Abstract
ExPress glaucoma filtration device (GFD) has recently become available in India as a surgical option for glaucoma patients. We retrospectively evaluated the outcome of ExPress GFD in 12 eyes with advanced glaucoma with intraocular pressures (IOPs) not controlled on maximal tolerable medical therapy. The mean preoperative IOP of 29.58 ± 7.13 mmHg decreased to 17.0 ± 2.67 and 17.40 ± 0.89 mmHg at 6 and 12 months after surgery. Absolute success (IOP ≤ 18 mmHg, with no additional glaucoma medications) was achieved in eight cases (66.7%) and qualified success (IOP ≤ 18 mmHg, with additional glaucoma medications) in two cases (16.7%) at 1-year after surgery. Early intervention was needed in 4 patients; two underwent anterior chamber reformation while the other two required needling. Two patients required resurgery. There was no significant change in the best corrected visual acuity postoperatively (P = 0.37). ExPress GFD does not seem to offer a benefit over standard trabeculectomy in patients with advanced glaucomatous disease in terms of IOP control or complication rate. However, due to the small sample size with a heterogeneous mixture of primary and secondary glaucoma's, we await further studies with a larger sample size and long-term follow-up, to see how the device performs.
Keywords: Advanced glaucoma, ExPress shunt, trabeculectomy and ExPress shunt
Trabeculectomy is the most commonly performed incisional procedure for intraocular pressure (IOP) reduction in glaucoma patients. Short-term complications after trabeculectomy include anterior chamber (AC) shallowing, hypotony, and choroidal detachment. Long-term complications include bleb leaks, blebitis/endophthalmitis, overhanging blebs, and bleb failure. These potential complications threaten vision thereby demonstrating the need for safer surgical procedures to manage glaucoma.
The Ex-PRESS glaucoma filtration device (GFD) (Alcon Laboratories, Fort Worth, TX, USA) was developed as an alternative to trabeculectomy. It is a miniature stainless steel device, introduced to offer a simple and safer alternative to the classic trabeculectomy with the proposed advantages of inducing minimal inflammation (no iridectomy required), decreasing early postoperative complications and a reduced requirement for postoperative hypotensive medications.[1] There are however, no reports on its efficacy in patients from the Indian sub-continent.
Material and Methods
Our study is a retrospective, noncomparative case series, including a total of 12 patients with advanced glaucomatous optic neuropathy and visual field changes (Hoddap-Parrish-Anderson criteria);[2] not controlled on maximal tolerable medical therapy after obtaining an informed written consent. Express GFD, Model P-50 (Alcon Laboratories, Inc., Fort Worth, TX, USA) was implanted in all patients by the same surgeon (TD).
Surgical technique
A conjunctival and a scleral flap (5 mm × 5 mm rectangular flap) were created as for a standard fornix based trabeculectomy. As the scleral flap was lifted, care was taken to identify the center of the “blue line” adjacent to the clear cornea, which corresponds to the location of the trabecular meshwork. A 26G needle was inserted into the AC through the center of the “blue line” at an angle parallel to the iris plane, and the preloaded shunt placed in the AC through the needle track using the inserter [Fig. 1]. The scleral flap was sutured with 10-0 nylon. One to three sutures were typically required depending on the flow, which was tested by inflating the AC with a balanced salt solution with a 27G or 30G canula through the temporal paracentesis. The conjunctival flap was closed with 8-0 polyglactin (Vicryl).
Figure 1.
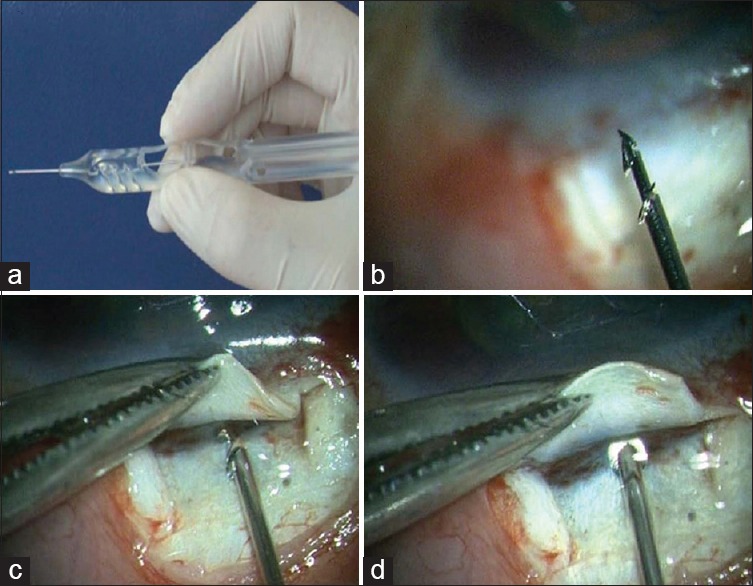
(a) The ExPress delivery system (EDS) - A specially designed preloaded disposable introducer for the ExPress mini glaucoma shunt. (b) High magnification view of the tip of the EDS showing the shunt. (c and d) The shunt is inserted into the anterior chamber through the center of the “blue line” at an angle parallel to the iris plane via a preplaced track made with 26G need
Clinical outcome assessment included Goldmann applanation tonometry, number of drugs required to attain IOP control, any associated complications and visual acuity. Data were available for follow-up at 3 months, 6 months, and 12 months postoperatively. Criteria for success were defined as follows: Absolute success – IOP ≤18 mmHg without any medication, qualified success – IOP ≤18 mmHg with ocular hypotensive medications.
Statistical analysis
Statistical analysis was performed using SPSS 15.0 (IBM® SPSS® Statistics Version 15). Eyes were taken as individual units of analysis in our study. Data are presented in mean ± standard deviation and frequency percentage. Paired t-test was used for IOP. To compare visual acuity and number of medications used, Wilcoxon signed-rank test was used. A P < 0.05 was considered statistically significant.
Results
The mean age of the patients was 55.75 ± 17.10 years (range 15–80 years), and there were six males and six females. The diagnosis varied, 4 patients had primary angle closure glaucoma, five had primary open-angle glaucoma (POAG); and 1 patient each was diagnosed as juvenile open-angle glaucoma, uveitic glaucoma, and neovascular glaucoma (NVG) (post-Avastin and panretinal photocoagulation). Eight of these patients were pseudophakic.
The mean preoperative IOP was 29.92 ± 6.78 mmHg (range 24–42) on an average of 3.25 ± 0.45 (range 3–4) anti-glaucoma drugs. This decreased to 17.54 ± 8.66 (range 12–32); 17.0 ± 2.67 (range 15–24); and 17.50 ± 0.85 (range 16–18) mmHg at 3, 6, and 12 months respectively, after surgery. The mean postoperative medication at last follow-up was 0.40 ± 0.84 drugs (range 0–2); P = 0.04 [Table 1]. Absolute success was noted in 8/12 (66.7%) eyes and qualified success in 2/12 eyes (16.7%), at 1-year after surgery.
Table 1.
Demographics with pre- and post-operative details
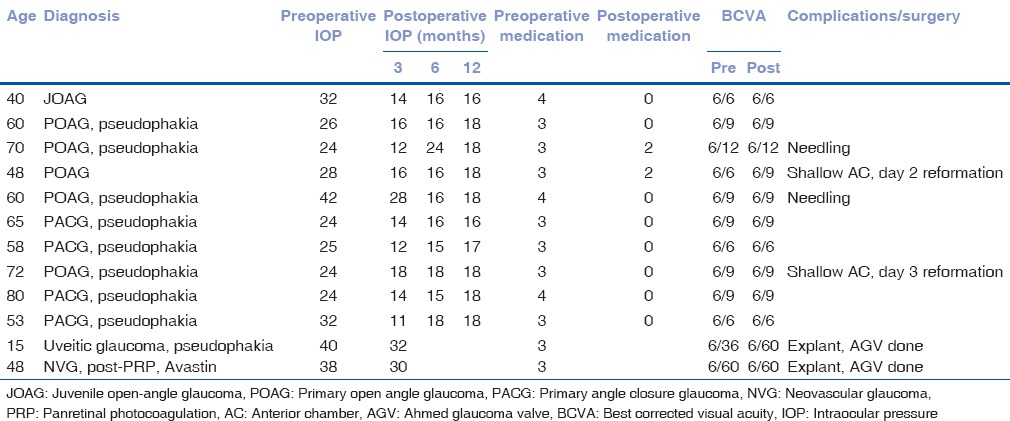
Best-corrected visual acuity (BCVA) was tested using Snellen chart, which was converted to LogMAR for statistical analysis and showed no significant change in the BCVA. Preoperative BCVA was 0.21 ± 0.28 (range 0.0–1.0) and postoperative BCVA was 0.23 ± 0.27 (range 0.0–1.0); P = 0.37.
Complications noted were shallow AC in 2 patients (16.7%) on postoperative day 2 and 3 respectively, for which AC reformation was done.
In two of our cases, with uveitic glaucoma and NVG, there was an early failure with fibrinous reaction and recurrent hyphema. In the uveitic patient, implant corneal touch was noted (8.3%) and in the NVG patient, implant iris touch was noted (8.3%). In both these patients, the implant was removed, and an Ahmed glaucoma valve (AGV) inserted to control the IOP. Two patients (16.7%) required needling with 5-flourouracil at 6–8 weeks postoperatively.
Discussion
The ExPress GFD is a new method for standardizing trabeculectomy with outcomes quite similar to trabeculectomy reported in the literature, which has gained popularity in the Caucasian population with over 70,000 implants worldwide. However, it is an expensive alternative to trabeculectomy, and there are no reports on its efficacy in our population.
De Jong et al. reported a success rate of 81.8% (IOP ≤18 mmHg) at 1-year and 66.7% at 3 years follow-up, in POAG patients.[3] Salim et al. reported a surgical success (IOP ≤18 mmHg) of 80.0% in the African American group and 83.3% in the white group with the device in POAG patients.[4] Hirooka et al. reported surgical success in 94.8% of 231 eyes at the end of 3 years follow-up.[5] Other authors have reported results quite similar to trabeculectomy in terms of IOP control.[6,7] Maris et al. reported a success rate of 90.0% (IOP <21 mmHg) at an average follow-up of 10.8 ± 3.1 months.[8] In our study, we achieved an absolute (IOP ≤18 mmHg) and qualified success of IOP control in 8/12 (66.7%) and 2/12 (83.3%) eyes at last follow-up, that is, at 12 months.
Hypotony related complications can occur after the implant if suturing is not adequate, as the implant alone will not prevent this complication as there is a free flow through it. In our patients, hypotony with shallow AC was noted in 2 patients in the early postoperative period, which did not resolve on medical management and required an AC re-formation. Various authors have reported low rates of hypotony with the ExPress GFD, ranging from as low as 1–4%[4,8,9] to 15–47.2%.[7,9] Hirooka et al. reported that all instances of hypotony during the early postoperative period resolved spontaneously, with no eyes developing flat AC with lens-cornea touch.[5]
Other complications noted by de Jong et al. were choroidal effusion in 7.5%, shallow AC in 20%, and bleb leak in 2.5%.[3] Maris et al. reported choroidal effusion in 8 patients, shallow AC in 2 patients, hypotonous maculopathy in 4 patients, hyphema in 4 patients, bleb leak in 6 patients, and endophthalmitis in 2 patients.[8] Seider et al. noted choroidal effusion in 33.3% and bleb leak in 30.5%.[7] Marzette and Herndon reported choroidal effusion in 4 patients, shallow AC in 5 patients, and hypotonous maculopathy in 3 patients.[9] The most common complication noted by Hirooka et al. was tube blockage, early hypotony and bleb leak.[5] In our case series, implant corneal touch was noted in 1 patient (8.3%) and implant iris touch in 1 patient (8.3%). In both these patients, the implant was removed, and an AGV inserted to control the IOP. De Jong also reported, the removal of an ExPress shunt due to malposition of the shunt into the iris.[10]
Our results of ExPress, GFD in eyes in Indian patients with the advanced glaucomatous disease are not encouraging. This may be attributed to several reasons: A higher fibrotic response in our eyes which were on maximal medical therapy, especially the ones with secondary glaucoma. Furthermore, the learning curves maybe a factor in the initial few cases. In addition, drainage achieved with a 50 um ostium may be inadequate for such eyes requiring a target IOP in the low teens and may promote an earlier wound healing. However, none of the patients in our study could achieve IOP in low teens. An implant with a 200 um ostium (not available in India) may have produced a better outcome. Finally, our sample size is small with a heterogeneous mixture of primary and secondary glaucoma's and, therefore, these results require further validation in a larger sample size.
The limitations of our study are that it is retrospective with a small, heterogeneous sample size, and lack of a control group. Comparison of different types of glaucoma with different success rates for filtering procedures is ideally not appropriate. However, the sample size of this study was limited by the cost of the device, which many patients could not afford. We therefore, recommend a prospective randomized controlled trial with a larger sample size in future.
We propose that there is no merit of currently replacing the standard of care for glaucoma surgery that is, trabeculectomy with the ExPress implant as this steeply raises the cost of surgery without offering significant benefits in terms of efficacy and safety. In addition, it unduly raises the expectation of patients who feel that getting an expensive implant may give a better outcome as compared to a standard trabeculectomy surgery.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Dahan E, Ben Simon GJ, Lafuma A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: A prospective, randomised study. Eye (Lond) 2012;26:703–10. doi: 10.1038/eye.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodapp E, Parrish RK, 2nd, Anderson DR. St Louis: The CV Mosby Co; 1993. Clinical Decisions in Glaucoma; pp. 52–61. [Google Scholar]
- 3.de Jong L, Lafuma A, Aguadé AS, Berdeaux G. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in primary open-angle glaucoma. Clin Ophthalmol. 2011;5:527–33. doi: 10.2147/OPTH.S18565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salim S, Du H, Boonyaleephan S, Wan J. Surgical outcomes of the Ex-PRESS glaucoma filtration device in African American and white glaucoma patients. Clin Ophthalmol. 2012;6:955–62. doi: 10.2147/OPTH.S32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanner EM, Netland PA, Sarkisian SR, Jr, Du H. Ex-PRESS miniature glaucoma device implanted under a scleral flap alone or combined with phacoemulsification cataract surgery. J Glaucoma. 2009;18:488–91. doi: 10.1097/IJG.0b013e31818fb44e. [DOI] [PubMed] [Google Scholar]
- 6.Hendrick AM, Kahook MY. Ex-PRESS mini glaucoma shunt: Surgical technique and review of clinical experience. Expert Rev Med Devices. 2008;5:673–7. doi: 10.1586/17434440.5.6.673. [DOI] [PubMed] [Google Scholar]
- 7.Seider MI, Rofagha S, Lin SC, Stamper RL. Resident-performed Ex-PRESS shunt implantation versus trabeculectomy. J Glaucoma. 2012;21:469–74. doi: 10.1097/IJG.0b013e3182182bfb. [DOI] [PubMed] [Google Scholar]
- 8.Maris PJ, Jr, Ishida K, Netland PA. Comparison of trabeculectomy with Ex-PRESS miniature glaucoma device implanted under scleral flap. J Glaucoma. 2007;16:14–9. doi: 10.1097/01.ijg.0000243479.90403.cd. [DOI] [PubMed] [Google Scholar]
- 9.Marzette L, Herndon LW. A comparison of the Ex-PRESS™ mini glaucoma shunt with standard trabeculectomy in the surgical treatment of glaucoma. Ophthalmic Surg Lasers Imaging. 2011;42:453–9. doi: 10.3928/15428877-20111017-03. [DOI] [PubMed] [Google Scholar]
- 10.de Jong LA. The Ex-PRESS glaucoma shunt versus trabeculectomy in open-angle glaucoma: A prospective randomized study. Adv Ther. 2009;26:336–45. doi: 10.1007/s12325-009-0017-6. [DOI] [PubMed] [Google Scholar]


