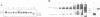Abstract
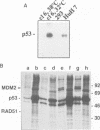
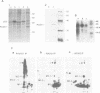
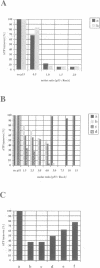
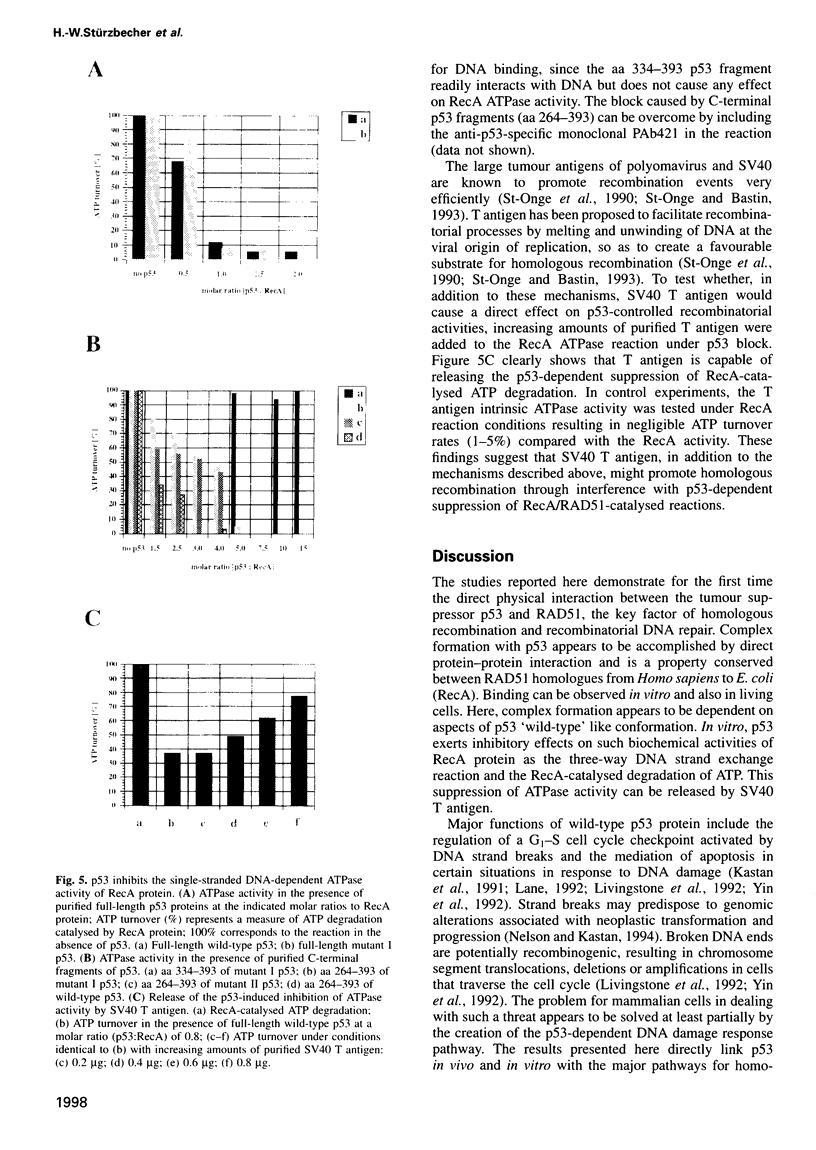
The tumour suppressor p53 prevents tumour formation after DNA damage by halting cell cycle progression to allow DNA repair or by inducing apoptotic cell death. Loss of wild-type p53 function renders cells resistant to DNA damage-induced cell cycle arrest and ultimately leads to genomic instabilities including gene amplifications, translocations and aneuploidy. Some of these chromosomal lesions are based on mechanisms that involve recombinatorial events. Here we report that p53 physically interacts with key factors of homologous recombination: the human RAD51 protein and its prokaryotic homologue RecA. In vitro, wild-type p53 inhibits defined biochemical activities of RecA protein, such as three-way DNA strand exchange and single strand DNA-dependent ATPase activity. In vivo, temperature-sensitive p53 forms complexes with RAD51 only in wild-type but not in mutant conformation. These observations suggest that functional wild-type p53 may select directly the appropriate pathway for DNA repair and control the extent and timing of the production of genetic variation via homologous recombination. Gene amplification an other types of chromosome rearrangements involved in tumour progression might occur not only as result of inappropriate cell proliferation but as a direct consequence of a defect in p53-mediated control of homologous recombination processes due to mutations in the p53 gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almon E., Goldfinger N., Kapon A., Schwartz D., Levine A. J., Rotter V. Testicular tissue-specific expression of the p53 suppressor gene. Dev Biol. 1993 Mar;156(1):107–116. doi: 10.1006/dbio.1993.1062. [DOI] [PubMed] [Google Scholar]
- Bakalkin G., Selivanova G., Yakovleva T., Kiseleva E., Kashuba E., Magnusson K. P., Szekely L., Klein G., Terenius L., Wiman K. G. p53 binds single-stranded DNA ends through the C-terminal domain and internal DNA segments via the middle domain. Nucleic Acids Res. 1995 Feb 11;23(3):362–369. doi: 10.1093/nar/23.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalkin G., Yakovleva T., Selivanova G., Magnusson K. P., Szekely L., Kiseleva E., Klein G., Terenius L., Wiman K. G. p53 binds single-stranded DNA ends and catalyzes DNA renaturation and strand transfer. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):413–417. doi: 10.1073/pnas.91.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R. K., Siegl B., Quellhorst S., Brandner G., Braun D. G. Monoclonal antibodies against simian virus 40 nuclear large T tumour antigen: epitope mapping, papova virus cross-reaction and cell surface staining. EMBO J. 1984 Jul;3(7):1485–1491. doi: 10.1002/j.1460-2075.1984.tb02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile G., Aker M., Mortimer R. K. Nucleotide sequence and transcriptional regulation of the yeast recombinational repair gene RAD51. Mol Cell Biol. 1992 Jul;12(7):3235–3246. doi: 10.1128/mcb.12.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson F. E., Stasiak A., West S. C. Purification and characterization of the human Rad51 protein, an analogue of E. coli RecA. EMBO J. 1994 Dec 1;13(23):5764–5771. doi: 10.1002/j.1460-2075.1994.tb06914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite A. W., Blair G. E., Nelson C. C., McGovern J., Bellett A. J. Adenovirus E1b-58 kD antigen binds to p53 during infection of rodent cells: evidence for an N-terminal binding site on p53. Oncogene. 1991 May;6(5):781–787. [PubMed] [Google Scholar]
- Dunderdale H. J., West S. C. Recombination genes and proteins. Curr Opin Genet Dev. 1994 Apr;4(2):221–228. doi: 10.1016/s0959-437x(05)80048-6. [DOI] [PubMed] [Google Scholar]
- Dutta A., Ruppert J. M., Aster J. C., Winchester E. Inhibition of DNA replication factor RPA by p53. Nature. 1993 Sep 2;365(6441):79–82. doi: 10.1038/365079a0. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Fiscella M., Ullrich S. J., Zambrano N., Shields M. T., Lin D., Lees-Miller S. P., Anderson C. W., Mercer W. E., Appella E. Mutation of the serine 15 phosphorylation site of human p53 reduces the ability of p53 to inhibit cell cycle progression. Oncogene. 1993 Jun;8(6):1519–1528. [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays S. L., Firmenich A. A., Berg P. Complex formation in yeast double-strand break repair: participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6925–6929. doi: 10.1073/pnas.92.15.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. C., Tokiwa T., Bennett W., Metcalf R. A., Welsh J. A., Sun T., Harris C. C. p53 gene mutation and integrated hepatitis B viral DNA sequences in human liver cancer cell lines. Carcinogenesis. 1993 May;14(5):987–992. doi: 10.1093/carcin/14.5.987. [DOI] [PubMed] [Google Scholar]
- Hupp T. R., Meek D. W., Midgley C. A., Lane D. P. Regulation of the specific DNA binding function of p53. Cell. 1992 Nov 27;71(5):875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
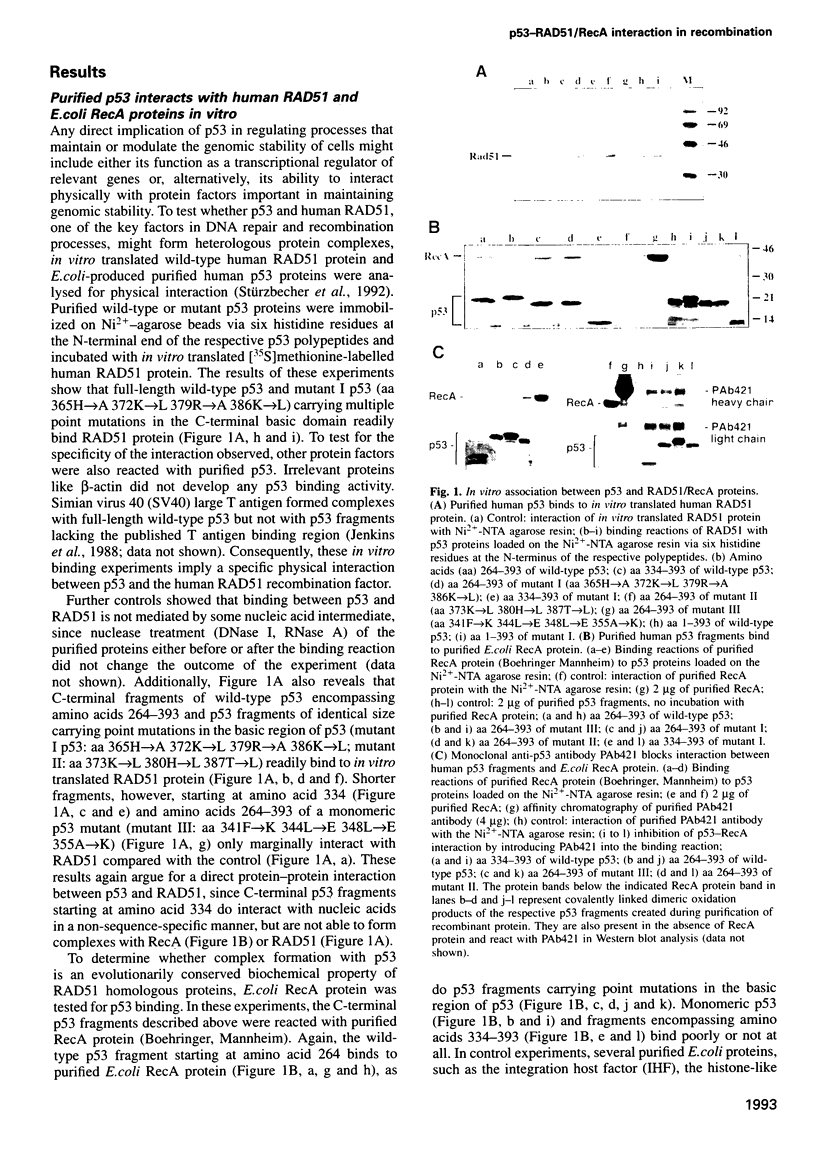
- Jenkins J. R., Chumakov P., Addison C., Stürzbecher H. W., Wade-Evans A. Two distinct regions of the murine p53 primary amino acid sequence are implicated in stable complex formation with simian virus 40 T antigen. J Virol. 1988 Oct;62(10):3903–3906. doi: 10.1128/jvi.62.10.3903-3906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. C., Yew P. R., Berk A. J. Domains required for in vitro association between the cellular p53 and the adenovirus 2 E1B 55K proteins. Virology. 1990 Dec;179(2):806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Lane D. P. Cancer. A death in the life of p53. Nature. 1993 Apr 29;362(6423):786–787. doi: 10.1038/362786a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Sung M. T. Use of N-chlorosuccinimide/urea for the selective cleavage of tryptophanyl peptide bonds in proteins. Cytochrome c. J Biol Chem. 1977 Jul 25;252(14):4976–4980. [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Milne G. T., Ho T., Weaver D. T. Modulation of Saccharomyces cerevisiae DNA double-strand break repair by SRS2 and RAD51. Genetics. 1995 Mar;139(3):1189–1199. doi: 10.1093/genetics/139.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Yoshimura Y., Yamamoto A., Murata K., Mori M., Yamamoto H., Matsushiro A. A mouse homolog of the Escherichia coli recA and Saccharomyces cerevisiae RAD51 genes. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6577–6580. doi: 10.1073/pnas.90.14.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. G., Kastan M. B. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994 Mar;14(3):1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberosler P., Hloch P., Ramsperger U., Stahl H. p53-catalyzed annealing of complementary single-stranded nucleic acids. EMBO J. 1993 Jun;12(6):2389–2396. doi: 10.1002/j.1460-2075.1993.tb05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Yu X., Shinohara A., Egelman E. H. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science. 1993 Mar 26;259(5103):1896–1899. doi: 10.1126/science.8456314. [DOI] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Patschinsky T., Bister K. Structural analysis of normal and transforming mil(raf) proteins: effect of 5'-truncation on phosphorylation in vivo or in vitro. Oncogene. 1988 Oct;3(4):357–364. [PubMed] [Google Scholar]
- Patschinsky T., Deppert W. Phosphorylation of p53 in primary, immortalised and transformed Balb/c mouse cells. Oncogene. 1990 Jul;5(7):1071–1076. [PubMed] [Google Scholar]
- Picksley S. M., Vojtesek B., Sparks A., Lane D. P. Immunochemical analysis of the interaction of p53 with MDM2;--fine mapping of the MDM2 binding site on p53 using synthetic peptides. Oncogene. 1994 Sep;9(9):2523–2529. [PubMed] [Google Scholar]
- Radding C. M. Helical interactions in homologous pairing and strand exchange driven by RecA protein. J Biol Chem. 1991 Mar 25;266(9):5355–5358. [PubMed] [Google Scholar]
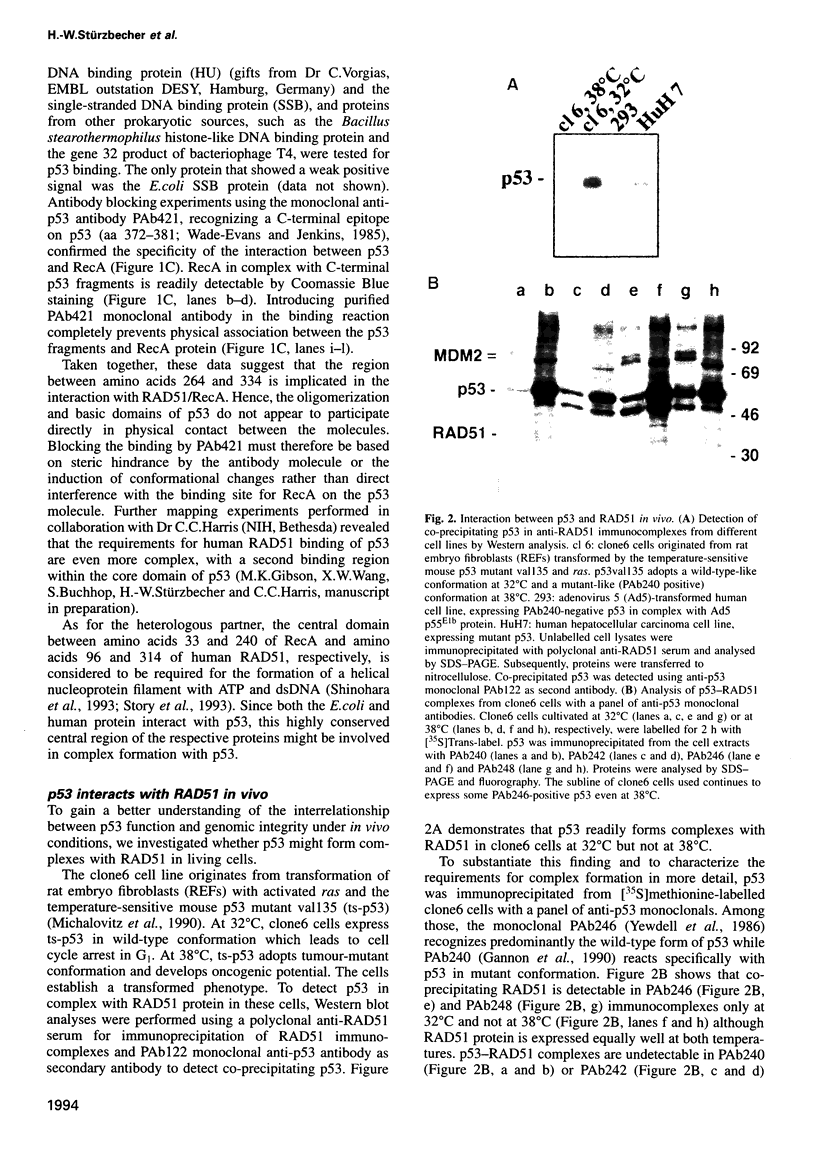
- Roth D. B., Lindahl T., Gellert M. Repair and recombination. How to make ends meet. Curr Biol. 1995 May 1;5(5):496–499. doi: 10.1016/s0960-9822(95)00101-1. [DOI] [PubMed] [Google Scholar]
- Schwartz D., Goldfinger N., Rotter V. Expression of p53 protein in spermatogenesis is confined to the tetraploid pachytene primary spermatocytes. Oncogene. 1993 Jun;8(6):1487–1494. [PubMed] [Google Scholar]
- Selva E. M., New L., Crouse G. F., Lahue R. S. Mismatch correction acts as a barrier to homeologous recombination in Saccharomyces cerevisiae. Genetics. 1995 Mar;139(3):1175–1188. doi: 10.1093/genetics/139.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Matsuda Y., Ushio N., Ikeo K., Ogawa T. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet. 1993 Jul;4(3):239–243. doi: 10.1038/ng0793-239. [DOI] [PubMed] [Google Scholar]
- St-Onge L., Bastin M. Amplification mediated by polyomavirus large T antigen defective in replication. J Virol. 1993 Aug;67(8):5025–5029. doi: 10.1128/jvi.67.8.5025-5029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge L., Bouchard L., Laurent S., Bastin M. Intrachromosomal recombination mediated by papovavirus large T antigens. J Virol. 1990 Jun;64(6):2958–2966. doi: 10.1128/jvi.64.6.2958-2966.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegenga W. T., Shvarts A., van Laar T., van der Eb A. J., Jochemsen A. G. Altered phosphorylation and oligomerization of p53 in adenovirus type 12-transformed cells. Oncogene. 1995 Jul 6;11(1):49–57. [PubMed] [Google Scholar]
- Story R. M., Bishop D. K., Kleckner N., Steitz T. A. Structural relationship of bacterial RecA proteins to recombination proteins from bacteriophage T4 and yeast. Science. 1993 Mar 26;259(5103):1892–1896. doi: 10.1126/science.8456313. [DOI] [PubMed] [Google Scholar]
- Stürzbecher H. W., Brain R., Addison C., Rudge K., Remm M., Grimaldi M., Keenan E., Jenkins J. R. A C-terminal alpha-helix plus basic region motif is the major structural determinant of p53 tetramerization. Oncogene. 1992 Aug;7(8):1513–1523. [PubMed] [Google Scholar]
- Stürzbecher H. W., Maimets T., Chumakov P., Brain R., Addison C., Simanis V., Rudge K., Philp R., Grimaldi M., Court W. p53 interacts with p34cdc2 in mammalian cells: implications for cell cycle control and oncogenesis. Oncogene. 1990 Jun;5(6):795–781. [PubMed] [Google Scholar]
- Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science. 1994 Aug 26;265(5176):1241–1243. doi: 10.1126/science.8066464. [DOI] [PubMed] [Google Scholar]
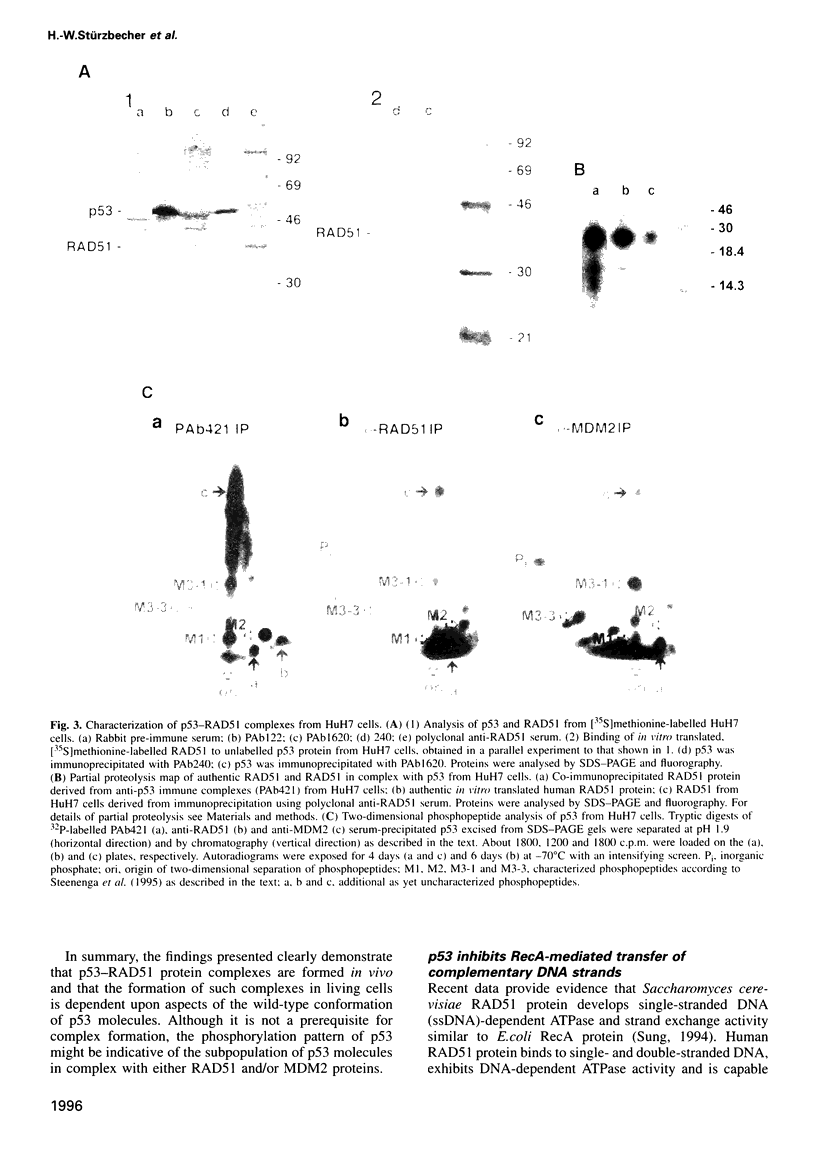
- Sung P., Robberson D. L. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell. 1995 Aug 11;82(3):453–461. doi: 10.1016/0092-8674(95)90434-4. [DOI] [PubMed] [Google Scholar]
- Wade-Evans A., Jenkins J. R. Precise epitope mapping of the murine transformation-associated protein, p53. EMBO J. 1985 Mar;4(3):699–706. doi: 10.1002/j.1460-2075.1985.tb03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W., Forrester K., Yeh H., Feitelson M. A., Gu J. R., Harris C. C. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Eckhart W. Phosphorylation sites in the amino-terminal region of mouse p53. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4231–4235. doi: 10.1073/pnas.89.10.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C. The processing of recombination intermediates: mechanistic insights from studies of bacterial proteins. Cell. 1994 Jan 14;76(1):9–15. doi: 10.1016/0092-8674(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Gannon J. V., Lane D. P. Monoclonal antibody analysis of p53 expression in normal and transformed cells. J Virol. 1986 Aug;59(2):444–452. doi: 10.1128/jvi.59.2.444-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Morita T., Yamamoto A., Matsushiro A. Cloning and sequence of the human RecA-like gene cDNA. Nucleic Acids Res. 1993 Apr 11;21(7):1665–1665. doi: 10.1093/nar/21.7.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]