Abstract
Constellation Pharmacology is a cell-based high-content phenotypic-screening platform that utilizes subtype-selective pharmacological agents to elucidate the cell-specific combinations (“constellations”) of key signaling proteins that define specific cell types. Heterogeneous populations of native cells, in which the different individual cell types have been identified and characterized, are the foundation for this screening platform. Constellation Pharmacology is useful for screening small molecules or for deconvoluting complex mixtures of biologically-active natural products. This platform has been used to purify natural products and discover their molecular mechanisms. In the on-going development of Constellation Pharmacology, there is a positive-feedback loop between the pharmacological characterization of cell types and screening for new drug candidates. As Constellation Pharmacology is used to discover compounds with novel targeting-selectivity profiles, those new compounds then further help to elucidate the constellations of specific cell types, thereby increasing the content of this high-content platform.
Keywords: Constellation Pharmacology, somatosensory neuron, DRG, conotoxin, high-content screen
INTRODUCTION
The advent of molecular biology produced optimism that both the therapeutic benefits and side effects of drugs could be understood mechanistically at the molecular level. In fact, molecular pharmacology and genetics have largely been successful in elucidating many of these mechanisms. However, productivity in therapeutic drug-discovery and development has not been commensurate with these striking advances in molecular biology. One consequence is that there has been a resurgence of interest in phenotypic screening for drug discovery, both at the whole-organism level and at the cellular level (1–3). Prior to the molecular-biology revolution, drug discovery was typically conducted through a form of phenotypic screening (3), often at the whole-organism level. Compounds that serendipitously produced desired therapeutic effects, with tolerable levels of side effects, were utilized in clinical practice. Notably, many of these compounds were natural products that had been honed by evolutionary forces to target a specific protein with high potency and selectivity (4, 5), although most drug mechanisms were a black box.
Although the terms “phenotypic screening” and “high-content screening” are often used synonymously, it is noteworthy that phenotypic screens, either at the whole-organism level or at the cellular level, are not truly high-content screens if the phenotypes elicited by novel compounds are uninterpretable or if the mechanisms are intractable. In this article, we detail how Constellation Pharmacology is a novel cell-based high-content phenotypic screening platform that not only interrogates numerous molecular mechanisms simultaneously, but also ties phenotypes elicited by pharmacological compounds to molecular targets and mechanisms in native cell types.
CORE CONCEPTS OF CONSTELLATION PHARMACOLOGY
Toxin cabals and their cognate constellations
The core concepts of Constellation Pharmacology arose from studies aimed at understanding the effects of the venoms of cone snails on their fish prey. A major conclusion from these studies was that individual venom components that the snail injected into a fish acted synergistically (6, 7). We began to realize that the snails were essentially using a combination drug strategy to attain the physiological endpoints needed to capture their prey efficiently.
We first elucidated the molecular mechanisms of individual venom components that inhibit neuromuscular transmission, which all fish-hunting cone snails appear to require. These venom components were called the “motor cabal” (6, 7). Cabals are secret societies that attempt to overthrow existing authority. Thus, the term “cabal” seemed appropriate for a group of toxins that act together in a synergistic manner to undermine the normal physiological state of the envenomated prey. Each individual venom peptide in the motor cabal targeted a different signaling protein complex involved in neuromuscular transmission (6, 7).
We refer to the molecular targets of a venom cabal as its cognate “constellation” (8–11). A constellation refers to a group of functionally linked ion channels, receptors or other signaling proteins. For neuromuscular signaling to occur, voltage-gated calcium channels at the pre-synaptic terminus must open before neurotransmitter is released, which then elicits opening of the post-synaptic nicotinic acetylcholine receptor (nAChR). This in turn causes depolarization at the post-synaptic terminus, which triggers activation of voltage-gated sodium channels resulting in the muscle action potential that ultimately triggers muscle contraction. Inhibiting any one of these sequentially-acting signaling components causes an inhibition of muscle contraction. Most importantly, these receptors and ion channels are functionally linked to each other and comprise the target “constellation” of the motor cabal.
Therapeutic cabals and constellations
Although the initial concepts of cabals and constellations came from toxin cabals and prey capture, the concepts of therapeutic cabals and constellations are a logical extension of these biological insights. If venom toxins can be used synergistically to facilitate prey capture, then certainly therapeutic compounds can also be used synergistically to facilitate a desired therapeutic endpoint. In some cases, this has already been demonstrated in clinical medicine. For example, patients who are HIV positive are often prescribed a combination or cocktail of drugs that act on a variety of molecular targets through diverse mechanisms. Some drugs in the cocktail inhibit viral entry (fusion) into cells, whereas others block the activity of viral enzymes, including reverse transcriptases, integrases and proteases (12, 13).
In order to extend the concepts of cabals and constellations to drug discovery, our first step has been to define cell-specific constellations. The cell, as the fundamental unit of life, is also the first level of significant integration of molecular components in biological systems. A specific cell type, with a specific physiological function, is therefore a potential entity for drug targeting, and therefore also a meaningful unit for phenotypic screening. Differences in cell-specific gene expression create distinct cell types with divergent physiological properties, functions and roles. In neurons, the ion channels and receptors expressed within a particular neuronal cell type are functionally linked to each other in order to integrate signaling inputs that result in a physiologically relevant output, such as action-potential firing and neurotransmitter release. The function of each cell-specific constellation is to produce the integrated physiological signaling of that neuronal cell type.
A relevant example of cell-specific constellations is found in the comparison of somatosensory neurons and sympathetic neurons of the peripheral nervous system. It has been shown that a gain-of-function mutation in one voltage-gated Na channel subtype, NaV1.7 (causing a depolarizing shift in resting membrane potential) produces hyper-excitability in a subset of somatosensory neurons, in which NaV1.7 is functionally coupled to another voltage-gated Na channel, NaV1.8 (14, 15). In these somatosensory neurons, the gain-of-function mutation in NaV1.7 causes the neurons to depolarize to a point at which NaV1.8 channels open, causing action potentials to fire. In contrast, the same gain-of-function mutation in sympathetic neurons causes hypo-excitability of those neurons because NaV1.8 is not expressed in sympathetic neurons (14, 15). Therefore, the NaV1.7 gain-of-function mutation merely causes those neurons to depolarize to a point at which NaV1.7 channels are inactivated, resulting in neuronal hypo-excitability. This example of cell-specific constellations is relevant to drug discovery and development, since targeting NaV1.7 in these different neuronal cell types would be expected to result in different physiological effects. Shifting the drug-discovery paradigm from molecular targets to cellular targets or higher levels of biological organization may be necessary to successfully treat some diseases.
Target-based screening, as a reductionistic paradigm, begins with the premise that one drug should be developed for one molecular target for one disease. This premise has been challenged by the proponents of network pharmacology, who argue that networks of signaling pathways are more relevant drug targets than single molecules (1, 16, 17). One example of this concept is synthetic lethality (17, 18), i.e. the fact that two individual gene deletions may not produce a phenotype, but their simultaneous deletion is lethal, suggesting that many signaling networks have redundancies that can compensate for loss of a single component, but not a loss of two components. This principle may be applied to drug discovery, where two drugs that are individually ineffective (because of redundancies in signaling networks) could be effective in combination, i.e. a therapeutic cabal.
The advocates of network pharmacology have argued that most therapeutic drugs interact with multiple targets and that polypharmacology should be optimized by medicinal chemistry for therapeutic benefit, rather than avoided (1, 17, 19, 20). However, in many cases, through medicinal chemistry, it may be very difficult to modify a single compound to achieve a set of beneficial molecular interactions, while eliminating other deleterious molecular interactions. The use of therapeutic cabals may be a more effective approach because each drug in the cabal may be administered at an optimally selective dose for its respective molecular target, which also minimizes off-target side effects. Notably, all binding interactions are concentration dependent, and therefore off-target side effects will be minimized when each drug can be administered at the minimal concentration required for therapeutic efficacy. With a single compound that has different binding affinities for different targets, it may be difficult to achieve a desired polypharmacology, while avoiding intolerable off-target side effects. In contrast, the development of drug cocktails or therapeutic cabals that act synergistically and specifically on cell-specific signaling pathways has the potential to reduce off-target side effects because each drug within a cocktail could be used at a lower concentration whenever there is functional synergy between the drug targets.
The principles of network pharmacology predict that phenotypic screening should have certain advantages over target-based screening. First, the best phenotypic screens have the potential to elucidate the broad spectrum of biochemical interactions and biological effects elicited by a compound. Second, network pharmacology re-introduces an old, but intuitively obvious concept: that the broad biological profile of a drug is more important than any particular molecular interaction (17).
THE HIGH CONTENT OF CONSTELLATION PHARMACOLOGY
Specialized roles of cell types
The foundation of Constellation Pharmacology is a population of diverse cell types that provide the content of this high-content platform. We illustrate this principle with somatosensory neurons of the dorsal root ganglia (DRG). Their physiological roles are easily understood; based on the different sensory modalities mediated by these neurons, there may be as many as 30 different neuronal cell types within the mammalian DRG. For example, these neurons may be divided into at least the following broad classes: thermosensors, mechanosensors, proprioceptors, pruriceptors and nociceptors (21–24), as shown in Figure 1. Furthermore, these neuronal cell classes may be subdivided into more narrowly defined subclasses. For example, there are thermosensors that mediate the sensation of heat and others that mediate the sensation of cold (23, 25). Such neuronal subtypes may be further differentiated by their threshold response to a stimulus (9, 10, 23, 25, 26) (Figure 1). Nociceptors, which mediate painful sensations, typically require higher-threshold stimuli to fire action potentials than low-threshold neurons that report non-painful sensations. Additionally, some nociceptive neurons are polymodal, responding to multiple types of noxious stimuli (23, 27). In virtually all cases, broad cell classes can be subdivided into more narrowly defined subclasses, each of which presumably has a specific physiological role (9–11, 24, 28, 29).
Figure 1.
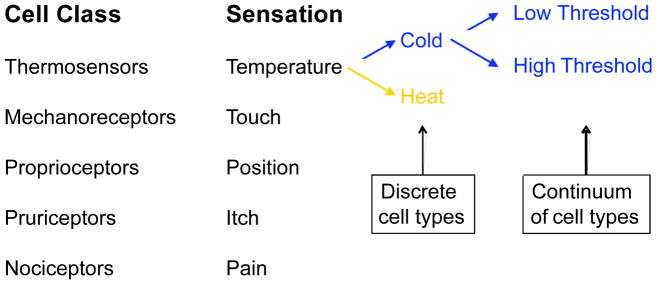
Somatosensory neurons (e.g. DRG neurons) are collectively one example of a heterogeneous cell population that can be used for Constellation Pharmacology. This cell population, like presumably all other native cell populations, is comprised of broad cell classes that each encompass more narrowly define cellular subclasses. Although there is presently debate about the degree to which various cell populations contain discrete cell types vs. a continuum of cell types, there is evidence that both claims are true, at least to some degree. For example, there are somatosensory neurons that appear to mediate a single sensory modality. However, these also appear to encompass cells with a range of thresholds for firing action potentials, from low threshold (innocuous stimuli) to high threshold (noxious or painful stimuli) (9). There are also somatosensory neurons that are polymodal, mediating multiple types of sensory modalities.
Constellation Pharmacology employs functional calcium imaging in combination with diverse sets of subtype-selective pharmacological agents to parse heterogeneous cell populations into cellular subclasses. Figure 2 demonstrates that a subset of small-diameter DRG neurons respond to the application of menthol, an agonist of TRPM8 channels. Their response to menthol indicates that they are cold-sensitive DRG neurons. Additionally, Figure 2 shows that we also employ non-pharmarcological markers of cell type in combination with Constellation Pharmacology. In this case, we stained live cells with isolectin B4, a marker for a subset of small-diameter DRG neurons with unmyelinated C fibers (30).
Figure 2.
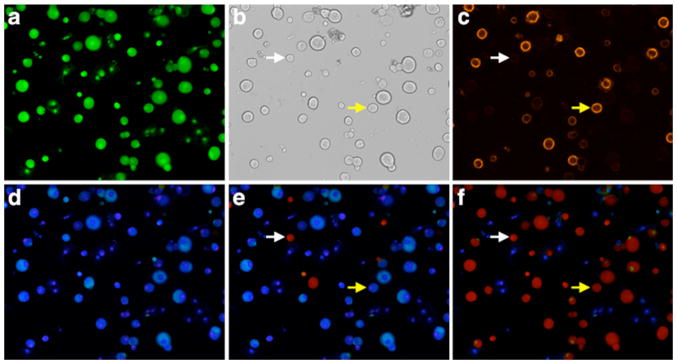
Images of a culture of dissociated lumbar DRG neurons from mice. All images are from a single field of view. (a) The green fluorescence of cells loaded with Fura-2 dye (380 nm excitation and 510 nm emission). (b) Brightfield image. (c) Live cells stained with isolectin B4 conjugated to Alexa-Fluor 546, which is reported to label a subset of primarily small-diameter neurons with unmyelinated C-fibers (30). (d) Pseudocolored ratiometric image of cells loaded with Fura-2 dye at rest (ratio of 510 nm emission obtained from alternating excitation with 340 nm and 380 nm light). (e) Pseudocolored ratiometric image of cells loaded with Fura-2 dye, just after stimulation with menthol, which activated a subset of primarily small-diameter neurons. The red color indicates a relatively high concentration of cytosolic calcium, whereas blue indicates a relatively low concentration of cytosolic calcium. (f) Pseudocolored ratiometric image of cells loaded with Fura-2 dye, just after a depolarizing stimulus (high concentration of extracellular potaasium). Notably all of the neurons (red) responded to this stimulus but some very small-diameter non-neuronal cells did not respond (blue), as expected. (b,c,e,f) White arrow points to a small-diameter neuron that responded to menthol and depolarization, but did not stain with isolectin B4. Yellow arrow points to a neuron that labeled with isolectin B4 and responded to depolarization, but did not respond to menthol.
In the on-going development of Constellation Pharmacology, we may subject a cell population to a series of pharmacological challenges, while simultaneously monitoring the individual responses of 100 – 200 cells by calcium imaging. For each individual cell, a trace of the type illustrated in Figure 3 is obtained. A particular set of pharmacological agents may elicit different response phenotypes in different cell types, which become phenotypic fingerprints that demarcate and define each cell type.
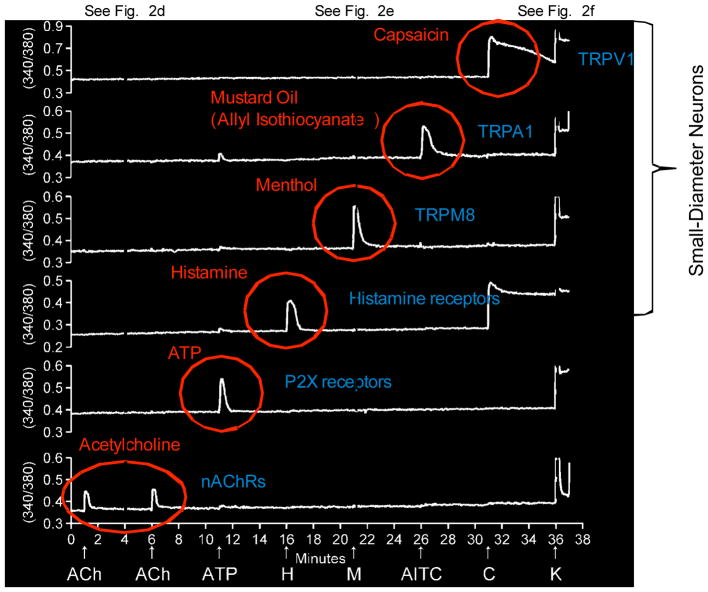
Figure 3.
Selected calcium-imaging traces from an experiment in which responses from more than 100 mouse DRG cells were monitored simultaneously. Each trace represents the response of a single cell. Traces from six cells were chosen. Each arrow represents the 15 second application of each respective pharmacological agent. Notably, the response profiles or phenotypes (black traces) to various pharmacological agents (red) tell us something about the distinct physiological roles of these different cell types. For example, the small subset of cells that respond to menthol (M) are putatively the somatosensory neurons that mediate the sensation of cold (cold thermosensor). The neurons that respond to histamine (H) are putatively neurons that mediate the sensation of itch (pruriceptors) and possibly include pain-sensing neurons also (nociceptors). The neurons that respond to capsaicin (C) and mustard oil (AITC) are putative nociceptors. The aforementioned cell types are all predominantly small-diameter neurons. The neurons that respond only to acetylcholine (ACh) or ATP are typically large-diameter neurons that include low-threshold mechanoreceptors and proprioceptors (that detect position or motion in a body part). The receptors and ion channels that respond to these stimuli are listed in blue type in the figure. Many other neurons in these cultures respond to more than one stimulus. The dashed lines that refer to Figure 2 represent the cytosolic calcium concentration of cells at rest (see Fig. 2d), after stimulation with menthol (see Fig. 2e), and after depolarization with potassium (see Fig. 2f). This figure was modified from (11) (we need to get permission from PNAS to modify and reproduce it).
Cell-specific constellations
The central concept of Constellation Pharmacology is to differentiate between cell types by their cell-specific constellations of key signaling proteins, using a set of pharmacological agents, including both agonists (as shown in Figure 3) and antagonists. If 8 different pharmacological agents are applied (as shown in Figure 3), then in ~1 hour of experimental time, 1600 assays (200 cells X 8 pharmacological challenges) are effectively conducted simultaneously. Ideally, either the agonist or antagonist for a particular signaling protein or protein complex will be highly selective, thus reporting the functional expression of a specific protein-complex subtype within a specific cell type. Accordingly, this general approach develops the content of this high-content platform.
For neuronal cells, plasma-membrane receptors and ion channels are among the most important determining factors of cell-type identity (31). In Figure 3, we demonstrate that various receptor agonists are useful for activation of specific classes and subclasses of somatosensory neurons. For example, cold thermosensors respond to menthol (TRPM8 agonist) (9, 10, 32); pruriceptors respond to either histamine (histamine receptor agonist) or chloroquine (agonist of certain Mas-related GPCRs) (33, 34), and nociceptors may respond to a variety of receptor agonists that include capsaicin (TRPV1 agonist) (35–37), mustard oil (ally isothiocyanate, TRPA1 agonist) (21, 38) or ATP (P2X and P2Y receptor agonist) (39, 40). However, these generalizations are somewhat oversimplified and it is important to recognize that responsiveness to just one of these agonists typically does not demarcate a specific somatosensory neuronal cell type. In most cases, cell classes that respond to one stimulus (pharmacological or physiological) can be subdivided into more narrowly defined subclasses based on their responsiveness to other types of stimuli that reveal their cell-specific constellations (9–11, 24, 28, 29). It is the cell-specific constellation that defines the cell type and determines its physiological function.
Inevitably, many molecular targets are widely distributed and found in a diverse set of different cell types (9–11, 24, 28, 29). Other molecular isoforms of ion channels and receptors may be completely absent from any cell present in a given anatomical locus. These two extremes are not particularly useful for differentiating between cell types, but those molecular targets that are restricted to one or a few of the cellular subclasses present in the heterogeneous mixture of cells may be the most valuable cell-type markers for Constellation Pharmacology, helping to differentiate certain cell types from all the other cell types present in the population.
One good example of the principles just described is from somatosensory neurons that transmit the sensation of cold temperature to the brain (9, 10). Although these neurons comprise a small minority of somatosensory neurons, they are relatively easy to identify. Even in culture, cold-thermosensor neurons (which detect non-painful cold) and cold-nociceptor neurons (which detect painful cold) respond to menthol and to cold temperatures (9, 10). The cold thermosensors will respond to temperatures that are regarded as innocuous cold (i.e., temperatures above 15°C). In contrast, cold nociceptors only respond to noxious cold temperatures (<15°C) (32, 41, 42). These two neuronal subclasses can be distinguished from each other by their differential responses to innocuous cold temperatures and from other sensory neurons by their responses to noxious cold temperatures.
What has emerged from the study of these two different neuronal subclasses is that they differ in their expression of almost every family of receptor and ion channel that has been assessed, using a battery of highly selective pharmacological agents (9, 10). The striking divergence in the spectrum of receptors and ion channels in the cold-sensitive neurons suggests that at the molecular level, the constellations of any two neuronal subclasses will differ. As the number of subtype-selective pharmacological agents increases, it may ultimately become possible to find a combination of two pharmacological agents that uniquely identifies any specific cell type at any given anatomical locus. The pharmacological differentiation of neuronal cell types, by their cell-specific constellations, appears to be feasible for any locus of the nervous system, with the studies carried out on DRG neurons already providing the proof-of-principle evidence.
Constant and variable components of cell types
Although many experiments have shown that a neuron maintains its identity even after in vitro culturing (31), it is important to recognize that some of the molecular components of specific cell types are not entirely static, but may vary over time. Notably, a recent review defines a neuronal cell type as a “conserved molecular ground state” that has core (constant) components that define the cell type but other components may vary (31). For instance, the expression of some signaling proteins within a particular cell type may change as a function of normal processes (e.g. development, conditioning, learning, or other environmental factors) and pathological processes (e.g. disease, injury, aging, etc.).
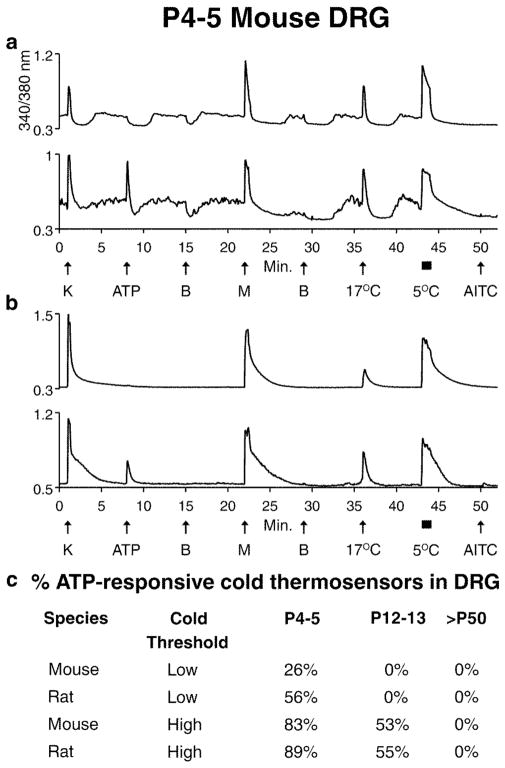
One advantage of Constellation Pharmacology is that it enables the identification and study of specific neuronal cell types at different time points or across species. Some comparative cellular physiology has already validated the utility of the Constellation Pharmacology platform in this respect. Cold-thermosensor neurons from mouse and rat DRG were investigated for expression of ATP receptors from neonatal through adult animals. In both mouse and rat, most cold-thermosensor neurons expressed ATP receptors in neonates but the expression disappeared in these neurons as the animals matured (Figure 4) (9). Interestingly, the rate of disappearance of the ATP receptors varied by the temperature threshold of the cold-thermosensor neurons. The rate of disappearance was faster in the low-threshold cold thermosensors than in the high-threshold cold thermosensors, although there was a complete loss of ATP receptors in fully mature mouse and rat low- and high-threshold cold thermosensors (Figure 4) (9). A similar comparison of cold-thermosensors from rat and mouse revealed a striking interspecies difference. The large majority of cold-thermosensor neurons in rats expressed the TRPA1 channel, while the vast majority of mouse cold-thermosensor neurons did not (9).
Figure 4.
(a & b) Selected calcium-imaging trace from one minor subclass of somatosensory neurons with variant forms: cold thermosensors. (a) Low-threshold cold thermosensors responded to menthol and innocuous cold temperature (e.g. 17 °C). Notably, the baseline calcium levels of these cells fluctuate with minor changes in temperature (at room temperature). Specifically, replacing the static-bath solution produces a transient slight warming, followed by evaporative cooling (9). (b) high-threshold cold thermosensors also responded to menthol and innocuous cold temperature (e.g. 17 °C). Notably, the baseline calcium levels of these cells are relatively stable, because they have higher threshold cold responses. (a & b) In neonatal mice, some of these neurons responded to ATP, as shown in the traces. (c) The ATP-receptor expression in neonatal cold thermosensors was observed in both mice and rats, and in both species the expression was lost as the animals matured. This figure was reproduced from (9) (we need to get permission from PNAS to reproduce it).
In principle, Constellation Pharmacology can also be used to assess changes that occur in specific cell types as a function of disease progression. This immediately raises the possibility of exploring disease mechanisms that are poorly understood, from neurodegenerative diseases to various forms of cancer. The progression from normal to pathological cell types could lead to a sharper definition of transitions between intermediate cellular states in the progression of disease, thus providing an opportunity to identify additional drug targets to inhibit any critical cellular transition.
From single cells to functional networks
The characterization of single cells is a critical step in the development of Constellation Pharmacology. An additional benefit of Constellation Pharmacology is the potential to elucidate the functional roles of cell types within functional networks. This can be illustrated by recent work (43) in which Constellation Pharmacology was applied to the ventral respiratory column (VRC), an area in the brainstem that generates the respiratory rhythm. As part of the pharmacological characterization to discriminate between different cell types in the VRC, a neuronal subclass responsive to substance P, histamine and bradykinin was identified. Prior work on the VRC network that controls the respiratory rhythm (known as the pre-Bötzinger complex) had revealed that inspiratory neurons in the circuit were modulated by substance P. However, it was not known that histamine and bradykinin could also directly modulate the activity of these inspiratory neurons. This hypothesis, suggested by Constellation Pharmacology, was tested and confirmed by electrophysiology on the slice preparation (43). This work also suggested that Constellation Pharmacology may eventually be extended beyond dissociated cells to the characterization of cell types within functional cellular networks, i.e. brain slice preparation or other intact tissue preparations.
SCREENING APPLICATIONS OF CONSTELLATION PHARMACOLOGY
We are using Constellation Pharmacology to identify and characterize compounds that have unique targeting selectivity. As demonstrated below, Constellation Pharmacology has particularly high promise for accelerating the discovery of ligands that selectively target a specific ion channel, transporter or receptor subtype. Furthermore, it is useful for purifying each of the biochemically diverse components present in complex biological mixtures and for deconvoluting their functionally diverse biological activities, particularly when the total amount of material available is limiting. Natural products are obvious partners for phenotypic screening, not only because the starting material may be limiting, but because these natural compounds often have built-in potency and selectivity for therapeutic targets.
Constellation Pharmacology is an attractive alternative to target-based screening for venom components and other natural products. Only limited amounts of venom are available from most animals, with a few exceptions, such as the large snakes, which can be routinely milked. Venom from a single animal may contain more than one hundred components, all of which have been honed by evolutionary forces to bind a single molecular target with high potency and selectivity. Screening venoms or venom components against a single molecular target of interest is an inefficient use of these scarce resources, which may be evaluated more efficiently in high-content phenotypic screens.
Venom peptides
The Constellation Pharmacology platform has been used for bioassay-guided purification of the components of several Conus venoms. Typically, multiple bioactive peptides can be detected in each venom. As shown in Figure 5 for Conus musicus and tessulatus venoms, we have screened venoms through an iterative approach, starting with crude venom, followed by biochemical fractionation of the venom. Either crude venom or venom fractions were applied to the DRG cell culture to observe the various response phenotypes elicited in different cell types.
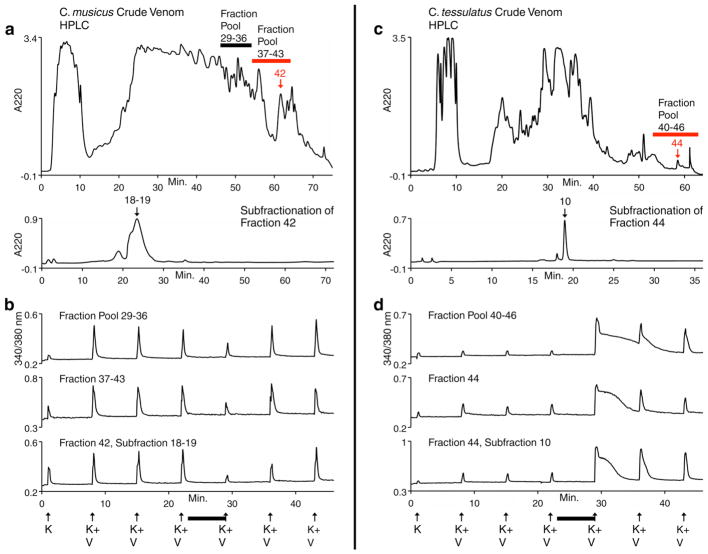
Figure 5.
Purification of novel peptides from C. musicus and C. tessulatus, using Constellation Pharmacology. (a) HPLC fractionation of crude C. musicus venom. Fraction 42 was further fractionated and the activity was isolated to subfractions 18–19. Further subfractionation was required to purify the active component (not shown). (b) Selected calcium-imaging traces from individual cells demonstrating that the active component from fraction 42 blocked the response to a depolarizing stimulus. (c) HPLC fractionation of crude C. tessulatus venom. Fraction 44 was further fractionated and the activity was isolated to subfraction 10. (d) Selected calcium-imaging traces from individual cells demonstrating that the active component from fraction 44 amplified the response to a depolarizing stimulus. (b & d) The initial depolarizing stimulus (at minute 1), was 20 mM extracellular potassium (K). At subsequent time points, the depolarizing stimulus was 20 mM extracellular potassium with 20 mM veratridine (K+V). This stimulus was used for screening venom fractions, in order to better detect blockers of voltage-gated Na channels. The black horizontal bar indicates when each respective venom fraction was present in the bath solution.
Figure 5a & b demonstrates that two fraction pools from C. musicus venom blocked the response to a depolarizing stimulus. From one of these fraction pools (37–43), we were able to follow the activity of one component though purification (Figure 5a & b). This component was a novel peptide with the following sequence: ZRGECRPNGTGCGKDLOLFGCCSGWCLFVCV# (Z = pyroglutamate, O = 4-trans-hydroxyproline, and # = amidated C-terminus). In contrast to the peptide from C. musicus, Figure 5c & d, demonstrates that one fraction pool from C. tessulatus venom amplified the response to a depolarizing stimulus. The active component of this fraction pool was also a novel peptide with the following sequence: CAAFGSFCGLPGLVDCCSGRCFIVCLL. This peptide has been further characterized as a novel δ-conotoxin (δ-conotoxin TsVIA) that delays inactivation of voltage-gated Na channels, consistent with its amplification of a depolarizing stimulus (44). This demonstrates the utility of Constellation Pharmacology for detecting diverse activities of venom components, by which those components may be purified biochemically.
The excitatory activity elicited by δ-conotoxin TsVIA affected almost all DRG neurons and was detected when the crude venom of C. tessulatus was assayed. The activity of this peptide was followed through multiple venom subfractionations (purification steps) until the peptide was purified to homogeneity. The purified peptide retained the broad-spectrum excitatory activity (Figure 5c & d). Both the biological activity observed using the Constellation Pharmacology platform, as well as the sequence of the peptide, suggested that the peptide was a δ-conotoxin. This was verified by electrophysiology using Xenopus oocytes that express the cloned mouse NaV1.6 channel, a Na channel subtype that is prevalent in axons. The δ-conotoxin that was purified from C. tessulatus venom is the first member of this family from a worm-hunting cone snail, and the sequence of the peptide proved to have broad significance (44).
Constellation Pharmacology may also be used to prioritize which venom components to purify or to evaluate for molecular targeting selectivity. Through molecular cloning, it is now straightforward to obtain an enormous diversity of venom-peptide sequences. The synthesis of these peptides, while requiring specialized expertise, can be achieved in a relatively straightforward way. However, it is often difficult to decide which compounds to pursue for further characterization. Ideally, we would prioritize ligands that have unique molecular targeting selectivity and/or mechanisms of action.
Constellation Pharmacology was recently used to identify that a novel family of venom peptides (P-like crassipeptides) from crassispirine snails exhibited a different targeting-selectivity profile than various conotoxins from cone snails (45). Some conopeptides exhibited effects in an overlapping set of cells, but the crassipeptides elicited or altered responses in a unique subset of small-diameter capsaicin-sensitive neurons. This cellular-targeting profile was unprecedented in our hands and also consistent with the activity of other potassium-channel blockers tested by Constellation Pharmacology (45). Consequently, one of the crassipeptides was confirmed by electrophysiology to block the shaker potassium channel (45). Thus, Constellation Pharmacology serves both as a high-content screening platform for purification and characterization of the components of complex venoms but also for differentiating the molecular targeting selectivity profiles of venom components.
Other natural products
Like venom peptides, small molecule natural products are produced as complex mixtures that likely have evolved to produce precise phenotypic responses in their targets. Their remarkable biological activities have been extensively exploited in drug discovery (46). We sought to explore whether natural products from bacteria could be screened using Constellation Pharmacology. One concern was that the many components in crude extracts might interfere with accurate interpretation of the assay. However, experience with hundreds of unfractionated bacterial extracts showed that the cellular responses still reflected the impact of one or a few very active components (47). In initial studies prior to the development of Constellation Pharmacology, in collaboration with Alan Light and co-workers, we found that a particular bacterial extract led to modest modulation of a subset of DRG neurons. The activity was traced to a series of novel small molecules, the pulicatins, which were solely responsible for the activity (48). Using other methods, it was shown that the pulicatins bind with nanomolar affinity to human serotonin receptor subtype 5HT2B, but not to related receptors. This finding led to further synthetic analogs that are currently under evaluation in biological models. Another crude extract from bacteria led to an apparently irreversible block of the response of DRG neurons to capsaicin, which was traced to action on the TRPV1 receptor (49). Other hit compounds were also discovered by this method (50).
While this initial work led to active compounds, determining the molecular target was very time consuming as in most phenotypic assays. The development of Constellation Pharmacology has enabled a more rapid focus on potentially interesting molecules. The first application of the method to small molecule natural products involved small molecule polyketides, the nocapyrones (51). The nocapyrones were applied to DRG neurons using an early version of Constellation Pharmacology. Capsaicin-responsive neurons were activated by nocapyrones, while other neurons in the DRG showed a decreased response to KCl when treated with nocapyrones. This early work demonstrated the advantages of Constellation Pharmacology as applied to small-molecule natural products, in addition to venoms.
Small molecule natural products exhibit several potential complications that are not found with venom peptides. For example, small molecules diffuse through membranes, and they can interact with components in the cytoplasm as well as extracellular proteins. They also commonly interfere with membranes or act as ionophores. These types of indirect effects can generally be recognized using Constellation Pharmacology. As with the venom peptides, there is a positive feedback loop between the discovery of compounds that elicit novel cellular response phenotypes and the further characterization of cell types using those novel pharmacological agents.
Molecular target identification
In order to develop an outstanding high-content screening assay, the phenotypic effect elicited by each compound should be traceable to specific molecular mechanisms in specific cell types. In general, the difficulty in target identification is the major drawback of phenotypic screening (2, 52), and perhaps the rate-limiting step. We have previously and now continue to put considerable effort into identifying each of the cell types present in a heterogeneous population and the cell-specific constellations of each cell type so that we can more easily identify the molecular mechanisms underlying the phenotypic effects elicited by novel pharmacological agents screened in this system.
One recent extension of Constellation Pharmacology is the use of cells from reporter mice in these assays. These mice have one or more cell types labeled with soluble fluorescent proteins expressed in the cytosol (Figure 6). This facilitates the identification and characterization of a specific cell type, but more importantly, the fluorescence of these cell types allow them to be sorted by FACS for a comprehensive evaluation of their transcriptomes. The transcriptomes can then be used to help identify the molecular targets and mechanisms of novel compounds that elicit phenotypic effects in those cell types. By identifying signaling components that are functionally expressed in particular cell types, novel drug targets may be discovered. For example, the elucidation of the constellations expressed within nociceptive neurons may reveal potential drug targets for pain. Perhaps most importantly, as broad cell-specific constellations are elucidated, each of the novel drug targets may become assayable in tandem, thereby continuously improving the utility of Constellation Pharmacology.
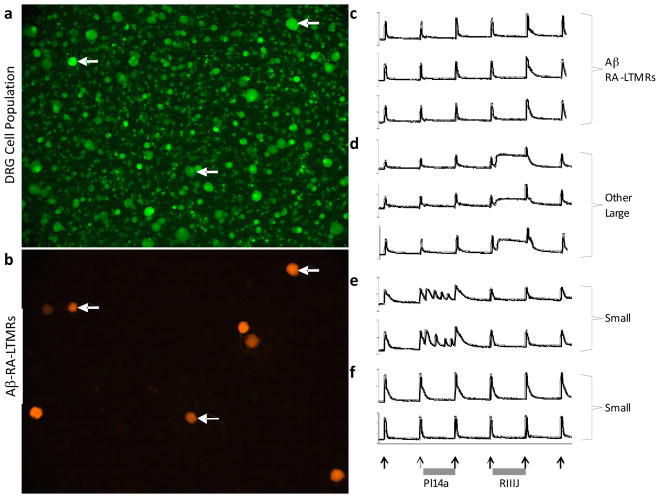
Figure 6.
In addition to the pharmacological profiling of cell types, we are combining Constellation Pharmacology with the genetic labeling of cell types. (a) Shown is a field of view of DRG cells loaded with Fura-2 dye. (b) The same field of view shown in A is shown here. In collaboration with the Ginty lab at Harvard, this experiment shows that a subset of large-diameter DRG neurons were fluorescently labeled with tdTomato. These neurons are rapidly adapting low-threshold mechanoreceptors with (heavily myelinated) Aβ fibers (Aβ-RA-LTMRs) (53). (c) It is noteworthy that in this experiment a conotoxin blocker of KV1.6 channels (κJ-conotoxin pl14a) did not affect the Aβ-RA-LTMRs but a conotoxin blocker of KV1.2 channels (κM-conotoxin RIIIJ) modestly amplified the response to a depolarizing stimulus (high concentration of extracellular potassium, as indicated by arrows) in the Aβ-RA-LTMRs. (d–f) Other neuronal cell types, corresponding to either large-diameter or small-diameter neurons, exhibited different phenotypes in response to these stimuli, as shown in the figure.
SUMMARY
Constellation Pharmacology is a multi-faceted platform that is useful for both drug discovery and for the identification and study of cell types. These reciprocally synergistic efforts imply that Constellation Pharmacology can be continuously improved. Constellation Pharmacology has the potential to comprehensively elucidate the cell-specific constellations of every cell type in a heterogeneous cell population and to evaluate changes that occur in cell-specific constellations as a function of disease progression. As a screening platform, Constellation Pharmacology can be used to screen and deconvolute complex biological mixtures (e.g. venoms or bacterial extracts) or synthetic small molecules in order to identify compounds that exhibit unique molecular and cellular targeting-selectivity profiles. Comparison to known pharmacologically-active compounds is facile, providing a direct pathway to discover compounds with the most novel targeting selectivity. Furthermore, cell-type-specific transcriptomes, to be used in conjunction with Constellation Pharmacology, facilitate molecular-target identification. Finally, it is possible to focus drug discovery and development on compounds that target a specific cell type, to achieve a desired therapeutic benefit, through the concerted and synergistic action of a therapeutic cabal on a therapeutic constellation.
Acknowledgments
The research of the authors was supported by GM48677 (RWT and BMO) and ICBG grant U01TW008163 (EWS).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Kell DB. Finding novel pharmaceuticals in the systems biology era using multiple effective drug targets, phenotypic screening and knowledge of transporters: where drug discovery went wrong and how to fix it. FEBS J. 2013;280:5957–80. doi: 10.1111/febs.12268. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Bogyo M. Target deconvolution techniques in modern phenotypic profiling. Curr Opin Chem Biol. 2013;17:118–26. doi: 10.1016/j.cbpa.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507–19. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- 4.Teichert RW, Olivera BM. Natural products and ion channel pharmacology. Future Med Chem. 2010;2:731–44. doi: 10.4155/fmc.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson I, Anderson EA. Chemistry. The renaissance of natural products as drug candidates. Science. 2005;310:451–3. doi: 10.1126/science.1116364. [DOI] [PubMed] [Google Scholar]
- 6.Olivera BM. E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Mol Biol Cell. 1997;8:2101–9. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terlau H, Shon K, Grilley M, Stocker M, Stühmer W, Olivera BM. Strategy for rapid immobilization of prey by a fish-hunting cone snail. Nature. 1996;381:148–51. doi: 10.1038/381148a0. [DOI] [PubMed] [Google Scholar]
- 8.Smith NJ, Hone AJ, Memon T, Bossi S, Smith TE, et al. Comparative functional expression of nAChR subtypes in rodent DRG neurons. Frontiers in cellular neuroscience. 2013;7:225. doi: 10.3389/fncel.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teichert RW, Memon T, Aman JW, Olivera BM. Using constellation pharmacology to define comprehensively a somatosensory neuronal subclass. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2319–24. doi: 10.1073/pnas.1324019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teichert RW, Raghuraman S, Memon T, Cox JL, Foulkes T, et al. Characterization of two neuronal subclasses through constellation pharmacology. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12758–63. doi: 10.1073/pnas.1209759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teichert RW, Smith NJ, Raghuraman S, Yoshikami D, Light AR, Olivera BM. Functional profiling of neurons through cellular neuropharmacology. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1388–95. doi: 10.1073/pnas.1118833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harbor perspectives in medicine. 2012;2:a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolis DM, Hazuda DJ. Combined approaches for HIV cure. Current opinion in HIV and AIDS. 2013;8:230–5. doi: 10.1097/COH.0b013e32835ef089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rush AM, Dib-Hajj SD, Liu S, Cummins TR, Black JA, Waxman SG. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8245–50. doi: 10.1073/pnas.0602813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waxman SG. Sodium channels, the electrogenisome and the electrogenistat: lessons and questions from the clinic. The Journal of physiology. 2012;590:2601–12. doi: 10.1113/jphysiol.2012.228460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrold JM, Ramanathan M, Mager DE. Network-based approaches in drug discovery and early development. Clin Pharmacol Ther. 2013;94:651–8. doi: 10.1038/clpt.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–90. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 18.Ooi SL, Pan X, Peyser BD, Ye P, Meluh PB, et al. Global synthetic-lethality analysis and yeast functional profiling. Trends Genet. 2006;22:56–63. doi: 10.1016/j.tig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–20. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina-Franco JL, Giulianotti MA, Welmaker GS, Houghten RA. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov Today. 2013;18:495–501. doi: 10.1016/j.drudis.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–43. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nature reviews Neuroscience. 2007;8:114–27. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 23.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belmonte C, Viana F. Molecular and cellular limits to somatosensory specificity. Molecular pain. 2008;4:14. doi: 10.1186/1744-8069-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neuroscience and biobehavioral reviews. 2010;34:177–84. doi: 10.1016/j.neubiorev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci. 2009;29:3120–31. doi: 10.1523/JNEUROSCI.4778-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmermann K, Hein A, Hager U, Kaczmarek JS, Turnquist BP, et al. Phenotyping sensory nerve endings in vitro in the mouse. Nature protocols. 2009;4:174–96. doi: 10.1038/nprot.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard A, Sorensen SA, Lein ES. Shifting the paradigm: new approaches for characterizing and classifying neurons. Curr Opin Neurobiol. 2009;19:530–6. doi: 10.1016/j.conb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Wichterle H, Gifford D, Mazzoni E. Neuroscience. Mapping neuronal diversity one cell at a time. Science. 2013;341:726–7. doi: 10.1126/science.1235884. [DOI] [PubMed] [Google Scholar]
- 30.Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkuhler J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. The Journal of comparative neurology. 2007;502:325–36. doi: 10.1002/cne.21311. [DOI] [PubMed] [Google Scholar]
- 31.Fishell G, Heintz N. The neuron identity problem: form meets function. Neuron. 2013;80:602–12. doi: 10.1016/j.neuron.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 32.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–8. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–65. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nature neuroscience. 2013;16:910–8. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annual review of neuroscience. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 36.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 37.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 38.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giniatullin R, Nistri A. Desensitization properties of P2X3 receptors shaping pain signaling. Frontiers in cellular neuroscience. 2013;7:245. doi: 10.3389/fncel.2013.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabbretti E. ATP P2X3 receptors and neuronal sensitization. Frontiers in cellular neuroscience. 2013;7:236. doi: 10.3389/fncel.2013.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hensel H, Zotterman Y. The effect of menthol on the thermoreceptors. Acta physiologica Scandinavica. 1951;24:27–34. doi: 10.1111/j.1748-1716.1951.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 42.Rainville P, Chen CC, Bushnell MC. Psychophysical study of noxious and innocuous cold discrimination in monkey. Experimental brain research. Experimentelle Hirnforschung Experimentation cerebrale. 1999;125:28–34. doi: 10.1007/s002210050654. [DOI] [PubMed] [Google Scholar]
- 43.Raghuraman S, Garcia AJ, III, Anderson T, Twede V, Curtice KJ, et al. Defining modulatory inputs into CNS neuronal subclasses through functional pharmacological profiling. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1404421111. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aman JW, Imperial J, Ueberheide B, Zhang M-M, Aguilar M, et al. Insights into the origins of fish-hunting in venomous cone snails from studies of Conus tessulatus. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1424435112. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imperial J, Cabang A, Song J, Raghuraman S, Gajewiak J, et al. A Family of Excitatory Peptide Toxins from Venomous Crassispirine Snails: Using Constellation Pharmacology to Assess Bioactivity. Toxicon. 2014 doi: 10.1016/j.toxicon.2014.06.014. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peraud O, Biggs JS, Hughen RW, Light AR, Concepcion GP, et al. Microhabitats within venomous cone snails contain diverse actinobacteria. Appl Environ Microbiol. 2009;75:6820–6. doi: 10.1128/AEM.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Z, Antemano RR, Hughen RW, Tianero MD, Peraud O, et al. Pulicatins A-E, neuroactive thiazoline metabolites from cone snail-associated bacteria. J Nat Prod. 2010;73:1922–6. doi: 10.1021/np100588c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Z, Reilly CA, Antemano R, Hughen RW, Marett L, et al. Nobilamides A-H, long-acting transient receptor potential vanilloid-1 (TRPV1) antagonists from mollusk-associated bacteria. J Med Chem. 2011;54:3746–55. doi: 10.1021/jm101621u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Z, Marett L, Hughen RW, Flores M, Forteza I, et al. Neuroactive diol and acyloin metabolites from cone snail-associated bacteria. Bioorg Med Chem Lett. 2013;23:4867–9. doi: 10.1016/j.bmcl.2013.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Z, Torres JP, Ammon MA, Marett L, Teichert RW, et al. A bacterial source for mollusk pyrone polyketides. Chem Biol. 2013;20:73–81. doi: 10.1016/j.chembiol.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tashiro E, Imoto M. Target identification of bioactive compounds. Bioorg Med Chem. 2012;20:1910–21. doi: 10.1016/j.bmc.2011.10.081. [DOI] [PubMed] [Google Scholar]
- 53.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–27. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]