Abstract
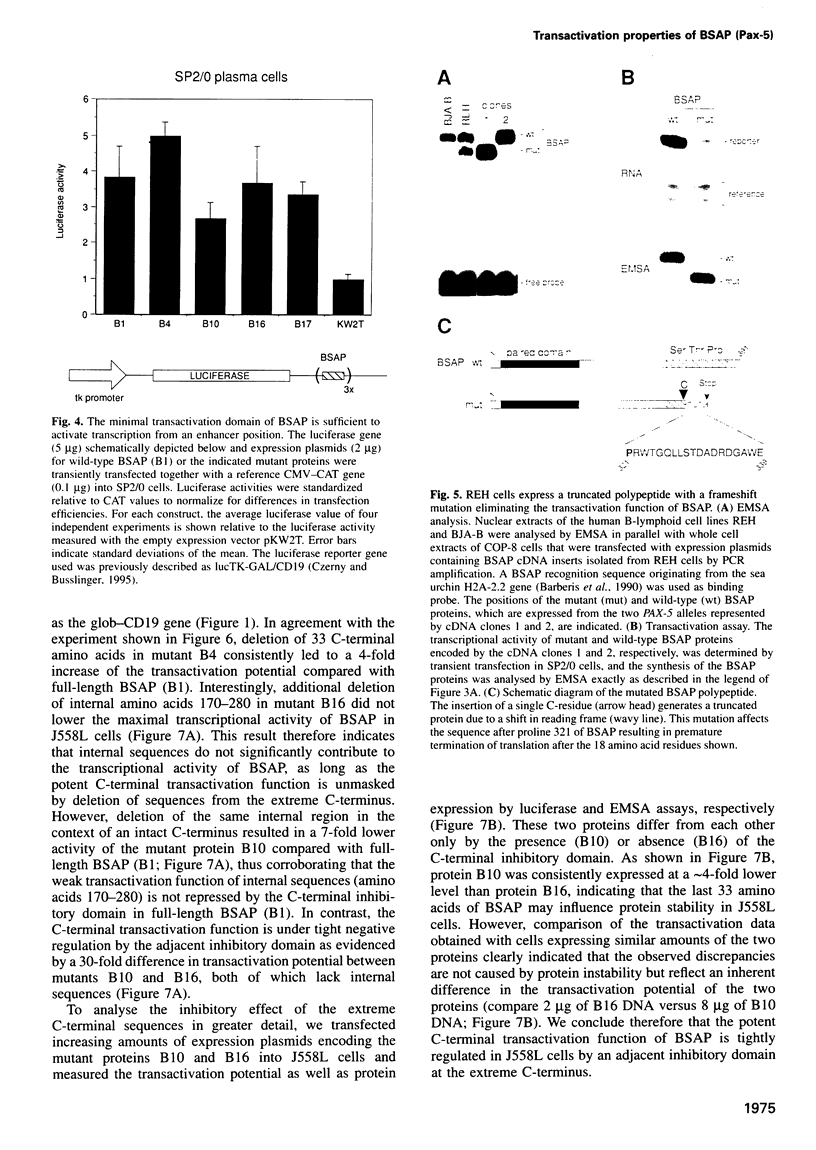
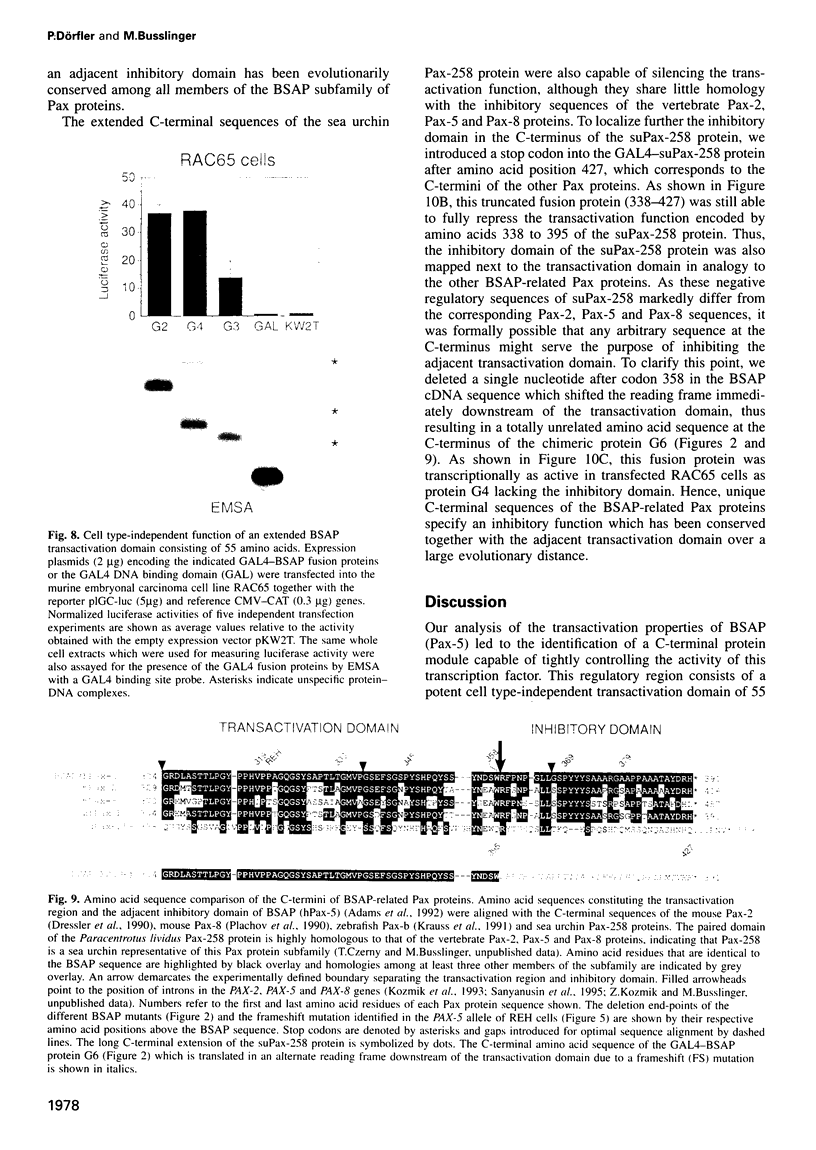
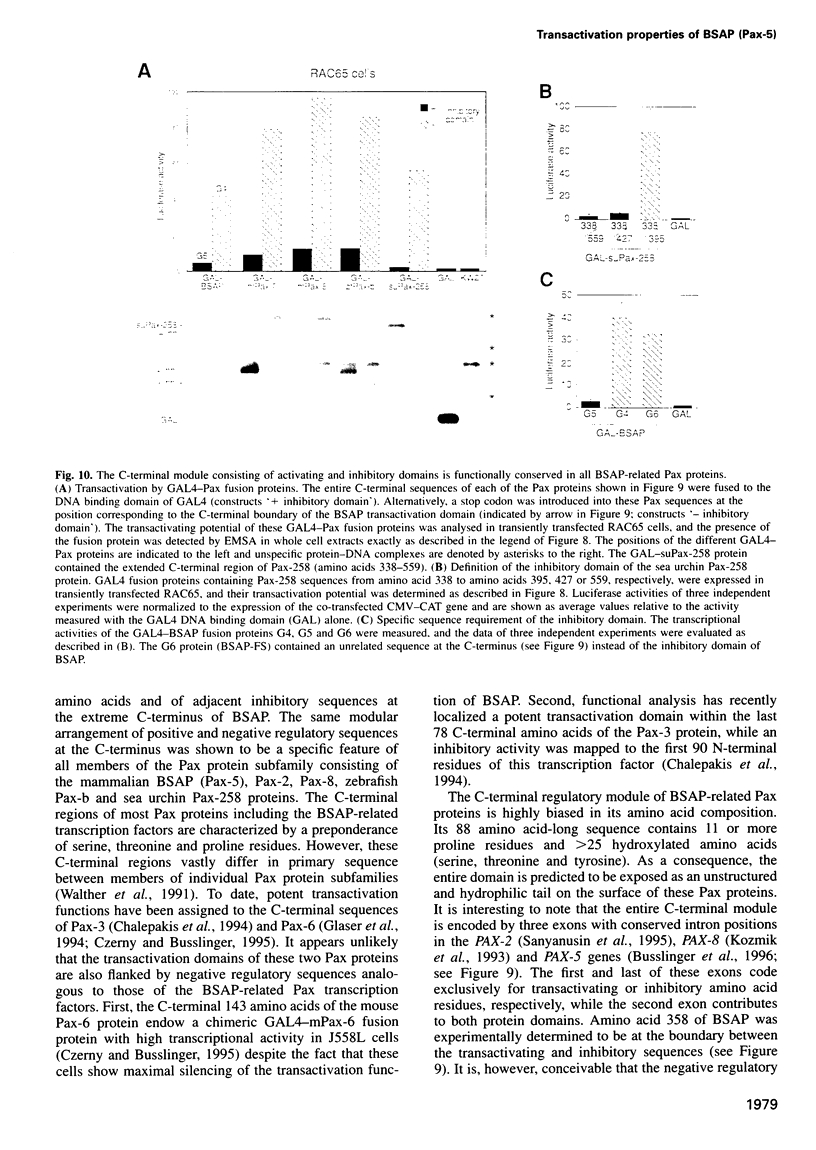
Pax-5 encodes the transcription factor BSAP which plays an essential role in early B cell development and midbrain patterning. In this study we have analysed the structural requirements for transcriptional activation by BSAP. In vitro mutagenesis and transient transfection experiments indicate that the C-terminal serine/threonine/proline-rich region of BSAP contains a potent transactivation domain of 55 amino acids which is active from promoter and enhancer positions. This transactivation domain was found to be inactivated by a naturally occurring frameshift mutation in one PAX-5 allele of the acute lymphoblastic leukemia cell line REH. The function of the transactivation domain is negatively regulated by adjacent sequences from the extreme C-terminus. The activating and inhibitory domains function together as an independent regulatory module in different cell types as shown by fusion to the GAL4 DNA binding domain. The same arrangement of positively and negatively acting sequences has been conserved in the mammalian Pax-2 and Pax-8, the zebrafish Pax-b as well as the sea urchin Pax-258 proteins. These data demonstrate that the transcriptional competence of a subfamily of Pax proteins is determined by a C-terminal regulatory module composed of activating and inhibitory sequences.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B., Dörfler P., Aguzzi A., Kozmik Z., Urbánek P., Maurer-Fogy I., Busslinger M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992 Sep;6(9):1589–1607. doi: 10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Park A., Tjian R. The cell-type-specific activator region of c-Jun juxtaposes constitutive and negatively regulated domains. Genes Dev. 1992 Aug;6(8):1493–1502. doi: 10.1101/gad.6.8.1493. [DOI] [PubMed] [Google Scholar]
- Barberis A., Superti-Furga G., Vitelli L., Kemler I., Busslinger M. Developmental and tissue-specific regulation of a novel transcription factor of the sea urchin. Genes Dev. 1989 May;3(5):663–675. doi: 10.1101/gad.3.5.663. [DOI] [PubMed] [Google Scholar]
- Barberis A., Widenhorn K., Vitelli L., Busslinger M. A novel B-cell lineage-specific transcription factor present at early but not late stages of differentiation. Genes Dev. 1990 May;4(5):849–859. doi: 10.1101/gad.4.5.849. [DOI] [PubMed] [Google Scholar]
- Bergers G., Graninger P., Braselmann S., Wrighton C., Busslinger M. Transcriptional activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the first intron. Mol Cell Biol. 1995 Jul;15(7):3748–3758. doi: 10.1128/mcb.15.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp D., Jamet E., Baumgartner S., Burri M., Noll M. Isolation of two tissue-specific Drosophila paired box genes, Pox meso and Pox neuro. EMBO J. 1989 Nov;8(11):3447–3457. doi: 10.1002/j.1460-2075.1989.tb08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. J., Sutherland J. A., Cook A., Bannister A. J., Kouzarides T. An inhibitor domain in c-Fos regulates activation domains containing the HOB1 motif. EMBO J. 1995 Jan 3;14(1):124–131. doi: 10.1002/j.1460-2075.1995.tb06982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Urbánek P. The role of BSAP (Pax-5) in B-cell development. Curr Opin Genet Dev. 1995 Oct;5(5):595–601. doi: 10.1016/0959-437x(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Chalepakis G., Fritsch R., Fickenscher H., Deutsch U., Goulding M., Gruss P. The molecular basis of the undulated/Pax-1 mutation. Cell. 1991 Sep 6;66(5):873–884. doi: 10.1016/0092-8674(91)90434-z. [DOI] [PubMed] [Google Scholar]
- Chalepakis G., Jones F. S., Edelman G. M., Gruss P. Pax-3 contains domains for transcription activation and transcription inhibition. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12745–12749. doi: 10.1073/pnas.91.26.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny T., Busslinger M. DNA-binding and transactivation properties of Pax-6: three amino acids in the paired domain are responsible for the different sequence recognition of Pax-6 and BSAP (Pax-5). Mol Cell Biol. 1995 May;15(5):2858–2871. doi: 10.1128/mcb.15.5.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny T., Schaffner G., Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993 Oct;7(10):2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- Dressler G. R., Deutsch U., Chowdhury K., Nornes H. O., Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990 Aug;109(4):787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Dubendorff J. W., Whittaker L. J., Eltman J. T., Lipsick J. S. Carboxy-terminal elements of c-Myb negatively regulate transcriptional activation in cis and in trans. Genes Dev. 1992 Dec;6(12B):2524–2535. doi: 10.1101/gad.6.12b.2524. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fickenscher H. R., Chalepakis G., Gruss P. Murine Pax-2 protein is a sequence-specific trans-activator with expression in the genital system. DNA Cell Biol. 1993 Jun;12(5):381–391. doi: 10.1089/dna.1993.12.381. [DOI] [PubMed] [Google Scholar]
- Glaser T., Jepeal L., Edwards J. G., Young S. R., Favor J., Maas R. L. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994 Aug;7(4):463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., Hamano T., Asofsky R., Herber-Katz E., Hedrick S., Schwartz R. H., Paul W. E. I region-restricted antigen presentation by B cell-B lymphoma hybridomas. Nature. 1982 Jul 15;298(5871):283–284. doi: 10.1038/298283a0. [DOI] [PubMed] [Google Scholar]
- Jayaraman P. S., Hirst K., Goding C. R. The activation domain of a basic helix-loop-helix protein is masked by repressor interaction with domains distinct from that required for transcription regulation. EMBO J. 1994 May 1;13(9):2192–2199. doi: 10.1002/j.1460-2075.1994.tb06496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowenz-Leutz E., Twamley G., Ansieau S., Leutz A. Novel mechanism of C/EBP beta (NF-M) transcriptional control: activation through derepression. Genes Dev. 1994 Nov 15;8(22):2781–2791. doi: 10.1101/gad.8.22.2781. [DOI] [PubMed] [Google Scholar]
- Kozmik Z., Kurzbauer R., Dörfler P., Busslinger M. Alternative splicing of Pax-8 gene transcripts is developmentally regulated and generates isoforms with different transactivation properties. Mol Cell Biol. 1993 Oct;13(10):6024–6035. doi: 10.1128/mcb.13.10.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z., Wang S., Dörfler P., Adams B., Busslinger M. The promoter of the CD19 gene is a target for the B-cell-specific transcription factor BSAP. Mol Cell Biol. 1992 Jun;12(6):2662–2672. doi: 10.1128/mcb.12.6.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S., Johansen T., Korzh V., Fjose A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development. 1991 Dec;113(4):1193–1206. doi: 10.1242/dev.113.4.1193. [DOI] [PubMed] [Google Scholar]
- Murata Y., Kim H. G., Rogers K. T., Udvadia A. J., Horowitz J. M. Negative regulation of Sp1 trans-activation is correlated with the binding of cellular proteins to the amino terminus of the Sp1 trans-activation domain. J Biol Chem. 1994 Aug 12;269(32):20674–20681. [PubMed] [Google Scholar]
- Nerlov C., Ziff E. B. Three levels of functional interaction determine the activity of CCAAT/enhancer binding protein-alpha on the serum albumin promoter. Genes Dev. 1994 Feb 1;8(3):350–362. doi: 10.1101/gad.8.3.350. [DOI] [PubMed] [Google Scholar]
- Neurath M. F., Strober W., Wakatsuki Y. The murine Ig 3' alpha enhancer is a target site with repressor function for the B cell lineage-specific transcription factor BSAP (NF-HB, S alpha-BP). J Immunol. 1994 Jul 15;153(2):730–742. [PubMed] [Google Scholar]
- Noll M. Evolution and role of Pax genes. Curr Opin Genet Dev. 1993 Aug;3(4):595–605. doi: 10.1016/0959-437x(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Plachov D., Chowdhury K., Walther C., Simon D., Guenet J. L., Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990 Oct;110(2):643–651. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- Pratt M. A., Kralova J., McBurney M. W. A dominant negative mutation of the alpha retinoic acid receptor gene in a retinoic acid-nonresponsive embryonal carcinoma cell. Mol Cell Biol. 1990 Dec;10(12):6445–6453. doi: 10.1128/mcb.10.12.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
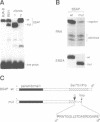
- Rosenfeld C., Goutner A., Choquet C., Venuat A. M., Kayibanda B., Pico J. L., Greaves M. F. Phenotypic characterisation of a unique non-T, non-B acute lymphoblastic leukaemia cell line. Nature. 1977 Jun 30;267(5614):841–843. doi: 10.1038/267841a0. [DOI] [PubMed] [Google Scholar]
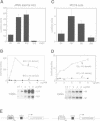
- Rothman P., Li S. C., Gorham B., Glimcher L., Alt F., Boothby M. Identification of a conserved lipopolysaccharide-plus-interleukin-4-responsive element located at the promoter of germ line epsilon transcripts. Mol Cell Biol. 1991 Nov;11(11):5551–5561. doi: 10.1128/mcb.11.11.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyanusin P., Schimmenti L. A., McNoe L. A., Ward T. A., Pierpont M. E., Sullivan M. J., Dobyns W. B., Eccles M. R. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet. 1995 Apr;9(4):358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- Schäfer B. W., Czerny T., Bernasconi M., Genini M., Busslinger M. Molecular cloning and characterization of a human PAX-7 cDNA expressed in normal and neoplastic myocytes. Nucleic Acids Res. 1994 Nov 11;22(22):4574–4582. doi: 10.1093/nar/22.22.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seipel K., Georgiev O., Schaffner W. Different activation domains stimulate transcription from remote ('enhancer') and proximal ('promoter') positions. EMBO J. 1992 Dec;11(13):4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Kroeger P. E., Morimoto R. I. The carboxyl-terminal transactivation domain of heat shock factor 1 is negatively regulated and stress responsive. Mol Cell Biol. 1995 Aug;15(8):4309–4318. doi: 10.1128/mcb.15.8.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Birshtein B. K. NF-HB (BSAP) is a repressor of the murine immunoglobulin heavy-chain 3' alpha enhancer at early stages of B-cell differentiation. Mol Cell Biol. 1993 Jun;13(6):3611–3622. doi: 10.1128/mcb.13.6.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D. L., Chalepakis G., Gruss P., Joyner A. L. Two Pax-binding sites are required for early embryonic brain expression of an Engrailed-2 transgene. Development. 1996 Feb;122(2):627–635. doi: 10.1242/dev.122.2.627. [DOI] [PubMed] [Google Scholar]
- Stuart E. T., Kioussi C., Gruss P. Mammalian Pax genes. Annu Rev Genet. 1994;28:219–236. doi: 10.1146/annurev.ge.28.120194.001251. [DOI] [PubMed] [Google Scholar]
- Treisman J., Harris E., Desplan C. The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 1991 Apr;5(4):594–604. doi: 10.1101/gad.5.4.594. [DOI] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbánek P., Wang Z. Q., Fetka I., Wagner E. F., Busslinger M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 1994 Dec 2;79(5):901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Vitelli L., Kemler I., Lauber B., Birnstiel M. L., Busslinger M. Developmental regulation of micro-injected histone genes in sea urchin embryos. Dev Biol. 1988 May;127(1):54–63. doi: 10.1016/0012-1606(88)90188-1. [DOI] [PubMed] [Google Scholar]
- Walther C., Guenet J. L., Simon D., Deutsch U., Jostes B., Goulding M. D., Plachov D., Balling R., Gruss P. Pax: a murine multigene family of paired box-containing genes. Genomics. 1991 Oct;11(2):424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- Westin G., Gerster T., Müller M. M., Schaffner G., Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987 Sep 11;15(17):6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannini M., Francis-Lang H., Plachov D., Di Lauro R. Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol. 1992 Sep;12(9):4230–4241. doi: 10.1128/mcb.12.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Rungger D., Voellmy R. Multiple layers of regulation of human heat shock transcription factor 1. Mol Cell Biol. 1995 Aug;15(8):4319–4330. doi: 10.1128/mcb.15.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]