Abstract
Background
Although BRCA1 and BRCA2 mutations account for only ∼27% of the familial aggregation of ovarian cancer (OvC), no OvC risk prediction model currently exists that considers the effects of BRCA1, BRCA2 and other familial factors. Therefore, a currently unresolved problem in clinical genetics is how to counsel women with family history of OvC but no identifiable BRCA1/2 mutations.
Methods
We used data from 1548 patients with OvC and their relatives from a population-based study, with known BRCA1/2 mutation status, to investigate OvC genetic susceptibility models, using segregation analysis methods.
Results
The most parsimonious model included the effects of BRCA1/2 mutations, and the residual familial aggregation was accounted for by a polygenic component (SD 1.43, 95% CI 1.10 to 1.86), reflecting the multiplicative effects of a large number of genes with small contributions to the familial risk. We estimated that 1 in 630 individuals carries a BRCA1 mutation and 1 in 195 carries a BRCA2 mutation. We extended this model to incorporate the explicit effects of 17 common alleles that are associated with OvC risk. Based on our models, assuming all of the susceptibility genes could be identified we estimate that the half of the female population at highest genetic risk will account for 92% of all OvCs.
Conclusions
The resulting model can be used to obtain the risk of developing OvC on the basis of BRCA1/2, explicit family history and common alleles. This is the first model that accounts for all OvC familial aggregation and would be useful in the OvC genetic counselling process.
Keywords: Genetic epidemiology, Ovarian Cancer, Risk prediction, Genome-wide, Genetic screening/counselling
Introduction
Ovarian cancer (OvC) is the third most common gynaecological cancer (http://www.cancerresearchuk.org/cancer-info/cancerstats/). It is well-established that OvC has a significant genetic component, with the risk to first-degree relatives of patients with OvC estimated to be approximately three times greater than the risk to women in the general population.1 2 High-penetrance mutations in BRCA1 and BRCA2 account for ∼27% of these familial cancers1 and another 10% are accounted for by rare variants in the MMR genes, RAD51C, RAD51D and BRIP1 (http://www.nature.com/icogs/primer/common-variation-and-heritability-estimates-for-breast-ovarian-and-prostate-cancers/).
Risk models that incorporate both BRCA1 and BRCA2 mutations and other sources of variation are required to provide accurate estimates of mutation carrier probabilities and cancer risk for use in genetic counselling. Existing risk-prediction models for familial OvC such as Breast and Ovarian Analysis of Disease Incidence and Carrrier Estimation Algorithm (BOADICEA) or BRCAPRO3 4 assume that all familial aggregation to OvC is due to BRCA1 and BRCA2 mutations but this does not reflect our understanding of OvC genetic susceptibility. As a consequence, these models may underestimate OvC risks in women without mutations in these genes. Therefore, how to counsel women with family history of OvC but without BRCA1 or BRCA2 mutations has remained a major unresolved question in clinical cancer genetics.
We have used data from a large, population-based series of cases diagnosed with OvC, the Studies of Epidemiology and Risk factors in Cancer Heredity (SEARCH), and segregation analysis methods to develop genetic models for OvC that incorporate the effects of BRCA1 and BRCA2 mutations and model the residual familial aggregation to OvC. The explicit effects of 17 common OvC susceptibility alleles, identified through genome-wide association studies (GWAS), were then incorporated into the algorithm. We finally considered the implications of our risk prediction model for OvC risk stratification in the general population and its use in OvC prevention.
Materials and methods
Study population
We used data on 1548 OvC cases (probands) recruited between 1999 and 2010, along with information on their first-degree and second-degree relatives ascertained through an epidemiological questionnaire. The probands were drawn from SEARCH, a large population-based study with cases ascertained through the Eastern Cancer Registration and Information Centre.1 5 Half-sibling status and relative type to the proband, age at cancer diagnosis, cancer site, vital status, status age (the age at death if deceased, the current age if alive) and year of birth were recorded for all probands and relatives.
BRCA1 and BRCA2 mutation screening
SEARCH OvC probands were screened for BRCA1 and BRCA2 mutations as part of a separate project to evaluate the contribution of rare, high-risk and moderate-risk variants to overall OvC risk in the general population.6 Briefly, this involved targeted sequence library preparation using multiplexed 48.48 Fluidigm access arrays and sequencing on an Illumina HiScan. BRCA1 and BRCA2 mutation status information was available on all 1548 probands. The following alterations were considered pathogenic: protein-truncating insertion/deletion variants, nonsense mutations, consensus splice-site variants and missense variants with reported damaging effect on protein function. For the purpose of our analysis, BRCA1 and BRCA2 mutation status were both recorded simply as mutation-positive or negative, with no distinction between different mutation types by location or functional effect.
Statistical analysis
Segregation analysis of OvC
Complex segregation analysis was used to fit genetic models to the occurrence of OvC in families, incorporating the explicit effects of BRCA1 and BRCA2 mutations on OvC risk. Female family members were followed from birth until the first of OvC diagnosis age, age at questionnaire, death age or age 80. We also considered breast cancer occurrence, but individuals were continued to be followed up for OvC after a breast cancer diagnosis in the analysis. Data on risk-reducing surgeries were not available in relatives of probands, and we were therefore unable to censor at these events. However, since this is a population-based study in which women with OvC diagnosis were recruited soon after diagnosis, and participants were not aware of their mutation status at the time of recruitment, we do not expect a high prevalence of risk-reducing surgeries at the time of pedigree collection.
To incorporate the effects of BRCA1 and BRCA2 mutations and to take account of changes in cancer incidences over time, the OvC incidence for a female i was assumed to depend on the underlying genetic effects through a model of the form
where 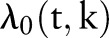 is the baseline incidence for individuals born in birth cohort k and
is the baseline incidence for individuals born in birth cohort k and 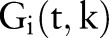 is the logarithm of the relative risk associated with BRCA1/BRCA2 mutation status g, for age t and cohort k; Mi is the logarithm of the relative risk associated with a third hypothetical major gene and Pi is the polygenic component. Pi is assumed to have a normal distribution with variance σ2 and mean zero and is approximated by the hypergeometric distribution to make it amenable to ‘peeling’.7
8 Eight sets of birth cohort and calendar-period-specific incidences for OvC in the general population were derived on the basis of incidences for England and Wales as described previously for the BOADICEA model.9 The eight cohorts included individuals born pre-1920 then in 10-year intervals up to post 1970. As the number of BRCA1 and BRCA2 mutation carriers in the SEARCH dataset was too small to obtain reliable cancer risk estimates for mutation carriers, we also used external estimates of the OvC and breast cancer relative risks for BRCA1 and BRCA2 carriers relative to population incidences, based on some of the largest studies available.10 Hence, the average BRCA1 and BRCA2 OvC and breast cancer incidences over all possible genetic effects in the model were fixed. The cohort-specific baseline incidences for BRCA1 and BRCA2 carriers were obtained for each cohort separately by constraining the average incidences over all possible genetic effects to agree with the external estimates.8 Similarly, the baseline incidences for non-mutation carriers were obtained by constraining the incidences over the BRCA1, BRCA2, other major gene and polygenic effects to agree with the population incidences (see online supplementary material methods).
is the logarithm of the relative risk associated with BRCA1/BRCA2 mutation status g, for age t and cohort k; Mi is the logarithm of the relative risk associated with a third hypothetical major gene and Pi is the polygenic component. Pi is assumed to have a normal distribution with variance σ2 and mean zero and is approximated by the hypergeometric distribution to make it amenable to ‘peeling’.7
8 Eight sets of birth cohort and calendar-period-specific incidences for OvC in the general population were derived on the basis of incidences for England and Wales as described previously for the BOADICEA model.9 The eight cohorts included individuals born pre-1920 then in 10-year intervals up to post 1970. As the number of BRCA1 and BRCA2 mutation carriers in the SEARCH dataset was too small to obtain reliable cancer risk estimates for mutation carriers, we also used external estimates of the OvC and breast cancer relative risks for BRCA1 and BRCA2 carriers relative to population incidences, based on some of the largest studies available.10 Hence, the average BRCA1 and BRCA2 OvC and breast cancer incidences over all possible genetic effects in the model were fixed. The cohort-specific baseline incidences for BRCA1 and BRCA2 carriers were obtained for each cohort separately by constraining the average incidences over all possible genetic effects to agree with the external estimates.8 Similarly, the baseline incidences for non-mutation carriers were obtained by constraining the incidences over the BRCA1, BRCA2, other major gene and polygenic effects to agree with the population incidences (see online supplementary material methods).
Since BRCA1 and BRCA2 mutations are also associated with increased breast cancer risks,10 11 we incorporated the effect of these mutations on breast cancer incidence. We assumed a similar model for the breast cancer incidence; however, the breast cancer incidence was assumed to depend on only the effects of BRCA1 and BRCA2 mutations.
In our analyses, we considered models with just the BRCA1 and BRCA2 effects, and models that additionally included a dominant, recessive or co-dominant hypothetical major gene and/or a polygenic component.
All the families used in the analysis consisted of women ascertained on the basis of OvC. Thus, to adjust for ascertainment bias,12–14 we employed an ascertainment assumption-free approach in which the likelihood of each family's joint phenotype was modelled as 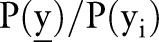 , where
, where 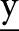 is the vector of all the family phenotypes including all phenotypic and genotypic information on the proband and yi is the phenotype of the proband. A sensitivity parameter was introduced, giving the probability of detecting a mutation if one existed, to take account of the fact that mutation screening methods used cannot detect large rearrangements in BRCA1 and BRCA2.6 A fixed value of 0.9 for both BRCA1 and BRCA2 was used in all models, but additional sensitivity analyses were performed.
is the vector of all the family phenotypes including all phenotypic and genotypic information on the proband and yi is the phenotype of the proband. A sensitivity parameter was introduced, giving the probability of detecting a mutation if one existed, to take account of the fact that mutation screening methods used cannot detect large rearrangements in BRCA1 and BRCA2.6 A fixed value of 0.9 for both BRCA1 and BRCA2 was used in all models, but additional sensitivity analyses were performed.
Maximum-likelihood estimates of the gene frequencies, polygenic standard deviation and the log relative risk for the hypothetical major gene were calculated using pedigree analysis software MENDEL.15 SEs for each parameter were obtained from the observed information matrix and were used to calculate 95% CIs. To assess goodness of fit, all of the models with a polygenic or major gene component were compared with the baseline model with just BRCA1 and BRCA2 effects using likelihood ratio tests (LRTs). Further LRTs were used to test for differences between the fit of nested models and the Akaike information criterion (AIC) equal to −2(log-likelihood – no. of parameters) was used to compare non-nested models.
OvC risk, mutation frequency and carrier numbers prediction
We used each of the models fitted to predict BRCA1 and BRCA2 mutation carrier frequencies and the risk of developing OvC in the future using the methods previously described in ref [11]. The predictions were used to compare the fit of the models as part of an internal validation. Although goodness-of-fit tests are not valid using the data generating dataset, we calculated χ2 goodness-of-fit tests that compared the observed and expected number of mutations and used these as an indicator of the model fit to the data. The expected number of mutation carriers was computed as the sum of the predicted BRCA1 and BRCA2 carrier probabilities across all SEARCH families.
We used the most parsimonious model to estimate risk of developing OvC for a 50-year-old woman to demonstrate the possible clinical implications for different scenarios of BRCA1 and BRCA2 carrier status and extent of family history. The results were compared with the corresponding predictions from the current BOADICEA model.
Incorporating SNPs into the risk prediction algorithm
We extended the most parsimonious model to also incorporate the explicit effects of the known common OvC susceptibility alleles following the methodology already published in the context of prostate cancer.16 The residual familial aggregation of OvC was accounted for in this model by a polygenic component reflecting the additive effects of a large number of genetic variants. The polygenic component Pi for each individual was divided into two parts for this purpose: a known-variant polygenic component Pk,i reflecting the polygenic risk score (PRS) due to 17 SNPs known to be associated with OvC17 and an unknown residual polygenic component PU,i. The two components were assumed to be independent and normally distributed with mean 0 and variance 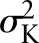 and
and 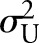 , respectively (see details in online supplementary material; methods).
, respectively (see details in online supplementary material; methods). 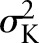 was calculated using previously described methods,16 based on the known allele frequencies and per-allele OR estimates.
was calculated using previously described methods,16 based on the known allele frequencies and per-allele OR estimates. 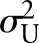 is then obtained as the difference between the total polygenic variance and variance of the PRS.
is then obtained as the difference between the total polygenic variance and variance of the PRS.
Distribution of OvC risk and implications for OvC prevention
The OvC risk associated with any individual common genetic variant is very small compared with rare variants like BRCA1. However, as there are thought to be many as yet undiscovered common variants and their effects are assumed to be additive on the logarithmic scale a woman with a high polygenic load is likely to have a greatly increased risk of OvC compared with someone with a low polygenic load. Being able to distinguish between high-risk and low-risk individuals in the population could be a valuable tool in clinical practice. Therefore, we considered the potential for risk prediction based both on known common variants and the total hypothesised polygenotype. We followed a similar approach to the methods described in ref. [18] (see online supplementary material for more details). We calculated the proportions of the population and of cancer cases at different levels of SNP risk and polygenic risk and plotted against each other for comparison purposes. This provides an informative measure of the relationship between risk distribution in the population and among cancer cases. In the hypothetical future when an individual's polygenic risk can be estimated with a high degree of accuracy, either from family history or because most of the currently theoretical polygenotype is accounted for by known variants, these measures could be used to estimate what proportions of the population would need to be monitored/screened/followed in order to detect a particular percentage of OvCs. This could also potentially contribute to stratifying population by OvC risk to enable targeting of effective screening/preventive intervention strategies for appropriate risk groups.
Results
Data from 1548 OvC cases recruited into the SEARCH study were used for our analyses. Female relatives of probands included 1340 mothers, 1404 sisters and 1144 daughters, of whom 80 were also diagnosed with OvC and 191 with breast cancer. The numbers of probands and their first-degree relatives, the number of OvCs diagnosed in each group and other sample characteristics are summarised in online supplementary table S1. All probands were screened for BRCA1 and BRCA2 mutations, identifying 44 and 62 carriers, respectively. The loci, minor allele frequencies and ORs of the 17 SNPs used in incorporating their effects into the final model are displayed in online supplementary table S2.
Segregation analyses for OvC incorporating the effects of BRCA1 and BRCA2 mutations
The results for the seven models that incorporate the explicit effects of BRCA1 and BRCA2 on OvC risk and that assume cohort-specific incidences are summarised in table 1. All the seven models that accounted for the residual familial aggregation to OvC (in addition to BRCA1 and BRCA2) provided significantly better fit than the model that included only BRCA1 and BRCA2 (p <2.8×10−5). The worst-fitting model for the residual familial aggregation of OvC, other than BRCA1 and BRCA2, was the major recessive and the most parsimonious was the polygenic model, with AICs of 5772.244 and 5764.372, respectively. Although the mixed models of inheritance all had slightly larger log-likelihoods, they did not improve the fit significantly over the model with only a polygenic component in addition to the BRCA1 and BRCA2 effects (LRT p values >0.14). In all models that included a hypothetical third major gene, the relative risk for the susceptible women was very high (ranging between ∼54 and ∼122). The estimated population allele frequency for BRCA1 and BRCA2 under the polygenic model were 0.08% (95% CI 0.06% to 0.11%) and 0.26% (95% CI 0.002% to 0.33%), respectively, with a SD of the polygenic component of 1.43 (95% CI 1.1 to 1.86).
Table 1.
Parameter estimates, goodness-of-fit measures and likelihood ratio tests (LRTs) of the seven cohort-specific models for breast and ovarian cancer
| Model | BRCA1 frequency (95% CI) | BRCA2 frequency (95% CI) | Major gene frequency (95% CI) | Major gene log relative risk (95% CI) | Polygenic SD (95% CI) | Log-likelihood | AIC | LRT p value |
|---|---|---|---|---|---|---|---|---|
| Base | 0.00081 (0.00061 to 0.0011) | 0.0026 (0.0020 to 0.0033) | – | – | – | −2892.237 | 5788.474 | 5.11E-06 |
| Major dominant | 0.00079 (0.00060 to 0.0011) | 0.0026 (0.0020 to 0.0032) | 0.00025 (0.000041 to 0.0015) | 4.8 (3.3 to 6.2) | – | −2880.343 | 5768.686 | 0.047 |
| Major recessive | 0.00080 (0.00060 to 0.0011) | 0.0026 (0.0020 to 0.0032) | 0.085 (0.017 to 0.33) | 4.0 (2.0 to 6.0) | – | −2882.122 | 5772.244 | 0.0079 |
| Major general | 0.00079 (0.00060 to 0.0011) | 0.0026 (0.0020 to 0.0032) | 0.00025 (0.00020 to 0.0033) | 4.8 (3.3 to 6.3) | – | −2880.335 | 5770.67 | 0.013 |
| 7.4 (−14.1 to 28.8) | ||||||||
| Polygenic | 0.00079 (0.00060 to 0.0011) | 0.0026 (0.0020 to 0.0033) | – | – | 1.43 (1.10 to 1.86) | −2879.186 | 5764.372 | 0.28 |
| Mixed dominant | 0.00079 (0.00059 to 0.0011) | 0.0026 (0.0020 to 0.0032) | 0.00023 (0.000023 to 0.0022) | 4.7 (2.8 to 6.6) | 1.09 (0.64 to 1.86) | −2877.289 | 5764.576 | 0.91 |
| Mixed recessive | 0.00079 (0.00060 to 0.0011) | 0.0026 (0.0020 to 0.0032) | 0.076 (0.020 to 0.25) | 3.7 (1.5 to 5.9) | 1.19 (0.74 to 1.91) | −2878.374 | 5768.806 | 0.14 |
| Mixed general | 0.00079 (0.00059 to 0.0011) | 0.0026 (0.0020 to 0.0032) | 0.00023 (0.000023 to 0.0023) | 4.7 (2.8 to 6.6) | 1.09 (0.64 to 1.86) | −2877.283 | 5766.566 | |
| 9.4 (−20.5 to 39.3) |
AIC, Akaike's information criterion; LRT p value, probability of the difference between log-likelihoods comparing each model against the mixed general model.
Predicted number of BRCA1 and BRCA2 mutation carriers and family members diagnosed with OvC
The expected numbers of BRCA1 and BRCA2 mutation carriers computed under each of the models are displayed in table 2. In line with magnitude of the log-likelihoods, all seven models gave similar predictions that were noticeably more accurate than the model that did not allow for additional residual familial aggregation other than the effects of BRCA1 and BRCA2. The polygenic model performed best for predicting the number of BRCA2 mutation carriers and there was a slight improvement in accuracy of BRCA1 number under the mixed models. In comparison, under the current implementation of BOADICEA, the predicted BRCA1 numbers were very close to the observed values but the number of BRCA2 carriers was substantially underpredicted (p value for difference between observed and expected number of mutations=4.64E-16).
Table 2.
Number of mutation carriers predicted by each model and comparison with observed numbers
| Model for the residual familial aggregation | Observed BRCA1 carriers | Expected BRCA1 carriers | Observed BRCA2 carriers | Expected BRCA2 carriers | χ2 value* |
|---|---|---|---|---|---|
| Baseline | 44 | 56.95 | 62 | 63.59 | 2.98 |
| Polygenic | 44 | 49.32 | 62 | 61.98 | 0.57 |
| Dominant major | 44 | 55.62 | 62 | 63.08 | 2.45 |
| Recessive major | 44 | 55.97 | 62 | 63.11 | 2.58 |
| General major | 44 | 55.62 | 62 | 63.08 | 2.45 |
| Dominant mixed | 44 | 48.07 | 62 | 61.01 | 0.36 |
| Recessive mixed | 44 | 49.08 | 62 | 61.10 | 0.54 |
| General mixed | 44 | 48.05 | 62 | 61.02 | 0.36 |
| BOADICEA | 44 | 45.76 | 62 | 23.03 | 66.01 |
*χ2 value, value of χ2 goodness-of-fit test.
BOADICEA, Breast and Ovarian Analysis of Disease Incidence and Carrrier Estimation Algorithm.
Similarly, when computing the expected number of families with a mother, a sister or mother and sister diagnosed with OvC, the predicted numbers were closer to that observed for the polygenic and mixed models of inheritance (see online supplementary table S3).
Predicting future OvC risks
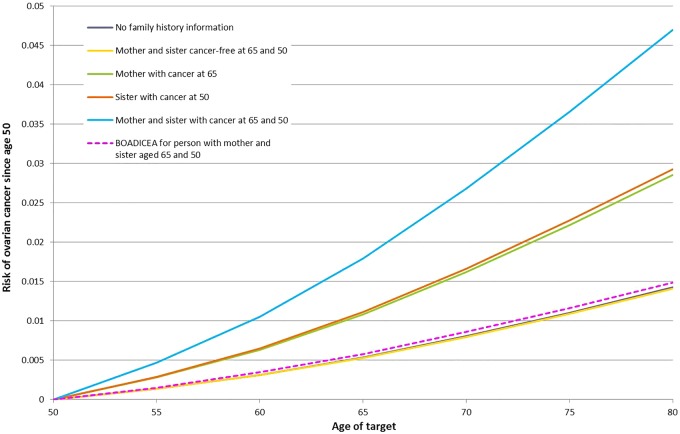
We estimated the probabilities of developing OvC for a 50-year-old woman born in 1940, with the following family histories: (i) no information on relatives; (ii) having a mother and sister cancer free at ages 65 and 50; (iii) mother and sister diagnosed with OvC at ages 65 and 50; and (iv) and (v) with both combinations of one diagnosed and one cancer free at these same ages. We compared these estimates with the risk estimates from the current version of BOADICEA.
Figure 1 displays the probabilities of developing OvC for a 50-year old woman without a BRCA1 or BRCA2 mutation. Under the best-fitting model, the risk of OvC increases with increasing number of relatives diagnosed with OvC. In contrast, the corresponding predictions under BOADICEA remain the same under all assumptions about family history. Similar patterns are observed when the index female is assumed to carry a BRCA1, or a BRCA2 mutation, where the risks in mutation carriers also depend on the exact family history information (see online supplementary figures S1 and S2). Under BOADICEA, the risks in mutation carriers are not modified by family history and are all very close to the corresponding risks predicted by our polygenic model algorithm for a women with no family history information.
Figure 1.
Predicted risks of ovarian cancer over time to a woman born in the 1940 birth cohort without a BRCA1 or BRCA2 mutation by family history. The predicted ovarian cancer risks under the most parsimonious model vary by extent of family history of ovarian cancer. In contrast, under the Breast and Ovarian Analysis of Disease Incidence and Carrrier Estimation Algorithm the predicted ovarian cancer risks remain the same under all scenarios.
Incorporating common alleles into the model
The loci, minor allele frequencies and ORs for the 17 SNPs considered are displayed in online supplementary table S2. Under the assumptions that the effects of the SNPs on OvC are all mutually independent and the same for BRCA1 carriers, BRCA2 carriers and non-carriers, each observed SNP profile was translated into a PRS. This PRS was assumed to have a centralised normal distribution with a variance of 0.0915, explaining about 4.5% of the total polygenic variance in our model.
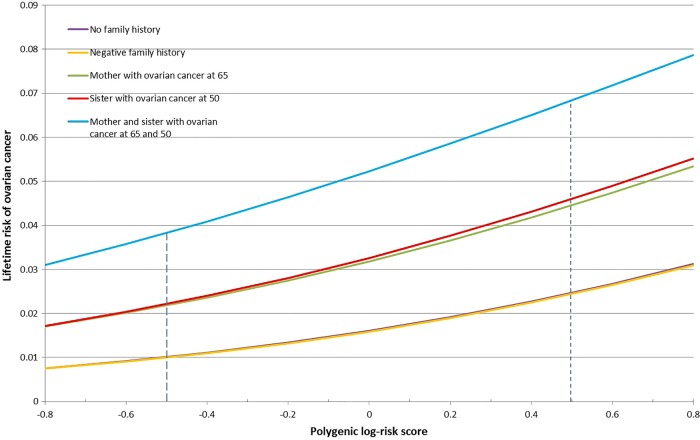
The lifetime risks of OvC to a 20-year-old non-BRCA1/2 mutation carrier, conditional on known PRS and family history of OvC, are shown in figure 2. As expected, the lifetime risk of developing OvC rose exponentially with increasing PRS. For example, the lifetime risk of OvC for a woman without a BRCA1 or BRCA2 mutation but with two affected first-degree relatives is predicted to be >5% if she is at the top 50% of the PRS distribution.
Figure 2.
Lifetime risks of ovarian cancer to a 20-year-old born in the 1940 birth cohort without a BRCA1 or BRCA2 mutation with different polygenic risk score (PRS) and family history. Graph of the change in probabilities of developing ovarian cancer by age 80 as PRS increases from −0.8 to 0.8, to a 20 year old with five different family histories. The two dotted lines, at −0.496 and 0.496, indicate the PRS of those at the 5th and 95th percentile of risk.
Examples of age-specific risks for a 50-year-old woman at the 5th and 95th percentiles of the PRS and by different family history assumptions are shown in online supplementary figures S3–S5
Implications of the polygenic model for OvC prevention
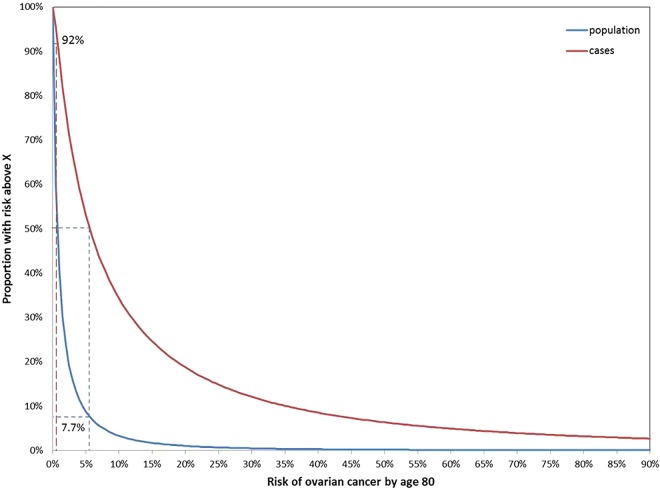
For a polygenic log-risk with the SD of 1.434, estimated under the best-fitting segregation analysis model, and assuming a baseline population OvC risk of 0.02 by age 80, the half of the population at higher risk accounts for 92% of all OvCs. Figure 3 displays the proportion of the population that have a risk greater than a given level and the proportion of the cases predicted to occur within this subgroup. From these curves, it can be seen that 50% of all cancers occur in the 7.7% of the population with a risk of 5.6% or more.
Figure 3.
Proportion of population above a specified absolute risk of ovarian cancer and proportion of cases occurring in that fraction of the population. Half the population have an absolute risk of ovarian cancer greater than 0.72% by age 80 and 92% of all cases occur in this half of the population. Half of all cancers occur in the 7.7% of the population with risk higher than 5.6%.
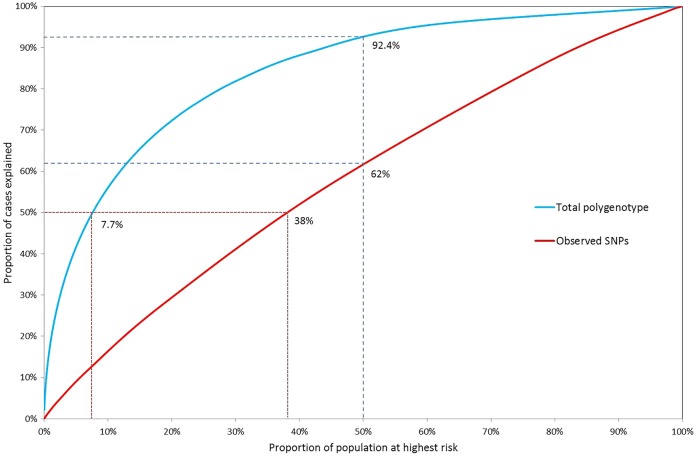
In figure 4, the population proportions are plotted against the case proportions accounted for, for the polygenic log-risk distributions and the combined SNP-effect distributions. The total known variance of the effects of 17 known SNPs is 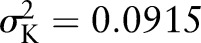 (see online supplementary material and methods). Due to the low known variance, the distinction between population and case risk is very low for the 17 SNPs alone.
(see online supplementary material and methods). Due to the low known variance, the distinction between population and case risk is very low for the 17 SNPs alone.
Figure 4.
Proportion of cases accounted for by a given proportion of the population above a specified risk of ovarian cancer according to the total polygenic risk and the observed 17 SNP distribution. Under the total polygenic risk distribution, 50% of cancers occur in the 7.7% of the population at highest risk and 92.4% of cancers occur in the half of the population at greater-than-average risk, whereas under the 17 SNP only 62% of cancers occur in the 50% at higher risk and 50% of cases are spread among almost 40% of the population at highest risk.
Discussion
We used complex segregation analysis to develop a risk-prediction model for familial OvC that incorporates the effects of BRCA1 and BRCA2 mutations, family history and several newly established common OvC susceptibility alleles using data from a population-based study of OvC cases in the UK. Our model accounts for the familial aggregation of OvC and helps inform a major unresolved clinical question on how to counsel women with family history of OvC but without BRCA1 or BRCA2 mutations.
The most parsimonious model included the effects of BRCA1 and BRCA2 mutations together with a polygenic component. This suggests that most of the familial aggregation not accounted for by BRCA1 and BRCA2 consists of the effects of a large number of genetic variants, each having small contributions to the OvC familial risk. This is in line with results from recent OvC GWAS19–22 that have demonstrated that common low-risk OvC susceptibility alleles exist. Parallel studies in breast cancer suggested that thousands of such genetic susceptibility alleles are likely to exist, which explain a substantial fraction of the unexplained genetic variability.23 A similar model is likely to apply to OvC. A model that included an additional, dominantly inherited, high-penetrance gene had the highest likelihood. Such a model could reflect the joint effects of other rare OvC susceptibility variants that confer higher risks collectively. However, our analysis may be underpowered as this model did not fit significantly better than the polygenic model.
Previous OvC segregation analyses24 25 that accounted for BRCA1 and BRCA2 mutations were based on 10-fold smaller sample set of high-risk OvC families and did not investigate polygenic models for the residual familial aggregation of OvC. In contrast to the present study, those studies found no significant evidence of a third high-penetrance gene in addition to BRCA1 and BRCA2. The difference could be explained primarily by the much lower power of those analyses due the smaller sample size but also due to the fact the ascertainment adjustment involved conditioning on all family phenotypes that imposed a much greater penalty in comparison to the present analysis that used families selected only on the OvC status of the index case.
Under the best-fitting model, the BRCA1 and BRCA2 mutation frequencies were estimated to be 0.00079 and 0.0026, respectively, corresponding to a carrier frequency of 1 in 630 population for BRCA1 and 1 in 195 population for BRCA2. These were higher than the BOADICEA estimates of 0.0006 for BRCA1 and 0.001 for BRCA2,10 but the difference was significant only for BRCA2 (p values 0.13 and 0.00002). This was also reflected in the significant underprediction of BRCA2 mutations under the BOADICEA model in the current dataset. This difference between the studies is probably partly due to the data sources and differences in the mutation screening techniques. The 2785 families used to fit the BOADICEA algorithm were ascertained primarily through population-based patients with breast cancer. This source of difference would be in line with the fact that BOADICEA was found to predict BRCA1 and BRCA2 mutations and breast cancer risk well in independent datasets of families with breast cancer.10 26–28 BOADICEA has not been evaluated so far in families ascertained on the basis of OvC only. Another possible factor is the mutation screening methods. The current study is based on currently available sequencing technologies6 that are estimated to be more sensitive in detecting mutations than the techniques used in the late 1990s.29 Moreover, the knowledge of which mutations are actually pathogenic has improved substantially over time.30 Both of these factors could contribute to higher mutation frequencies, although it is unclear why the difference is only significant for BRCA2. An alternative explanation could be a differential response rate for participating in the present study between mutation carriers and non-carriers. BRCA1 and BRCA2 mutations have both been associated with improved short-term OvC survival. In particular, BRCA2 mutation carriers have been reported to have a better prognosis.31 32 If women with short prognosis are more likely to participate in the study, this could potentially lead to an overestimation of the mutation frequency. However, data on response differences by prognostic characteristics are not available to assess this.
In the long term, we expect that these differences will be resolved by fitting a single algorithm to all available data that models comprehensively both the genetic susceptibility to breast cancer and OvC. However, at this stage this is not feasible based on current technologies due to computational complexities (in particular, the number of underlying genotypes in the models). The current approach aims to develop separate algorithms for the susceptibility to breast cancer and OvC that individually incorporate the explicit effects of all observed and unobserved genetic variants such that we obtain accurate risks of each cancer. Validation studies in independent datasets will determine the most appropriate model for use in each context. As technologies evolve over time, in the long term we expect to synthesise the models into a single algorithm.
In our analyses, we took account of OvCs occurring after a breast cancer diagnosis, assuming the OvC incidence remains the same before and after the breast cancer diagnosis. Repeating the analysis but censoring at the first cancer yielded similar results (eg, under the polygenic model BRCA1 mutation frequency was estimated to be 0.083% and BRCA2 mutation frequency was 0.27% with a polygenic SD of 1.46). Therefore, our results were not sensitive to these assumptions.
In our analysis, we aimed to include only epithelial OvCs. However, subsequent to the model fitting process, additional pathology information became available, which revealed 41 of the probands’ tumours to be non-epithelial OvCs. This consisted of one BRCA2 carrier and 40 non-carriers, were non-epithelial OvCs. Refitting the models using only epithelial OvCs had very little effect on results. Under the polygenic model, the estimated BRCA1 and BRCA2 mutation frequencies were 0.081% and 0.26%, polygenic SD was 1.44 and the estimated numbers of BRCA1 and BRCA2 carriers were 48.6 and 60.8, respectively.
Our models assumed that the mutation testing sensitivities were 0.9 for both BRCA1 and BRCA2. Obtaining exact estimates is difficult, but in practice mutation sensitivities could be lower. We refitted the models using a sensitivity parameter of 0.83 for BRCA1 and 0.76 for BRCA2.6 Under the polygenic model, the estimated BRCA1 and BRCA2 mutation frequencies were slightly higher at 0.086% and 0.3%, respectively, and the polygenic SD decreased slightly to 1.375, but none of these were significantly different than the results under a sensitivity of 0.9. These patterns are expected as the mutation frequency and mutation screening sensitivity parameters are confounded.
One possible source of bias in our analysis is the possibility of errors in the reporting of family cancer history. However, previous studies have found reported OvC history in first-degree relatives to be reasonably accurate (83.3% probability of agreement between reported OvC status in first-degree relatives and established status).33 34 Therefore, the fact that the OvC diagnoses in relatives are not confirmed is unlikely to have a great impact on our results. Another possible weakness of our study is the usage of external estimates of breast cancer and OvC relative risks to BRCA1 and BRCA2 mutation carriers. However, due to the small number of mutation carriers in the SEARCH dataset, it was not possible to estimate reliably the cancer risks for BRCA1 and BRCA2 mutation carriers. The estimates used were based on some of the largest studies available.10 35 Future studies should aim to analyse all the data jointly.
Under our models, the probabilities of developing OvC increase with the number of OvCs in relatives, while under BOADICEA10 the risks remain invariable, at values very close to those we predicted for someone with no recorded family history, which for non-BRCA1 or non-BRCA2 carriers is close to the population risk. This is due to the fact that BOADICEA, along with other previously developed algorithms such as BRCAPRO,3 uses only BRCA1 and BRCA2 mutations to model genetic susceptibility to OvC. As a result, under BOADICEA and BRCAPRO, OvC risks are determined only by the BRCA1 and BRCA2 mutation status, no matter what their family history is. Three quarters of OvC familial relative risk is not accounted for by BRCA1 and BRCA2 mutations;1 therefore, the present models are more realistic. As it stands, BOADICEA and BRCAPRO could underestimate the risk to many individuals with a family history of OvC but no identified mutations.
In all models incorporating a polygenic component or known SNPs, the effects were assumed to be the same for carriers of a BRCA1 or BRCA2 mutation and non-carriers. This assumption is supported by recent studies17 36 37 where all but one of the OvC loci identified through GWAS were found to be associated with risk to a similar relative extent in BRCA1 and BRCA2 carriers and non-carriers.38 If future studies identify additional BRCA1-specific or BRCA2-specific modifiers of risk, it should be possible to extend the present model to allow for this level of complexity.
Although we have incorporated the explicit effects of the common low-risk alleles, future efforts should focus on incorporating the explicit effects of other intermediate risk OvC susceptibility variants such as RAD51C, RAD51D and BRIP1.39–41 However, prior to incorporating those into risk prediction models, it is critical to obtain precise estimates of the risks conferred by such mutations that currently are not available.
We also used our models to investigate the possible implications for OvC risk stratification in the population. Using the parameters from the polygenic model, we estimate that 50% of OvCs occur within 7.7% of the population at highest risk. Meanwhile, half of the population at lower risk is forecast to contain only 1 in 13 cancer cases. Targeting the 10% at highest polygenic risk for preventative measures or excluding the low-risk half could therefore lead to a much more efficient distribution of resources. However, to achieve this will require that we identify all the genetic factors that contribute to polygenic inheritance. The almost flat curve in figure 4 from the SNP log-risk distribution, with 50% of the population at higher risk predicted to contain around only 60% of cases, suggests very low power to discriminate between high-risk and low-risk individuals on SNP profiles alone. It is perhaps not surprising as currently only 4.5% of the OvC polygenic variance is accounted for by known low-penetrance genetic variants. However, the currently known SNP profiles in combination with family history information and other risk factors for the disease are expected to have a greater impact for individualised OvC risk prediction, as demonstrated by our model.
Our model can be used in the genetic counselling process of women with family history of OvC as well as for counselling women both with and without BRCA1 or BRCA2 mutations. This would be helpful to both BRCA1 and BRCA2 carriers and non-carriers while making decisions regarding clinical interventions following counselling. Probabilities of developing OvC based on family history, BRCA1, BRCA2 mutation status and/or polygenic risk could be used to assess the risk to an individual and to discriminate between high-risk and low-risk individuals, which may in time prove useful for targeting appropriate interventions.
Future research
Although the mutation carrier probability algorithms produced very accurate estimates of the number of carriers in the SEARCH data, an external validation is needed to establish the performance of the model in independent datasets and to assess the model performance in predicting OvC risk in prospective studies. Future plans to extend the models include the addition of lifestyle and reproductive factors such as parity, breast feeding and oral contraceptive use,42 mutations in genes such as RAD51C, RAD51D and BRIP1 that are known to be associated with OvC risk,39–41 competing causes of mortality and differences in the associations of the various risk factors with different OvC morphological subtypes. The ultimate goal is to combine the models within the BOADICEA framework and develop a comprehensive breast and ovarian risk user-friendly prediction tool.
Supplementary Material
Acknowledgments
We thank all the study participants who contributed to this study and all the researchers, clinicians and technical and administrative staff who have made possible this work. In particular, we thank Craig Luccarini, the SEARCH team and the Eastern Cancer Registration and Information Centre. ACA is a Cancer Research UK Senior Cancer Research Fellow. IJ is a National Institute for Health Research Senior Investigator.
Footnotes
Correction notice: The license of this article has changed since publication to CC BY 4.0.
Contributors: Conception and design: ACA and PPDP. Analysis: SJ, HS, AL, ED, PH and CB. Interpretation of data: SJ, ACA, PPDP, RM, DFE and IJ. Acquisition of data: PPDP and DFE. Drafting the article: SJ, PPDP and ACA. Critical revision: all authors. Final approval: all authors.
Funding: This work has been supported by grants from Cancer Research UK (C1005/A12677, C12292/A11174, C490/A10119, C490/A10124) including the PROMISE research programme, the Eve Appeal and the UK National Institute for Health Research Biomedical Research Centre at the University of Cambridge.
Competing interests: IJ is a director of Abcodia.
Patient consent: Obtained.
Ethics approval: Cambridgeshire 4 research ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jervis S, Song H, Lee A, Dicks E, Tyrer J, Harrington P, Easton DF, Jacobs IJ, Pharoah PP, Antoniou AC. Ovarian cancer familial relative risks by tumour subtypes and by known ovarian cancer genetic susceptibility variants. J Med Genet 2014;51:108–13. 10.1136/jmedgenet-2013-102015 [DOI] [PubMed] [Google Scholar]
- 2.Stratton JF, Pharoah P, Smith SK, Easton D, Ponder BA. A systematic review and meta-analysis of family history and risk of ovarian cancer. Br J Obstet Gynaecol 1998;105:493–9. 10.1111/j.1471-0528.1998.tb10148.x [DOI] [PubMed] [Google Scholar]
- 3.Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet 1998;62:145–58. 10.1086/301670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniou AC, Pharoah PDP, McMullan G, Day NE, Stratton MR, Peto J, Ponder BJ, Easton DF. A comprehensive model for familial breast cancer incorporating BRCA1, BRCA2 and other genes. Br J Cancer 2002;86:76–83. 10.1038/sj.bjc.6600008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pharoah PDP, Tyrer J, Dunning AM, Easton DF, Ponder BAJ. Association between Common Variation in 120 Candidate Genes and Breast Cancer Risk. PLoS Genet 2007;3:e42 10.1371/journal.pgen.0030042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song H, Cicek MS, Dicks E, Harrington P, Ramus SJ, Cunningham JM, Fridley BL, Tyrer JP, Alsop J, Jimenez-Linan M, Gayther SA, Goode EL, Pharoah PD. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum Mol Genet 2014;23:4703–9. 10.1093/hmg/ddu172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange K. An approximate model of polygenic inheritance. Genetics 1997;147:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniou AC, Pharoah PD, McMullan G, Day NE, Ponder BA, Easton D. Evidence for further breast cancer susceptibility genes in addition to BRCA1 and BRCA2 in a population-based study. Genet Epidemiol 2001;21:1–18. 10.1002/gepi.1014 [DOI] [PubMed] [Google Scholar]
- 9.Lee AJ, Cunningham AP, Kuchenbaecker KB, Mavaddat N, Easton DF, Antoniou AC. BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer 2014;110:535–45. 10.1038/bjc.2013.730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoniou AC, Cunningham AP, Peto J, Evans DG, Lalloo F, Narod SA, Risch HA, Eyfjord JE, Hopper JL, Southey MC, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tryggvadottir L, Syrjakoski K, Kallioniemi OP, Eerola H, Nevanlinna H, Pharoah PD, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 2008;98:1457–66. 10.1038/sj.bjc.6604305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoniou AC, Pharoah PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 2004;91:1580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannings C, Thompson EA. Ascertainment in the sequential sampling of pedigrees. Clin Genet 1977;12:208–12. 10.1111/j.1399-0004.1977.tb00928.x [DOI] [PubMed] [Google Scholar]
- 13.Ewens WJ, Shute NC. A resolution of the ascertainment sampling problem. I. Theory. Theor Popul Biol 1986;30:388–412. 10.1016/0040-5809(86)90042-0 [DOI] [PubMed] [Google Scholar]
- 14.Shute NC, Ewens WJ. A resolution of the ascertainment sampling problem. III. Pedigrees. Am J Hum Genet 1988;43:387–95. [PMC free article] [PubMed] [Google Scholar]
- 15.Lange K, Weeks D, Boehnke M. Programs for pedigree analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol 1988;5:471–2. 10.1002/gepi.1370050611 [DOI] [PubMed] [Google Scholar]
- 16.Macinnis RJ, Antoniou AC, Eeles RA, Severi G, Al Olama AA, McGuffog L, Kote-Jarai Z, Guy M, O'Brien LT, Hall AL, Wilkinson RA, Sawyer E, Ardern-Jones AT, Dearnaley DP, Horwich A, Khoo VS, Parker CC, Huddart RA, Van As N, McCredie MR, English DR, Giles GG, Hopper JL, Easton DF. A risk prediction algorithm based on family history and common genetic variants: application to prostate cancer with potential clinical impact. Genet Epidemiol 2011;35:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchenbaecker KB, Ramus SJ, Tyrer J, Lee A, Shen HC, Beesley J, Lawrenson K, McGuffog L, Healey S, Lee JM, Spindler TJ, Lin YG, Pejovic T, Bean Y, Li Q, Coetzee S, Hazelett D, Miron A, Southey M, Terry MB, Goldgar DE, Buys SS, Janavicius R, Dorfling CM, van Rensburg EJ, Neuhausen SL, Ding YC, Hansen TV, Jonson L, Gerdes AM, Ejlertsen B, Barrowdale D, Dennis J, Benitez J, Osorio A, Garcia MJ, Komenaka I, Weitzel JN, Ganschow P, Peterlongo P, Bernard L, Viel A, Bonanni B, Peissel B, Manoukian S, Radice P, Papi L, Ottini L, Fostira F, Konstantopoulou I, Garber J, Frost D, Perkins J, Platte R, Ellis S, Godwin AK, Schmutzler RK, Meindl A, Engel C, Sutter C, Sinilnikova OM, Damiola F, Mazoyer S, Stoppa-Lyonnet D, Claes K, De Leeneer K, Kirk J, Rodriguez GC, Piedmonte M, O'Malley DM, de la Hoya M, Caldes T, Aittomaki K, Nevanlinna H, Collee JM, Rookus MA, Oosterwijk JC, Tihomirova L, Tung N, Hamann U, Isaccs C, Tischkowitz M, Imyanitov EN, Caligo MA, Campbell IG, Hogervorst FB, Olah E, Diez O, Blanco I, Brunet J, Lazaro C, Pujana MA, Jakubowska A, Gronwald J, Lubinski J, Sukiennicki G, Barkardottir RB, Plante M, Simard J, Soucy P, Montagna M, Tognazzo S, Teixeira MR, Pankratz VS, Wang X, Lindor N, Szabo CI, Kauff N, Vijai J, Aghajanian CA, Pfeiler G, Berger A, Singer CF, Tea MK, Phelan CM, Greene MH, Mai PL, Rennert G, Mulligan AM, Tchatchou S, Andrulis IL, Glendon G, Toland AE, Jensen UB, Kruse TA, Thomassen M, Bojesen A, Zidan J, Friedman E, Laitman Y, Soller M, Liljegren A, Arver B, Einbeigi Z, Stenmark-Askmalm M, Olopade OI, Nussbaum RL, Rebbeck TR, Nathanson KL, Domchek SM, Lu KH, Karlan BY, Walsh C, Lester J, Hein A, Ekici AB, Beckmann MW, Fasching PA, Lambrechts D, Van Nieuwenhuysen E, Vergote I, Lambrechts S, Dicks E, Doherty JA, Wicklund KG, Rossing MA, Rudolph A, Chang-Claude J, Wang-Gohrke S, Eilber U, Moysich KB, Odunsi K, Sucheston L, Lele S, Wilkens LR, Goodman MT, Thompson PJ, Shvetsov YB, Runnebaum IB, Durst M, Hillemanns P, Dork T, Antonenkova N, Bogdanova N, Leminen A, Pelttari LM, Butzow R, Modugno F, Kelley JL, Edwards RP, Ness RB, du Bois A, Heitz F, Schwaab I, Harter P, Matsuo K, Hosono S, Orsulic S, Jensen A, Kjaer SK, Hogdall E, Hasmad HN, Azmi MA, Teo SH, Woo YL, Fridley BL, Goode EL, Cunningham JM, Vierkant RA, Bruinsma F, Giles GG, Liang D, Hildebrandt MA, Wu X, Levine DA, Bisogna M, Berchuck A, Iversen ES, Schildkraut JM, Concannon P, Weber RP, Cramer DW, Terry KL, Poole EM, Tworoger SS, Bandera EV, Orlow I, Olson SH, Krakstad C, Salvesen HB, Tangen IL, Bjorge L, van Altena AM, Aben KK, Kiemeney LA, Massuger LF, Kellar M, Brooks-Wilson A, Kelemen LE, Cook LS, Le ND, Cybulski C, Yang H, Lissowska J, Brinton LA, Wentzensen N, Hogdall C, Lundvall L, Nedergaard L, Baker H, Song H, Eccles D, McNeish I, Paul J, Carty K, Siddiqui N, Glasspool R, Whittemore AS, Rothstein JH, McGuire V, Sieh W, Ji BT, Zheng W, Shu XO, Gao YT, Rosen B, Risch HA, McLaughlin JR, Narod SA, Monteiro AN, Chen A, Lin HY, Permuth-Wey J, Sellers TA, Tsai YY, Chen Z, Ziogas A, Anton-Culver H, Gentry-Maharaj A, Menon U, Harrington P, Lee AW, Wu AH, Pearce CL, Coetzee G, Pike MC, Dansonka-Mieszkowska A, Timorek A, Rzepecka IK, Kupryjanczyk J, Freedman M, Noushmehr H, Easton DF, Offit K, Couch FJ, Gayther S, Pharoah PP, Antoniou AC, Chenevix-Trench G. Identification of six new susceptibility loci for invasive epithelial ovarian cancer. Nat Genet 2015;47:164–71. 10.1038/ng.3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pharoah PD, Antoniou A, Bobrow M, Zimmern RL, Easton DF, Ponder BA. Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet 2002;31:33–6. 10.1038/ng853 [DOI] [PubMed] [Google Scholar]
- 19.Song H, Ramus SJ, Tyrer J, Bolton KL, Gentry-Maharaj A, Wozniak E, Anton-Culver H, Chang-Claude J, Cramer DW, DiCioccio R, Dork T, Goode EL, Goodman MT, Schildkraut JM, Sellers T, Baglietto L, Beckmann MW, Beesley J, Blaakaer J, Carney ME, Chanock S, Chen Z, Cunningham JM, Dicks E, Doherty JA, Durst M, Ekici AB, Fenstermacher D, Fridley BL, Giles G, Gore ME, De Vivo I, Hillemanns P, Hogdall C, Hogdall E, Iversen ES, Jacobs IJ, Jakubowska A, Li D, Lissowska J, Lubinski J, Lurie G, McGuire V, McLaughlin J, Medrek K, Moorman PG, Moysich K, Narod S, Phelan C, Pye C, Risch H, Runnebaum IB, Severi G, Southey M, Stram DO, Thiel FC, Terry KL, Tsai YY, Tworoger SS, Van Den Berg DJ, Vierkant RA, Wang-Gohrke S, Webb PM, Wilkens LR, Wu AH, Yang H, Brewster W, Ziogas A, Houlston R, Tomlinson I, Whittemore AS, Rossing MA, Ponder BA, Pearce CL, Ness RB, Menon U, Kjaer SK, Gronwald J, Garcia-Closas M, Fasching PA, Easton DF, Chenevix-Trench G, Berchuck A, Pharoah PD, Gayther SA. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet 2009;41:996–1000. 10.1038/ng.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolton KL, Tyrer J, Song H, Ramus SJ, Notaridou M, Jones C, Sher T, Gentry-Maharaj A, Wozniak E, Tsai YY, Weidhaas J, Paik D, Van Den Berg DJ, Stram DO, Pearce CL, Wu AH, Brewster W, Anton-Culver H, Ziogas A, Narod SA, Levine DA, Kaye SB, Brown R, Paul J, Flanagan J, Sieh W, McGuire V, Whittemore AS, Campbell I, Gore ME, Lissowska J, Yang HP, Medrek K, Gronwald J, Lubinski J, Jakubowska A, Le ND, Cook LS, Kelemen LE, Brook-Wilson A, Massuger LF, Kiemeney LA, Aben KK, van Altena AM, Houlston R, Tomlinson I, Palmieri RT, Moorman PG, Schildkraut J, Iversen ES, Phelan C, Vierkant RA, Cunningham JM, Goode EL, Fridley BL, Kruger-Kjaer S, Blaeker J, Hogdall E, Hogdall C, Gross J, Karlan BY, Ness RB, Edwards RP, Odunsi K, Moyisch KB, Baker JA, Modugno F, Heikkinenen T, Butzow R, Nevanlinna H, Leminen A, Bogdanova N, Antonenkova N, Doerk T, Hillemanns P, Durst M, Runnebaum I, Thompson PJ, Carney ME, Goodman MT, Lurie G, Wang-Gohrke S, Hein R, Chang-Claude J, Rossing MA, Cushing-Haugen KL, Doherty J, Chen C, Rafnar T, Besenbacher S, Sulem P, Stefansson K, Birrer MJ, Terry KL, Hernandez D, Cramer DW, Vergote I, Amant F, Lambrechts D, Despierre E, Fasching PA, Beckmann MW, Thiel FC, Ekici AB, Chen X, Johnatty SE, Webb PM, Beesley J, Chanock S, Garcia-Closas M, Sellers T, Easton DF, Berchuck A, Chenevix-Trench G, Pharoah PD, Gayther SA. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet 2010;42:880–4. 10.1038/ng.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goode EL, Chenevix-Trench G, Song H, Ramus SJ, Notaridou M, Lawrenson K, Widschwendter M, Vierkant RA, Larson MC, Kjaer SK, Birrer MJ, Berchuck A, Schildkraut J, Tomlinson I, Kiemeney LA, Cook LS, Gronwald J, Garcia-Closas M, Gore ME, Campbell I, Whittemore AS, Sutphen R, Phelan C, Anton-Culver H, Pearce CL, Lambrechts D, Rossing MA, Chang-Claude J, Moysich KB, Goodman MT, Dork T, Nevanlinna H, Ness RB, Rafnar T, Hogdall C, Hogdall E, Fridley BL, Cunningham JM, Sieh W, McGuire V, Godwin AK, Cramer DW, Hernandez D, Levine D, Lu K, Iversen ES, Palmieri RT, Houlston R, van Altena AM, Aben KK, Massuger LF, Brooks-Wilson A, Kelemen LE, Le ND, Jakubowska A, Lubinski J, Medrek K, Stafford A, Easton DF, Tyrer J, Bolton KL, Harrington P, Eccles D, Chen A, Molina AN, Davila BN, Arango H, Tsai YY, Chen Z, Risch HA, McLaughlin J, Narod SA, Ziogas A, Brewster W, Gentry-Maharaj A, Menon U, Wu AH, Stram DO, Pike MC, Beesley J, Webb PM, Chen X, Ekici AB, Thiel FC, Beckmann MW, Yang H, Wentzensen N, Lissowska J, Fasching PA, Despierre E, Amant F, Vergote I, Doherty J, Hein R, Wang-Gohrke S, Lurie G, Carney ME, Thompson PJ, Runnebaum I, Hillemanns P, Durst M, Antonenkova N, Bogdanova N, Leminen A, Butzow R, Heikkinen T, Stefansson K, Sulem P, Besenbacher S, Sellers TA, Gayther SA, Pharoah PD. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet 2010;42:874–9. 10.1038/ng.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pharoah PD, Tsai YY, Ramus SJ, Phelan CM, Goode EL, Lawrenson K, Buckley M, Fridley BL, Tyrer JP, Shen H, Weber R, Karevan R, Larson MC, Song H, Tessier DC, Bacot F, Vincent D, Cunningham JM, Dennis J, Dicks E, Aben KK, Anton-Culver H, Antonenkova N, Armasu SM, Baglietto L, Bandera EV, Beckmann MW, Birrer MJ, Bloom G, Bogdanova N, Brenton JD, Brinton LA, Brooks-Wilson A, Brown R, Butzow R, Campbell I, Carney ME, Carvalho RS, Chang-Claude J, Chen YA, Chen Z, Chow WH, Cicek MS, Coetzee G, Cook LS, Cramer DW, Cybulski C, Dansonka-Mieszkowska A, Despierre E, Doherty JA, Dork T, du BA, Durst M, Eccles D, Edwards R, Ekici AB, Fasching PA, Fenstermacher D, Flanagan J, Gao YT, Garcia-Closas M, Gentry-Maharaj A, Giles G, Gjyshi A, Gore M, Gronwald J, Guo Q, Halle MK, Harter P, Hein A, Heitz F, Hillemanns P, Hoatlin M, Hogdall E, Hogdall CK, Hosono S, Jakubowska A, Jensen A, Kalli KR, Karlan BY, Kelemen LE, Kiemeney LA, Kjaer SK, Konecny GE, Krakstad C, Kupryjanczyk J, Lambrechts D, Lambrechts S, Le ND, Lee N, Lee J, Leminen A, Lim BK, Lissowska J, Lubinski J, Lundvall L, Lurie G, Massuger LF, Matsuo K, McGuire V, McLaughlin JR, Menon U, Modugno F, Moysich KB, Nakanishi T, Narod SA, Ness RB, Nevanlinna H, Nickels S, Noushmehr H, Odunsi K, Olson S, Orlow I, Paul J, Pejovic T, Pelttari LM, Permuth-Wey J, Pike MC, Poole EM, Qu X, Risch HA, Rodriguez-Rodriguez L, Rossing MA, Rudolph A, Runnebaum I, Rzepecka IK, Salvesen HB, Schwaab I, Severi G, Shen H, Shridhar V, Shu XO, Sieh W, Southey MC, Spellman P, Tajima K, Teo SH, Terry KL, Thompson PJ, Timorek A, Tworoger SS, van Altena AM, van den Berg D, Vergote I, Vierkant RA, Vitonis AF, Wang-Gohrke S, Wentzensen N, Whittemore AS, Wik E, Winterhoff B, Woo YL, Wu AH, Yang HP, Zheng W, Ziogas A, Zulkifli F, Goodman MT, Hall P, Easton DF, Pearce CL, Berchuck A, Chenevix-Trench G, Iversen E, Monteiro AN, Gayther SA, Schildkraut JM, Sellers TA. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet 2013;45:362–70. 10.1038/ng.2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, Schmidt MK, Chang-Claude J, Bojesen SE, Bolla MK, Wang Q, Dicks E, Lee A, Turnbull C, Rahman N, Fletcher O, Peto J, Gibson L, Silva Idos S, Nevanlinna H, Muranen TA, Aittomäki K, Blomqvist C, Czene K, Irwanto A, Liu J, Waisfisz Q, Meijers-Heijboer H, Adank M, van der Luijt RB, Hein R, Dahmen N, Beckman L, Meindl A, Schmutzler RK, Müller-Myhsok B, Lichtner P, Hopper JL, Southey MC, Makalic E, Schmidt DF, Uitterlinden AG, Hofman A, Hunter DJ, Chanock SJ, Vincent D, Bacot F, Tessier DC, Canisius S, Wessels LFA, Haiman CA, Shah M, Luben R, Brown J, Luccarini C, Schoof N, Humphreys K, Li J, Nordestgaard BG, Nielsen SF, Flyger H, Couch FJ, Wang X, Vachon C, Stevens KN, Lambrechts D, Moisse M, Paridaens R, Christiaens MR, Rudolph A, Nickels S, Flesch-Janys D, Johnson N, Aitken Z, Aaltonen K, Heikkinen T, Broeks A, Van't Veer LJ, van der Schoot CE, Guénel P, Truong T, Laurent-Puig P, Menegaux F, Marme F, Schneeweiss A, Sohn C, Burwinkel B, Zamora MP, Perez JIA, Pita G, Alonso MR, Cox A, Brock IW, Cross SS, Reed MWR, Sawyer EJ, Tomlinson I, Kerin MJ, Miller N, Henderson BE, Schumacher F, Le Marchand L, Andrulis IL, Knight JA, Glendon G, Mulligan AM, Lindblom A, Margolin S, Hooning MJ, Hollestelle A, van den Ouweland AMW, Jager A, Bui QM, Stone J, Dite GS, Apicella C, Tsimiklis H, Giles GG, Severi G, Baglietto L, Fasching PA, Haeberle L, Ekici AB, Beckmann MW, Brenner H, Müller H, Arndt V, Stegmaier C, Swerdlow A, Ashworth A, Orr N, Jones M, Figueroa J, Lissowska J, Brinton L, Goldberg MS, Labrèche F, Dumont M, Winqvist R, Pylkäs K, Jukkola-Vuorinen A, Grip M, Brauch H, Hamann U, Brüning T, Radice P, Peterlongo P, Manoukian S, Bonanni B, Devilee P, Tollenaar R, Seynaeve C, van Asperen CJ, Jakubowska A, Lubinski J, Jaworska K, Durda K, Mannermaa A, Kataja V, Kosma VM, Hartikainen JM, Bogdanova NV, Antonenkova NN, Dörk T, Kristensen VN, Anton-Culver H, Slager S, Toland AE, Edge S, Fostira F, Kang D, Yoo KY, Noh DY, Matsuo K, Ito H, Iwata H, Sueta A, Wu AH, Tseng CC, Van Den Berg D, Stram DO, Shu XO, Lu W, Gao YT, Cai H, Teo SH, Yip CH, Phuah SY, Cornes BK, Hartman M, Miao H, Lim WY, Sng JH, Muir K, Lophatananon A, Stewart-Brown S, Siriwanarangsan P, Shen CY, Hsiung CN, Wu PE, Ding SL, Sangrajrang S, Gaborieau V, Brennan P, McKay J, Blot WJ, Signorello LB, Cai Q, Zheng W, Deming-Halverson S, Shrubsole M, Long J, Simard J, Garcia-Closas M, Pharoah PDP, Chenevix-Trench G, Dunning AM, Benitez J, Easton DF. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet 2013;45:353–61. e2 10.1038/ng.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gayther SA, Russell P, Harrington P, Antoniou AC, Easton DF, Ponder BA. The contribution of germline BRCA1 and BRCA2 mutations to familial ovarian cancer: no evidence for other ovarian cancer-susceptibility genes. Am J Hum Genet 1999;65:1021–9. 10.1086/302583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoniou AC, Gayther SA, Stratton JF, Ponder BA, Easton DF. Risk models for familial ovarian and breast cancer. Genet Epidemiol 2000;18:173–90. [DOI] [PubMed] [Google Scholar]
- 26.Fischer C, Kuchenbacker K, Engel C, Zachariae S, Rhiem K, Meindl A, Rahner N, Dikow N, Plendl H, Debatin I, Grimm T, Gadzicki D, Flottmann R, Horvath J, Schrock E, Stock F, Schafer D, Schwaab I, Kartsonaki C, Mavaddat N, Schlegelberger B, Antoniou AC, Schmutzler R. Evaluating the performance of the breast cancer genetic risk models BOADICEA, IBIS, BRCAPRO and Claus for predicting BRCA1/2 mutation carrier probabilities: a study based on 7352 families from the German Hereditary Breast and Ovarian Cancer Consortium. J Med Genet 2013;50:360–7. 10.1136/jmedgenet-2012-101415 [DOI] [PubMed] [Google Scholar]
- 27.Antoniou AC, Hardy R, Walker L, Evans DG, Shenton A, Eeles R, Shanley S, Pichert G, Izatt L, Rose S, Douglas F, Eccles D, Morrison PJ, Scott J, Zimmern RL, Easton DF, Pharoah PD. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet 2008;45:425–31. 10.1136/jmg.2007.056556 [DOI] [PubMed] [Google Scholar]
- 28.MacInnis RJ, Bickerstaffe A, Apicella C, Dite GS, Dowty JG, Aujard K, Phillips KA, Weideman P, Lee A, Terry MB, Giles GG, Southey MC, Antoniou AC, Hopper JL. Prospective validation of the breast cancer risk prediction model BOADICEA and a batch-mode version BOADICEACentre. Br J Cancer 2013;109:1296–301. 10.1038/bjc.2013.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.[No authors listed]. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer 2000;83:1301–8. 10.1054/bjoc.2000.1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Easton DF, Deffenbaugh AM, Pruss D, Frye C, Wenstrup RJ, Allen-Brady K, Tavtigian SV, Monteiro AN, Iversen ES, Couch FJ, Goldgar DE. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet 2007;81:873–83. 10.1086/521032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Candido-dos-Reis FJ, Song H, Goode EL, Cunningham JM, Fridley BL, Larson MC, Alsop K, Dicks E, Harrington P, Ramus SJ, de Fazio A, Mitchell G, Fereday S, Bolton KL, Gourley C, Michie C, Karlan B, Lester J, Walsh C, Cass I, Olsson H, Gore M, Benitez JJ, Garcia MJ, Andrulis I, Mulligan AM, Glendon G, Blanco I, Lazaro C, Whittemore AS, McGuire V, Sieh W, Montagna M, Alducci E, Sadetzki S, Chetrit A, Kwong A, Kjaer SK, Jensen A, Hogdall E, Neuhausen S, Nussbaum R, Daly M, Greene MH, Mai PL, Loud JT, Moysich K, Toland AE, Lambrechts D, Ellis S, Frost D, Brenton JD, Tischkowitz M, Easton DF, Antoniou A, Chenevix-Trench G, Gayther SA, Bowtell D, Pharoah PD. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res 2015;21:652–7. 10.1158/1078-0432.CCR-14-2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, Lambrechts D, Despierre E, Barrowdale D, McGuffog L, Healey S, Easton DF, Sinilnikova O, Benitez J, Garcia MJ, Neuhausen S, Gail MH, Hartge P, Peock S, Frost D, Evans DG, Eeles R, Godwin AK, Daly MB, Kwong A, Ma ES, Lazaro C, Blanco I, Montagna M, D'Andrea E, Nicoletto MO, Johnatty SE, Kjaer SK, Jensen A, Hogdall E, Goode EL, Fridley BL, Loud JT, Greene MH, Mai PL, Chetrit A, Lubin F, Hirsh-Yechezkel G, Glendon G, Andrulis IL, Toland AE, Senter L, Gore ME, Gourley C, Michie CO, Song H, Tyrer J, Whittemore AS, McGuire V, Sieh W, Kristoffersson U, Olsson H, Borg A, Levine DA, Steele L, Beattie MS, Chan S, Nussbaum RL, Moysich KB, Gross J, Cass I, Walsh C, Li AJ, Leuchter R, Gordon O, Garcia-Closas M, Gayther SA, Chanock SJ, Antoniou AC, Pharoah PD. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012;307:382–90. 10.1001/jama.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. Am J Prev Med 2003;24:190–8. 10.1016/S0749-3797(02)00593-7 [DOI] [PubMed] [Google Scholar]
- 34.Mai PL, Garceau AO, Graubard BI, Dunn M, McNeel TS, Gonsalves L, Gail MH, Greene MH, Willis GB, Wideroff L. Confirmation of family cancer history reported in a population-based survey. J Natl Cancer Inst 2011;103:788–97. 10.1093/jnci/djr114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003;72:1117–30. 10.1086/375033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramus SJ, Kartsonaki C, Gayther SA, Pharoah PD, Sinilnikova OM, Beesley J, Chen X, McGuffog L, Healey S, Couch FJ, Wang X, Fredericksen Z, Peterlongo P, Manoukian S, Peissel B, Zaffaroni D, Roversi G, Barile M, Viel A, Allavena A, Ottini L, Papi L, Gismondi V, Capra F, Radice P, Greene MH, Mai PL, Andrulis IL, Glendon G, Ozcelik H, Thomassen M, Gerdes AM, Kruse TA, Cruger D, Jensen UB, Caligo MA, Olsson H, Kristoffersson U, Lindblom A, Arver B, Karlsson P, Stenmark Askmalm M, Borg A, Neuhausen SL, Ding YC, Nathanson KL, Domchek SM, Jakubowska A, Lubinski J, Huzarski T, Byrski T, Gronwald J, Gorski B, Cybulski C, Debniak T, Osorio A, Duran M, Tejada MI, Benitez J, Hamann U, Rookus MA, Verhoef S, Tilanus-Linthorst MA, Vreeswijk MP, Bodmer D, Ausems MG, van Os TA, Asperen CJ, Blok MJ, Meijers-Heijboer HE, Peock S, Cook M, Oliver C, Frost D, Dunning AM, Evans DG, Eeles R, Pichert G, Cole T, Hodgson S, Brewer C, Morrison PJ, Porteous M, Kennedy MJ, Rogers MT, Side LE, Donaldson A, Gregory H, Godwin A, Stoppa-Lyonnet D, Moncoutier V, Castera L, Mazoyer S, Barjhoux L, Bonadona V, Leroux D, Faivre L, Lidereau R, Nogues C, Bignon YJ, Prieur F, Collonge-Rame MA, Venat-Bouvet L, Fert-Ferrer S, Miron A, Buys SS, Hopper JL, Daly MB, John EM, Terry MB, Goldgar D, Hansen T, Jonson L, Ejlertsen B, Agnarsson BA, Offit K, Kirchhoff T, Vijai J, Dutra-Clarke AV, Przybylo JA, Montagna M, Casella C, Imyanitov EN, Janavicius R, Blanco I, Lazaro C, Moysich KB, Karlan BY, Gross J, Beattie MS, Schmutzler R, Wappenschmidt B, Meindl A, Ruehl I, Fiebig B, Sutter C, Arnold N, Deissler H, Varon-Mateeva R, Kast K, Niederacher D, Gadzicki D, Caldes T, de la Hoya M, Nevanlinna H, Aittomaki K, Simard J, Soucy P, Spurdle AB, Holland H, Chenevix-Trench G, Easton DF, Antoniou AC. Genetic variation at 9p22.2 and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 2011;103:105–16. 10.1093/jnci/djq494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramus SJ, Antoniou AC, Kuchenbaecker KB, Soucy P, Beesley J, Chen X, McGuffog L, Sinilnikova OM, Healey S, Barrowdale D, Lee A, Thomassen M, Gerdes AM, Kruse TA, Jensen UB, Skytte AB, Caligo MA, Liljegren A, Lindblom A, Olsson H, Kristoffersson U, Stenmark-Askmalm M, Melin B, Domchek SM, Nathanson KL, Rebbeck TR, Jakubowska A, Lubinski J, Jaworska K, Durda K, Zlowocka E, Gronwald J, Huzarski T, Byrski T, Cybulski C, Toloczko-Grabarek A, Osorio A, Benitez J, Duran M, Tejada MI, Hamann U, Rookus M, van Leeuwen FE, Aalfs CM, Meijers-Heijboer HE, van Asperen CJ, van Roozendaal KE, Hoogerbrugge N, Collee JM, Kriege M, van der Luijt RB, Peock S, Frost D, Ellis SD, Platte R, Fineberg E, Evans DG, Lalloo F, Jacobs C, Eeles R, Adlard J, Davidson R, Eccles D, Cole T, Cook J, Paterson J, Douglas F, Brewer C, Hodgson S, Morrison PJ, Walker L, Porteous ME, Kennedy MJ, Pathak H, Godwin AK, Stoppa-Lyonnet D, Caux-Moncoutier V, de Pauw A, Gauthier-Villars M, Mazoyer S, Leone M, Calender A, Lasset C, Bonadona V, Hardouin A, Berthet P, Bignon YJ, Uhrhammer N, Faivre L, Loustalot C, Buys S, Daly M, Miron A, Terry MB, Chung WK, John EM, Southey M, Goldgar D, Singer CF, Tea MK, Pfeiler G, Fink-Retter A, Hansen T, Ejlertsen B, Johannsson OT, Offit K, Kirchhoff T, Gaudet MM, Vijai J, Robson M, Piedmonte M, Phillips KA, Van Le L, Hoffman JS, Ewart Toland A, Montagna M, Tognazzo S, Imyanitov E, Issacs C, Janavicius R, Lazaro C, Blanco I, Tornero E, Navarro M, Moysich KB, Karlan BY, Gross J, Olah E, Vaszko T, Teo SH, Ganz PA, Beattie MS, Dorfling CM, van Rensburg EJ, Diez O, Kwong A, Schmutzler RK, Wappenschmidt B, Engel C, Meindl A, Ditsch N, Arnold N, Heidemann S, Niederacher D, Preisler-Adams S, Gadzicki D, Varon-Mateeva R, Deissler H, Gehrig A, Sutter C, Kast K, Fiebig B, Schafer D, Caldes T, de la Hoya M, Nevanlinna H, Aittomaki K, Plante M, Spurdle AB, Neuhausen SL, Ding YC, Wang X, Lindor N, Fredericksen Z, Pankratz VS, Peterlongo P, Manoukian S, Peissel B, Zaffaroni D, Bonanni B, Bernard L, Dolcetti R, Papi L, Ottini L, Radice P, Greene MH, Mai PL, Andrulis IL, Glendon G, Ozcelik H, Pharoah PD, Gayther SA, Simard J, Easton DF, Couch FJ, Chenevix-Trench G. Ovarian cancer susceptibility alleles and risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Hum Mutat 2012;33:690–702. 10.1002/humu.22025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couch FJ, Wang X, McGuffog L, Lee A, Olswold C, Kuchenbaecker KB, Soucy P, Fredericksen Z, Barrowdale D, Dennis J, Gaudet MM, Dicks E, Kosel M, Healey S, Sinilnikova OM, Lee A, Bacot F, Vincent D, Hogervorst FB, Peock S, Stoppa-Lyonnet D, Jakubowska A, Radice P, Schmutzler RK, Domchek SM, Piedmonte M, Singer CF, Friedman E, Thomassen M, Hansen TV, Neuhausen SL, Szabo CI, Blanco I, Greene MH, Karlan BY, Garber J, Phelan CM, Weitzel JN, Montagna M, Olah E, Andrulis IL, Godwin AK, Yannoukakos D, Goldgar DE, Caldes T, Nevanlinna H, Osorio A, Terry MB, Daly MB, van Rensburg EJ, Hamann U, Ramus SJ, Toland AE, Caligo MA, Olopade OI, Tung N, Claes K, Beattie MS, Southey MC, Imyanitov EN, Tischkowitz M, Janavicius R, John EM, Kwong A, Diez O, Balmana J, Barkardottir RB, Arun BK, Rennert G, Teo SH, Ganz PA, Campbell I, van der Hout AH, van Deurzen CH, Seynaeve C, Gomez Garcia EB, van Leeuwen FE, Meijers-Heijboer HE, Gille JJ, Ausems MG, Blok MJ, Ligtenberg MJ, Rookus MA, Devilee P, Verhoef S, van Os TA, Wijnen JT, Frost D, Ellis S, Fineberg E, Platte R, Evans DG, Izatt L, Eeles RA, Adlard J, Eccles DM, Cook J, Brewer C, Douglas F, Hodgson S, Morrison PJ, Side LE, Donaldson A, Houghton C, Rogers MT, Dorkins H, Eason J, Gregory H, McCann E, Murray A, Calender A, Hardouin A, Berthet P, Delnatte C, Nogues C, Lasset C, Houdayer C, Leroux D, Rouleau E, Prieur F, Damiola F, Sobol H, Coupier I, Venat-Bouvet L, Castera L, Gauthier-Villars M, Leone M, Pujol P, Mazoyer S, Bignon YJ, Zlowocka-Perlowska E, Gronwald J, Lubinski J, Durda K, Jaworska K, Huzarski T, Spurdle AB, Viel A, Peissel B, Bonanni B, Melloni G, Ottini L, Papi L, Varesco L, Tibiletti MG, Peterlongo P, Volorio S, Manoukian S, Pensotti V, Arnold N, Engel C, Deissler H, Gadzicki D, Gehrig A, Kast K, Rhiem K, Meindl A, Niederacher D, Ditsch N, Plendl H, Preisler-Adams S, Engert S, Sutter C, Varon-Mateeva R, Wappenschmidt B, Weber BH, Arver B, Stenmark-Askmalm M, Loman N, Rosenquist R, Einbeigi Z, Nathanson KL, Rebbeck TR, Blank SV, Cohn DE, Rodriguez GC, Small L, Friedlander M, Bae-Jump VL, Fink-Retter A, Rappaport C, Gschwantler-Kaulich D, Pfeiler G, Tea MK, Lindor NM, Kaufman B, Shimon Paluch S, Laitman Y, Skytte AB, Gerdes AM, Pedersen IS, Moeller ST, Kruse TA, Jensen UB, Vijai J, Sarrel K, Robson M, Kauff N, Mulligan AM, Glendon G, Ozcelik H, Ejlertsen B, Nielsen FC, Jonson L, Andersen MK, Ding YC, Steele L, Foretova L, Teule A, Lazaro C, Brunet J, Pujana MA, Mai PL, Loud JT, Walsh C, Lester J, Orsulic S, Narod SA, Herzog J, Sand SR, Tognazzo S, Agata S, Vaszko T, Weaver J, Stavropoulou AV, Buys SS, Romero A, de la Hoya M, Aittomaki K, Muranen TA, Duran M, Chung WK, Lasa A, Dorfling CM, Miron A, Benitez J, Senter L, Huo D, Chan SB, Sokolenko AP, Chiquette J, Tihomirova L, Friebel TM, Agnarsson BA, Lu KH, Lejbkowicz F, James PA, Hall P, Dunning AM, Tessier D, Cunningham J, Slager SL, Wang C, Hart S, Stevens K, Simard J, Pastinen T, Pankratz VS, Offit K, Easton DF, Chenevix-Trench G, Antoniou AC. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013;9:e1003212 10.1371/journal.pgen.1003212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meindl A, Ditsch N, Kast K, Rhiem K, Schmutzler RK. Hereditary Breast and Ovarian Cancer: New Genes, New Treatments, New Concepts. DtschArzteblInt 2011;108:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, Bowden G, Kalmyrzaev B, Warren-Perry M, Snape K, Adlard JW, Barwell J, Berg J, Brady AF, Brewer C, Brice G, Chapman C, Cook J, Davidson R, Donaldson A, Douglas F, Greenhalgh L, Henderson A, Izatt L, Kumar A, Lalloo F, Miedzybrodzka Z, Morrison PJ, Paterson J, Porteous M, Rogers MT, Shanley S, Walker L, Eccles D, Evans DG, Renwick A, Seal S, Lord CJ, Ashworth A, Reis-Filho JS, Antoniou AC, Rahman N. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet 2011;43:879–82. 10.1038/ng.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafnar T, Gudbjartsson DF, Sulem P, Jonasdottir A, Sigurdsson A, Jonasdottir A, Besenbacher S, Lundin P, Stacey SN, Gudmundsson J, Magnusson OT, le Roux L, Orlygsdottir G, Helgadottir HT, Johannsdottir H, Gylfason A, Tryggvadottir L, Jonasson JG, de Juan A, Ortega E, Ramon-Cajal JM, Garcia-Prats MD, Mayordomo C, Panadero A, Rivera F, Aben KK, van Altena AM, Massuger LF, Aavikko M, Kujala PM, Staff S, Aaltonen LA, Olafsdottir K, Bjornsson J, Kong A, Salvarsdottir A, Saemundsson H, Olafsson K, Benediktsdottir KR, Gulcher J, Masson G, Kiemeney LA, Mayordomo JI, Thorsteinsdottir U, Stefansson K. Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet 2011;43:1104–7. 10.1038/ng.955 [DOI] [PubMed] [Google Scholar]
- 42.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. IV. The pathogenesis of epithelial ovarian cancer. Collaborative Ovarian Cancer Group. Am J Epidemiol 1992;136:1212–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.