Abstract
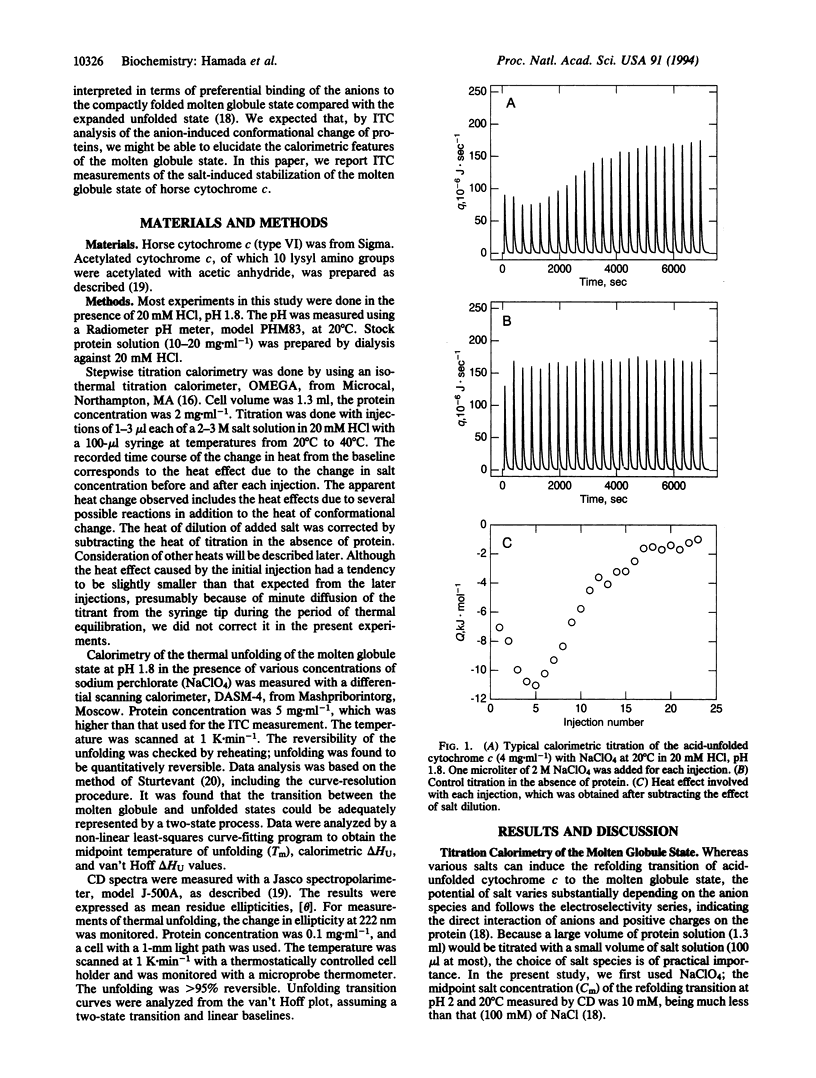
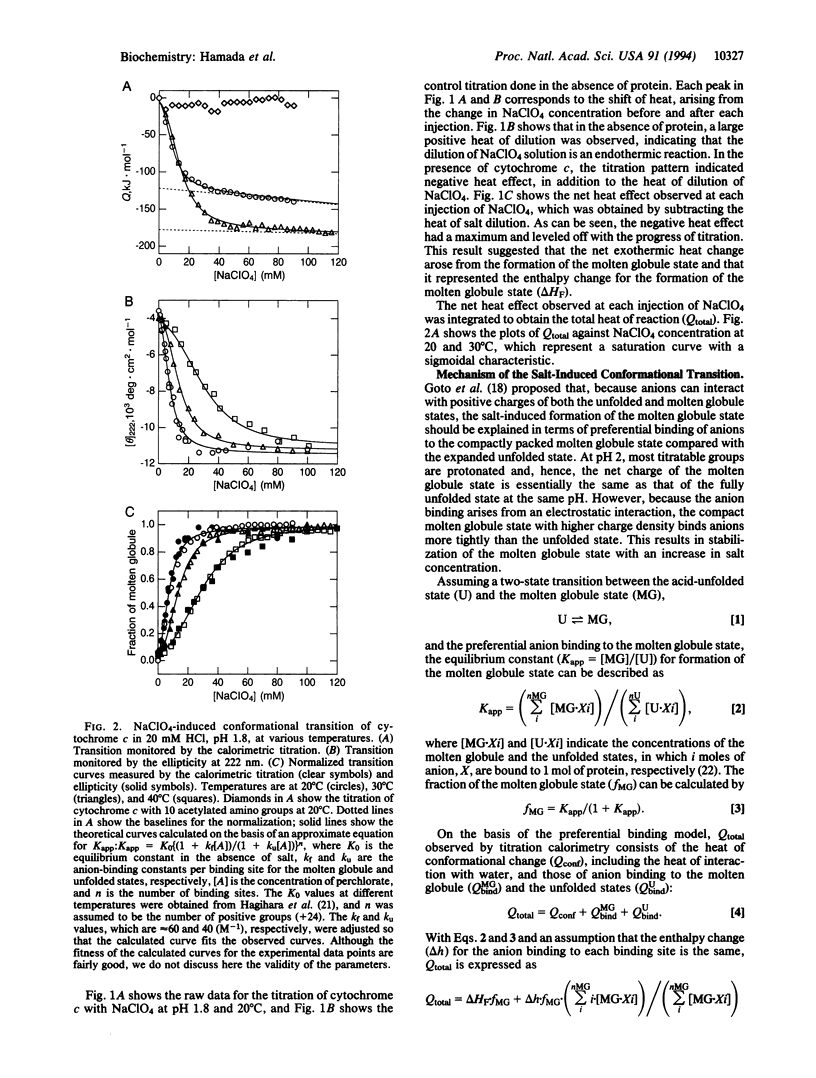
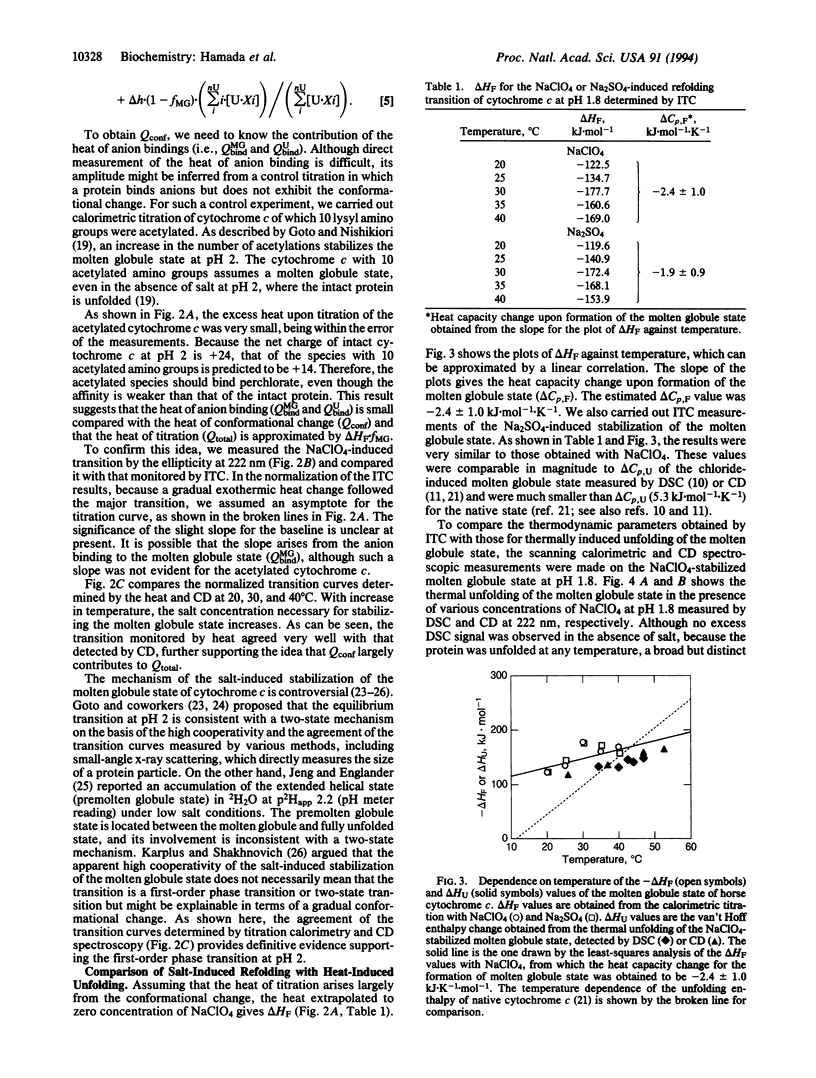
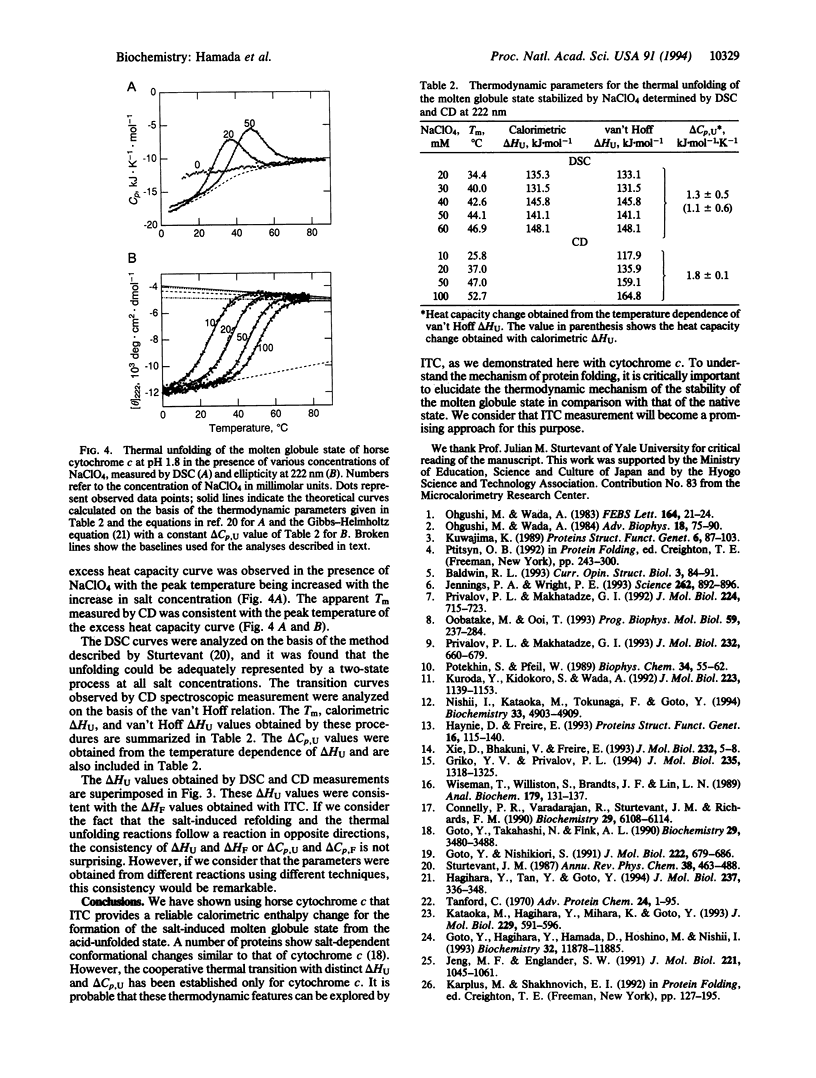
Although the molten globule state has been proposed as a major intermediate of protein folding, it has proven difficult to obtain thermodynamic data characterizing this state. To explore another approach for characterizing the molten globule state, salt-induced formation of the molten globule state of horse cytochrome c at pH 1.8 was studied by isothermal titration calorimetry. By titrating the acid-unfolded cytochrome c with sodium perchlorate, an exothermic reaction was observed. The titration curve obtained from the heat was cooperative and agreed well with the conformational transition curve measured by CD at 222 nm. This result indicated that the salt-induced conformation change is well approximated by a two-state transition between the acid-unfolded and molten globule states. The heat for formation of the molten globule state estimated by isothermal titration calorimetry was consistent with the enthalpy change for unfolding of the sodium perchlorate-stabilized molten globule state at pH 1.8, which was measured by differential scanning calorimetry and CD. These results indicate that the heat of titration largely reflects the enthalpy change of the conformational transition. From these results, we consider that isothermal titration calorimetry will become a useful approach for investigating the molten globule state.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Connelly P. R., Varadarajan R., Sturtevant J. M., Richards F. M. Thermodynamics of protein-peptide interactions in the ribonuclease S system studied by titration calorimetry. Biochemistry. 1990 Jun 26;29(25):6108–6114. doi: 10.1021/bi00477a031. [DOI] [PubMed] [Google Scholar]
- Goto Y., Hagihara Y., Hamada D., Hoshino M., Nishii I. Acid-induced unfolding and refolding transitions of cytochrome c: a three-state mechanism in H2O and D2O. Biochemistry. 1993 Nov 9;32(44):11878–11885. doi: 10.1021/bi00095a017. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nishikiori S. Role of electrostatic repulsion in the acidic molten globule of cytochrome c. J Mol Biol. 1991 Dec 5;222(3):679–686. doi: 10.1016/0022-2836(91)90504-y. [DOI] [PubMed] [Google Scholar]
- Goto Y., Takahashi N., Fink A. L. Mechanism of acid-induced folding of proteins. Biochemistry. 1990 Apr 10;29(14):3480–3488. doi: 10.1021/bi00466a009. [DOI] [PubMed] [Google Scholar]
- Griko Y. V., Privalov P. L. Thermodynamic puzzle of apomyoglobin unfolding. J Mol Biol. 1994 Jan 28;235(4):1318–1325. doi: 10.1006/jmbi.1994.1085. [DOI] [PubMed] [Google Scholar]
- Hagihara Y., Tan Y., Goto Y. Comparison of the conformational stability of the molten globule and native states of horse cytochrome c. Effects of acetylation, heat, urea and guanidine-hydrochloride. J Mol Biol. 1994 Apr 1;237(3):336–348. doi: 10.1006/jmbi.1994.1234. [DOI] [PubMed] [Google Scholar]
- Haynie D. T., Freire E. Structural energetics of the molten globule state. Proteins. 1993 Jun;16(2):115–140. doi: 10.1002/prot.340160202. [DOI] [PubMed] [Google Scholar]
- Jeng M. F., Englander S. W. Stable submolecular folding units in a non-compact form of cytochrome c. J Mol Biol. 1991 Oct 5;221(3):1045–1061. doi: 10.1016/0022-2836(91)80191-v. [DOI] [PubMed] [Google Scholar]
- Jennings P. A., Wright P. E. Formation of a molten globule intermediate early in the kinetic folding pathway of apomyoglobin. Science. 1993 Nov 5;262(5135):892–896. doi: 10.1126/science.8235610. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Hagihara Y., Mihara K., Goto Y. Molten globule of cytochrome c studied by small angle X-ray scattering. J Mol Biol. 1993 Feb 5;229(3):591–596. doi: 10.1006/jmbi.1993.1064. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Kidokoro S., Wada A. Thermodynamic characterization of cytochrome c at low pH. Observation of the molten globule state and of the cold denaturation process. J Mol Biol. 1992 Feb 20;223(4):1139–1153. doi: 10.1016/0022-2836(92)90265-l. [DOI] [PubMed] [Google Scholar]
- Kuwajima K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins. 1989;6(2):87–103. doi: 10.1002/prot.340060202. [DOI] [PubMed] [Google Scholar]
- Nishii I., Kataoka M., Tokunaga F., Goto Y. Cold denaturation of the molten globule states of apomyoglobin and a profile for protein folding. Biochemistry. 1994 Apr 26;33(16):4903–4909. doi: 10.1021/bi00182a019. [DOI] [PubMed] [Google Scholar]
- Ohgushi M., Wada A. 'Molten-globule state': a compact form of globular proteins with mobile side-chains. FEBS Lett. 1983 Nov 28;164(1):21–24. doi: 10.1016/0014-5793(83)80010-6. [DOI] [PubMed] [Google Scholar]
- Ohgushi M., Wada A. Liquid-like state of side chains at the intermediate stage of protein denaturation. Adv Biophys. 1984;18:75–90. doi: 10.1016/0065-227x(84)90007-8. [DOI] [PubMed] [Google Scholar]
- Oobatake M., Ooi T. Hydration and heat stability effects on protein unfolding. Prog Biophys Mol Biol. 1993;59(3):237–284. doi: 10.1016/0079-6107(93)90002-2. [DOI] [PubMed] [Google Scholar]
- Potekhin S., Pfeil W. Microcalorimetric studies of conformational transitions of ferricytochrome c in acidic solution. Biophys Chem. 1989 Sep 15;34(1):55–62. doi: 10.1016/0301-4622(89)80041-9. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Makhatadze G. I. Contribution of hydration and non-covalent interactions to the heat capacity effect on protein unfolding. J Mol Biol. 1992 Apr 5;224(3):715–723. doi: 10.1016/0022-2836(92)90555-x. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Makhatadze G. I. Contribution of hydration to protein folding thermodynamics. II. The entropy and Gibbs energy of hydration. J Mol Biol. 1993 Jul 20;232(2):660–679. doi: 10.1006/jmbi.1993.1417. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- Wiseman T., Williston S., Brandts J. F., Lin L. N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989 May 15;179(1):131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]



