Abstract
Harmonisation of regulations in the European Union and the European Economic Area, as of January 1, 2012, has led to an increase in the number of rescue dogs imported to Norway from Eastern European countries, in particular Romania. Today the only requirements for dogs entering Norway are rabies vaccination and prophylactic Echinococcus multilocularis treatment. The aim of this study was to investigate the antibody levels to rabies virus in vaccinated rescue dogs and to examine if the dogs had sufficient antibody response according to the recommended titre ≥0.5 IU/ml by the World Organisation for Animal Health (OIE). A significant proportion (53%, 95% CI (41% to 65%)) of imported rescue dogs from Eastern Europe were found to have inadequate titres after rabies vaccination. Moreover, 41 per cent of the dogs had antibody levels below or equal to 0.2 IU/ml, and among these, 14 dogs had titres ≤0.1 IU/ml, which is considered negative in the fluorescent antibody virus neutralisation assay. This study indicates that the present regulation increases the risk of introducing rabies from member states where rabies is still prevalent to countries considered free from rabies.
Keywords: Rabies, Dogs, Vaccines
Introduction
The transport of companion animals across borders provides a real threat for the spread and introduction of various infectious pathogens, including rabies virus. The European Union (EU) implemented a harmonised pet movement policy for non-commercial movement of dogs, cats and ferrets under EU regulation 998/2003 of the European Community (EU 2003). As member of the European Economic Area, Norway also follows this regulation. It states that (i) all animals should be identified by tattoo and/or microchip, (ii) be accompanied by a passport issued by a veterinarian authorised by the competent authority certifying valid anti-rabies vaccination, and (iii) a 21 day waiting period in case of primary vaccination. Until January 1, 2012, countries considered free of rabies were granted a temporary derogation from the policy, allowing them to implement specific regulations regarding the transport of pets across their borders. Until end of 2011, five countries (the UK, Ireland, Malta, Sweden and Norway) required an individual serological test for rabies neutralising antibodies before entry into the country (Fooks and others 2011). Today, identification by microchip, a passport certifying valid anti-rabies vaccination as well as prophylactic Echincococcus multilocularis treatment are the only entry requirement for dogs entering Norway. This change of movement policy has led to an increase in the number of rescue dogs imported from Eastern European countries for re-homing in Norway through advertisement on the internet. According to data recorded by the customs authority at Oslo Gardermoen airport, the non-commercial movement of dogs from the EU has increased from about 5000 in 2011 to approximately 7500 in 2012 (personal communication; Ole-Herman Tronerud, Norwegian Food Safety Authority, January 2015). Since serious infectious diseases such as echinococcosis and rabies are endemic in Eastern Europe, a report on the health hazards linked to import of rescue dogs to Norway was requested by the Norwegian Food Safety Authority (Norwegian Veterinary Institute 2013). The current paper reports the results of an investigation of the antibody level to rabies virus in vaccinated rescue dogs imported to Norway. The aim was to examine if the internationally accepted threshold antibody titre of ≥0.5 IU/ml was reached in these dogs.
Material and Methods
The criteria for inclusion in the study were that the dog (i) was considered a stray animal, that is, not under the direct control by a person, in its country of origin and (ii) had arrived from Romania, Hungary, the Balkans or the Baltic countries during 2012. Dog owners were encouraged to visit a veterinary clinic for blood sampling, analysis costs being covered by the Norwegian Food Safety Authority. A total of 75 blood samples were submitted to the Norwegian Veterinary Institute from veterinary clinics throughout the country and sent to the National Veterinary Institute in Sweden for analysis. The antibody responses were determined by the OIE approved fluorescent antibody virus neutralisation (FAVN) test (Cliquet and others 1998). A control group of 1766 owned dogs from Sweden, that had antibody titre analysis carried out at the same laboratory, was selected from a previous study. The dogs in this control group had received one injection of rabies vaccine and were sampled four months to six months after vaccination (Berndtsson and others 2011). An antibody titre ≥0.5 IU/ml is the internationally accepted threshold after rabies vaccination of dogs (OIE Terrestrial manual 2013). Titres ≤0.1 IU/ml are considered negative in the FAVN assay. The blood samples from the rescue dogs were accompanied by a submission form containing information on age, breed and sex. In addition, passport details such as date of vaccination (reported for 56 of 75 dogs) and vaccine label (reported for 38 of 75 dogs) was requested. A number of different vaccines, both monovalent and polyvalent products, were used such as Rabisin og Eurican DHPPi-LR (Merial, France), Nobivac Rabies og Nobivac DHPPi+LR (Merck, the Netherlands), Biocan R (Bioveta, Check Republic), Hexadog (M.C.I. Merial, Morocco), Vanguard Rabies (Pfizer, USA). Proportions and exact CIs were calculated using R V.2.12.0 with EpiR package, and group comparisons were done using Fisher's exact test.
Results
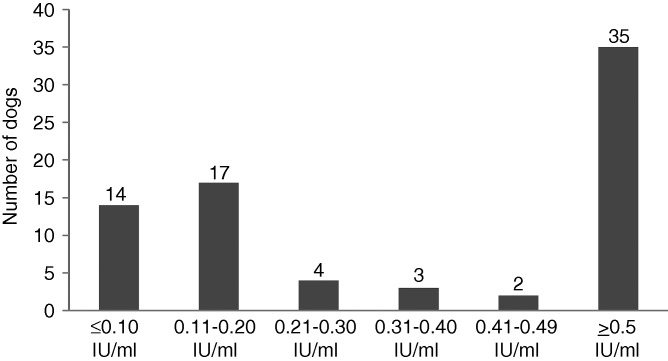
The screening of specific antibody titres to rabies virus in imported rescue dogs demonstrated that only 35 of the 75 dogs (proportion 47%, 95% exact CI (35% to 59%)) showed a satisfactory antibody level ≥0.5 IU/ml. In addition, 31 dogs (41% (30% to 53%)) had titres ≤0.2 IU/ml and among these, 14 dogs (19% (11% to 29%)) had titres ≤0.1 IU/ml, which is considered negative in the FAVN assay (Fig 1). Among the 56 dogs with a reported vaccination date, 50 per cent had antibody titre <0.5 IU/ml.
FIG 1:
Distribution of antibody titre to rabies virus in 75 imported rescue dogs with a certified valid anti-rabies vaccination. Titre ≥0.5 IU/ml is the internationally accepted threshold level after vaccination and antibody titre ≤0.1 IU/ml is considered negative (fluorescent antibody virus neutralisation test)
Sixty-three of the 75 dogs came from Romania, 8 came from Hungary, and for 4 dogs the country of origin was not reported. All dogs imported from Hungary had antibody titres ≥0.5 IU/ml.
The 1766 dogs used as control group were tested four months to six months after vaccination. To compare the level of antibodies detected in vaccinated rescue dogs and conventionally owned dogs the data were restricted from the rescue dogs to samples obtained four months to six months after injection (22 of 75 dogs). Of the conventionally owned dogs 85.7 per cent had antibody titres ≥0.5 IU/ml, as compared with 45.5 per cent of the rescue dogs (P<0.0001).
It was examined if the time between the date of vaccination and blood sampling influenced the antibody titres in the rescue dogs. The interval varied from 1 month to 12 months, and there was no correlation between antibody titre and time since vaccination (Fig 2). Furthermore there was no association between vaccine product and antibody titres, and all the different vaccines resulted in one or more dogs with titres <0.5 IU/ml.
FIG 2:

Antibody titre to rabies virus in 57 vaccinated dogs, shown as a function of time after vaccination. Titre ≥0.5 IU/ml is represented as 0.5 IU/ml
Discussion
This is the first known study to examine the anti-rabies titres in imported rescue dogs from Eastern Europe to a country within the European Economic Area after the new movement policy was implemented. According to information in the passports, all of the rescue dogs included in this study were vaccinated against rabies at least 21 days before arrival in Norway. However, more than half of the dogs did not achieve the satisfactory antibody level recommended by WHO and OIE. Most alarming was the finding that 19 per cent of the dogs had serum titres ≤0.1 IU/ml raising doubt about whether they had been vaccinated at all.
The two main objectives of antibody testing are to check if the animal (i) has been vaccinated according to recommendations, and (ii) has developed an adequate humoral immune response. Previous studies in conventional pet dogs show that the antibody response is influenced by vaccine product used, number of vaccine doses in the primary immunisation schedule, interval between vaccination and blood sampling, age, size and breed of the dog (Cliquet and others 2003, Mansfield and others 2004, Kennedy and others 2007, Jakel and others 2008). However, taking these aspects into consideration, more than 85 per cent of vaccinated dogs achieve an adequate immune response after one dosage of rabies vaccine (Fooks and others 2002, Council of Europe 2008, Van de Zande and others 2009, Berndtsson and others 2011). Several studies report that the interval between vaccination and testing will affect the proportion of dogs with titres above 0.5 IU/ml since peak antibody values are seen four weeks to six weeks postvaccination (Cliquet and others 2003, Mansfield and others 2004, Jakel and others 2008). Therefore analysis was restricted to only those dogs that had been vaccinated four months to six months before sampling to standardise with the control group (Berndtsson and others 2011), and the difference between imported rescue dogs and owned Swedish dogs was statistically significant. Only 45.5 per cent of the rescue dogs showed a sufficient antibody response four months to six months postvaccination compared with 87.5 per cent of the conventionally owned dogs. There is no systematic comparison of the rabies antibody titre and protection from challenge between immunosuppressed and healthy dogs (Morters and others 2014). Many rescue dogs have poor body condition, as well as deficiencies and underlying infections which might have a negative impact on the immune response (Davlin and Vonville 2012). Still, mass vaccination campaigns in free-roaming dogs are very successful (Cleaveland and others 2006, Thiptara and others 2011, Morters and others 2014), and one study in Peru demonstrated that 97 per cent of the free-roaming dogs had antibody titre ≥0.5 IU/ml 12 months postvaccination (Chomel and others 1988). The rabies vaccines used in the present study are all inactivated and approved for the European market. If stored or administered according to the manufacturers’ instructions, they should be expected to provide a satisfactory response in the majority of dogs examined in the present study. Hence, one might question if dogs with no detectable antibody responses have been properly vaccinated before rehoming and adoption to Norway.
The impact of these findings on human and animal health is complex to assess. In veterinary medicine, rabies vaccination is considered mainly a preventive measure, to be applied before dogs are exposed to rabies. The antibody titre of vaccinated animals before they move from a rabies-free area to a rabies-endemic area, indicates their level of immunity, and is therefore directly related to their future risk of infection (Aubert 1992). A three-week delay after vaccination usually ensures sufficient high levels of protective antibodies, and is therefore adequate for these types of movement. However, this is no longer the case when dogs are moved from rabies-endemic areas into rabies-free areas, particularly for free-roaming dogs which may unknowingly have been exposed to rabies virus before vaccination. The effect of vaccination on dogs already incubating rabies is debated, and seems to depend on type of challenge (dose, route, natural or experimental) and time between challenge and vaccination (Hanlon and others 2002, Manickama and others 2008, Wilson and others 2010). Antibody titres alone are not able to reveal if animals are infected or not (Hanlon and others 2002, Manickama and others 2008), unless it is known that the animal has been observed over a time period longer than the maximum incubation period for rabies in which case rabies can be ruled out. The present waiting time of 21 days following primary vaccination is considered too short to ensure that vaccinated dogs do not incubate rabies (EFSA, 2006). This has resulted in understandable concern in cases where people have been bitten by recently imported rescue dogs. Postexposure prophylaxis has been needed, and systematic pre-exposure prophylaxis for veterinarians is considered. It is worrying that the relaxation in movement policy has led to increased adoption of rescue dogs from member states where rabies is still prevalent. The majority of rescue dogs came from Romania where the number of reported rabies cases during 2012 was 318 in wild animals and 139 in domestic animals, including 52 dogs and 30 cats (FLI 2014). In these cases, the non-negligible risk that those dogs may have been exposed to the rabies virus before capture and rabies vaccination should be considered. In addition, these results suggest that the level of compliance with the regulation may be low. Goddard and others (2012) showed that a 20 per cent non-compliance to the present regulation decreased the predicted number of years between rabies introduction to the UK from 211 (90% CI 177 to 247) to 144 (90% CI 125 to 163), compared with full compliance. The present results suggest that compliance could be even lower. Low compliance with the regulation raises concern about other health issues as well, such as the treatment for Echinococcus multilocularis before entering free countries.
Acknowledgments
The authors thank Marit Forbord and Ole-Herman Tronerud at the Norwegian Food Safety Authority for initiating and providing data to this project, and the Norwegian Food Safety Authority for covering cost analyses. The authors are grateful to the dog owners for their contribution as well as veterinarians for collecting blood samples. The authors thank staff at the section for Immunology and Parasitology for organising the samples.
References
- AUBERT M. F. (1992) Practical significance of rabies antibodies in cats and dogs. Revue Scientifique et Technique 11, 735–760 [DOI] [PubMed] [Google Scholar]
- BERNDTSSON L. T., NYMAN A. K., RIVERA E. & KLINGEBORN B. (2011) Factors associated with the success of rabies vaccination of dogs in Sweden. Acta Veterinaria Scandinavica 53, 22 10.1186/1751-0147-53-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOMEL B., CHAPPUIS G., BULLON F., CARDENAS E., DE BEUBLAIN T. D., LOMBARD M. & GIAMBRUNO E. (1988) Mass vaccination campaign against rabies: are dogs correctly protected? The Peruvian experience. Reviews of Infectious Diseases 10(Suppl 4), S697–S702 10.1093/clinids/10.Supplement_4.S697 [DOI] [PubMed] [Google Scholar]
- CLEAVELAND S., KAARE M., KNOBEL D. & LAURENSON M. K. (2006) Canine vaccination--providing broader benefits for disease control. Veterinary Microbiology 117, 43–50 10.1016/j.vetmic.2006.04.009 [DOI] [PubMed] [Google Scholar]
- CLIQUET F., AUBERT M. & SAGNE L. (1998) Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. Journal of Immunological Methods 212, 79–87 10.1016/S0022-1759(97)00212-3 [DOI] [PubMed] [Google Scholar]
- CLIQUET F., VERDIER Y., SAGNE L., AUBERT M., SCHEREFFER J. L., SELVE M., WASNIEWSKI M. & SERVAT A. (2003) Neutralising antibody titration in 25,000 sera of dogs and cats vaccinated against rabies in France, in the framework of the new regulations that offer an alternative to quarantine. Revue Scientifique et Technique 22, 857–866 [DOI] [PubMed] [Google Scholar]
- COUNCIL OF EUROPE (2008) (2015) (Abstract)
- DAVLIN S. L. & VONVILLE H. M. (2012) Canine rabies vaccination and domestic dog population characteristics in the developing world: a systematic review. Vaccine 30, 3492–3502 10.1016/j.vaccine.2012.03.069 [DOI] [PubMed] [Google Scholar]
- EU (2003) European Union directive No. 998/2003.
- EFSA (2006) Assessement of the risk of rabies introduction into the UK, Ireland, Sweden and Malta as a consequence of abandoning serological tests measuring protective antibodies to rabies. EFSA Journal, 1–54
- FLI (2014) Rabies Information System of the WHO Collaboration Centre for Rabies Surveillance and Research. www.who-rabies-bulletin.org/Queries/Dynamic.aspx. (accessed 9 Jan 2015)
- FOOKS A. R., HORTON D. L., JOHNSON N., TOTH B. & ROBERTS H. C. (2011) Changes to pet travel rules: rabies, ticks and tapeworms. The Veterinary Record 169, 97–98 10.1136/vr.d4642 [DOI] [PubMed] [Google Scholar]
- FOOKS A. R., MCELHINNEY L. M., BROOKES S. M., JOHNSON N., KEENE V., PARSONS G. & SOLDAN A. (2002) Rabies antibody testing and the UK Pet Travel Scheme. The Veterinary Record 150, 428–430 [PubMed] [Google Scholar]
- GODDARD A. D., DONALDSON N. M., HORTON D. L., KOSMIDER R., KELLY L. A., SAYERS A. R., BREED A. C., FREULING C. M., MULLER T., SHAW S. E., HALLGREN G., FOOKS A. R. & SNARY E. L. (2012) A quantitative release assessment for the noncommercial movement of companion animals: risk of rabies reintroduction to the United kingdom. Risk Analysis 32, 1769–1783 10.1111/j.1539-6924.2012.01804.x [DOI] [PubMed] [Google Scholar]
- HANLON C. A., NIEZGODA M. & RUPPRECHT C. E. (2002) Postexposure prophylaxis for prevention of rabies in dogs. American Journal of Veterinary Research 63, 1096–1100 10.2460/ajvr.2002.63.1096 [DOI] [PubMed] [Google Scholar]
- JAKEL V., KONIG M., CUSSLER K., HANSCHMANN K. & THIEL H. J. (2008) Factors influencing the antibody response to vaccination against rabies. Developments in Biologicals 131, 431–437 [PubMed] [Google Scholar]
- KENNEDY L. J., LUNT M., BARNES A., MCELHINNEY L., FOOKS A. R., BAXTER D. N. & OLLIER W. E. (2007) Factors influencing the antibody response of dogs vaccinated against rabies. Vaccine 25, 8500–8507 10.1016/j.vaccine.2007.10.015 [DOI] [PubMed] [Google Scholar]
- MANICKAMA R., BASHEER M. D. & JAYAKUMAR R. (2008) Post-exposure prophylaxis (PEP) of rabies-infected Indian street dogs. Vaccine 26, 6564–6568 10.1016/j.vaccine.2008.09.053 [DOI] [PubMed] [Google Scholar]
- MANSFIELD K. L., BURR P. D., SNODGRASS D. R., SAYERS R. & FOOKS A. R. (2004) Factors affecting the serological response of dogs and cats to rabies vaccination. The Veterinary Record 154, 423–426 10.1136/vr.154.14.423 [DOI] [PubMed] [Google Scholar]
- MORTERS M. K., MCKINLEY T. J., HORTON D. L., CLEAVELAND S., SCHOEMAN J. P., RESTIF O., WHAY H. R., GODDARD A., FOOKS A. R., DAMRIYASA I. M. & WOOD J. L. (2014) Achieving population-level immunity to rabies in free-roaming dogs in Africa and Asia. PLoS Neglected Tropical Diseases 8, e3160 10.1371/journal.pntd.0003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMNES, I. S., KLEVAR, S., DAVIDSON, R. K., HØGÅSEN, H. R., LUND, A., 2013. Parasittologisk og serologisk undersøkelse av prøver fra gatehunder importert til Norge fra land i Øst-Europa (In Norwegian). 20 pp. Veterinærinstituttets rapportserie 15-2013 http://www.vetinst.no/Publikasjoner/Rapportserie/Rapportserie-2013/Parasittologisk-og-serologisk-undersoekelse-av-proever-fra-gatehunder-importert-til-Norge-fra-land-i-OEst-Europa (14.04.2015)
- OIE. Rabies: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2013 pp 1–26. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.13_RABIES.pdf.
- THIPTARA A., ATWILL E. R., KONGKAEW W. & CHOMEL B. B. (2011) Epidemiologic trends of rabies in domestic animals in southern Thailand, 1994–2008. The American journal of tropical medicine and hygiene 85, 138–145 10.4269/ajtmh.2011.10-0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DE ZANDE S., KAASHOEK M., HESSELINK W., SUTTON D. & NELL T. (2009) Comments to “Comparison of antibody responses after vaccination with two inactivated rabies vaccines” [Minke, J.M., et al., 2009. Vet. Microbiol. 133, 283–286]. Veterinary Microbiology 138, 202–203 10.1016/j.vetmic.2008.12.026 [DOI] [PubMed] [Google Scholar]
- WILSON P. J., OERTLI E. H., HUNT P. R. & SIDWA T. J. (2010) Evaluation of a postexposure rabies prophylaxis protocol for domestic animals in Texas: 2000–2009. Journal of the American Veterinary Medical Association 237, 1395–1401 10.2460/javma.237.12.1395 [DOI] [PubMed] [Google Scholar]