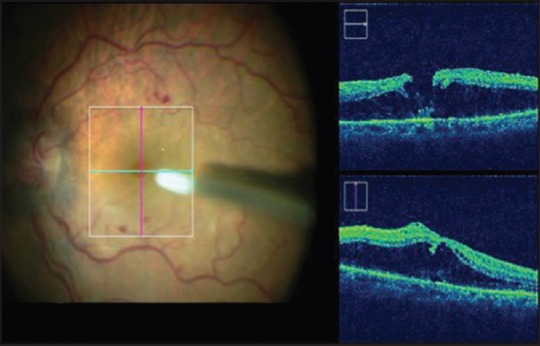
Cystoid macular edema with a lamellar macular hole
Figure 9.
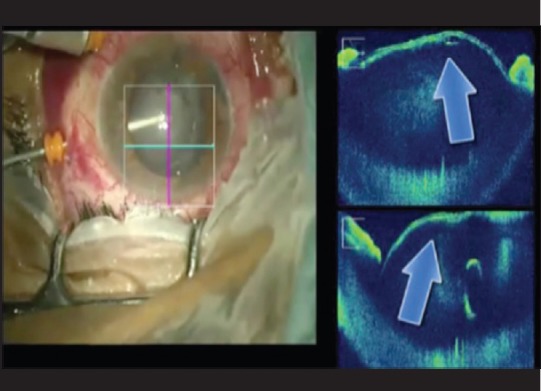
Monitoring the anterior capsule during pars plana lensectomy
Retinopathy of prematurity
The field of intra-operative OCT in ROP remains largely unexplored. Owing to the nature of the disease, it is likely to provide the surgeon the opportunity to assess the depth of the engaging preretinal membranes during lens sparing vitrectomy (LSV). With the MIOCT, the surgeon may be able to determine the extent of both horizontal and anteroposterior tractional elements, thereby allowing careful dissection or peeling while sparing the retinal elements. This could help in determining the appropriate plane for dissection and improving the chances of anatomical outcome in these cases. In addition, the use of MIOCT in ROP and other vascular retinopathies like familial exudative vitreoretinopathy may help image the flat neovascular fronds that often attend this pathology in its acute phase.[1] Especially, if LSV with endo laser is being considered, determining the location and extent of these neovascular fronds would be useful in preventing iatrogenic hemorrhage.
Some of the drawbacks of this system include:
Targeting the OCT field-of-view to the area of interest[15]
-
Visualization of the instrument and the underlying retina can be affected by the optical properties of surgical instruments which can cause obscuration due to back shadowing[9] [Fig. 10].
- Metallic instruments (e.g. forceps and needles) - high reflectivity with total shadowing below the instrument
- Polyamide material - moderate reflectivity with subtotal shadowing
- Silicone instrumentation - moderate reflectivity with minimal shadowing.
Figure 10.
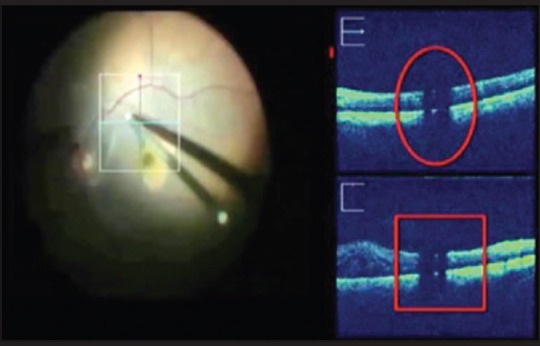
Obscuration of underlying tissue by instrument back shadowing is a limitation
With the evolution of the portability of the hand-held OCT technology from a dismantled hand-piece to integration into the assistant scope of the operating microscope, the terminology has evolved from intraoperative OCT to a microscope mounted OCT to an MIOCT. With the availability of a truly co-axial system that is integrated into the operating microscope in the MIOCT, it is finally possible to have a true intra-operative surgical assist. There is now potential to transform the OCT from a research tool into a surgical assistive device. Using this technology, we can achieve a greater understanding of the intraoperative retinal morphological changes by observing real-time instrument tissue interaction. This can help us better understand the variations in postoperative structural and functional results and, therefore, help prognosticate surgical outcomes more objectively.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Vinekar A, Sivakumar M, Shetty R, Mahendradas P, Krishnan N, Mallipatna A, et al. A novel technique using spectral-domain optical coherence tomography (Spectralis, SD-OCT + HRA) to image supine non-anaesthetized infants: Utility demonstrated in aggressive posterior retinopathy of prematurity. Eye (Lond) 2010;24:379–82. doi: 10.1038/eye.2009.313. [DOI] [PubMed] [Google Scholar]
- 2.Scott AW, Farsiu S, Enyedi LB, Wallace DK, Toth CA. Imaging the infant retina with a hand-held spectral-domain optical coherence tomography device. Am J Ophthalmol. 2009;147:364–373.e2. doi: 10.1016/j.ajo.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Ray R, Barañano DE, Fortun JA, Schwent BJ, Cribbs BE, Bergstrom CS, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011;118:2212–7. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31:1332–6. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 5.Hahn P, Migacz J, O’Connell R, Maldonado RS, Izatt JA, Toth CA. The use of optical coherence tomography in intraoperative ophthalmic imaging. Ophthalmic Surg Lasers Imaging. 2011;42 Suppl:S85–94. doi: 10.3928/15428877-20110627-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han S, Sarunic MV, Wu J, Humayun M, Yang C. Handheld forward-imaging needle endoscope for ophthalmic optical coherence tomography inspection. J Biomed Opt. 2008;13:020505. doi: 10.1117/1.2904664. [DOI] [PubMed] [Google Scholar]
- 7.Balicki M, Han JH, Iordachita I, Gehlbach P, Handa J, Taylor R, et al. Single fiber optical coherence tomography microsurgical instruments for computer and robot-assisted retinal surgery. Med Image Comput Comput Assist Interv. 2009;12:108–15. doi: 10.1007/978-3-642-04268-3_14. [DOI] [PubMed] [Google Scholar]
- 8.Tao YK, Ehlers JP, Toth CA, Izatt JA. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Opt Lett. 2010;35:3315–7. doi: 10.1364/OL.35.003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52:3153–9. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao YK, Ehlers JP, Toth CA, Izatt JA. Visualization of vitreoretinal surgical manipulations using intraoperative spectral domain optical coherence tomography. Proc SPIE. 2011;7889:78890F. [Google Scholar]
- 11.Hahn P, Migacz J, O’Connell R, Izatt JA, Toth CA. Unprocessed real-time imaging of vitreoretinal surgical maneuvers using a microscope-integrated spectral-domain optical coherence tomography system. Graefes Arch Clin Exp Ophthalmol. 2013;251:213–20. doi: 10.1007/s00417-012-2052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn P, Migacz J, O’Donnell R, Day S, Lee A, Lin P, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013;33:1328–37. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: Preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98:1329–32. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlers JP, Dupps WJ, Kaiser PK, Goshe J, Singh RP, Petkovsek D, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am J Ophthalmol. 2014;158:999–1007. doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers JP, Tao YK, Srivastava SK. The value of intraoperative optical coherence tomography imaging in vitreoretinal surgery. Curr Opin Ophthalmol. 2014;25:221–7. doi: 10.1097/ICU.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]


