Abstract
All cells use changes in intracellular calcium concentration ([Ca2+]i) to regulate cell signalling events. In neurons, with their elaborate dendritic and axonal arborizations, there are clear examples of both localized and widespread Ca2+ signals. [Ca2+]i changes that are generated by Ca2+ entry through voltage- and ligand-gated channels are the best characterized. In addition, the release of Ca2+ from intracellular stores can result in increased [Ca2+]i; the signals that trigger this release have been less well-studied, in part because they are not usually associated with specific changes in membrane potential. However, recent experiments have revealed dramatic widespread Ca2+ waves and localized spark-like events, particularly in dendrites. Here we review emerging data on the nature of these signals and their functions.
In most CNS neurons, the best-characterized intra-cellular calcium concentration ([Ca2+]i) changes follow from opening of voltage-gated calcium channels (VGCCs) or ligand-gated channels1–3. Action potentials generate widespread [Ca2+]i increases in axons and presynaptic terminals and, when they backpropagate, over large regions of the dendrites. Synaptic potentials evoke localized [Ca2+]i increases in the synaptic region. More localized Ca2+ signals result from Ca2+ entry through ligand-gated channels; the classic example is the entry of Ca2+ through NMDA receptors on postsynaptic spines. These signals are brief and of moderate amplitude, as the rise time of the Ca2+ transient is determined by the time course of the spike or synaptic potential, and [Ca2+]i is rapidly returned to resting levels through cytoplasmic buffers and efficient membrane and sarcoendoplasmic reticulum calcium ATPase (SERCA) pumps. In some circumstances, regenerative Ca2+ spikes or NMDA spikes, usually in the more distal parts of the dendrites, can generate much larger and longer lasting [Ca2+]i increases4,5.
Less is known about the [Ca2+]i changes that result from Ca2+ release from internal stores. Although pharmacological and immunohistochemical evidence of their presence and potential significance has been clear for many years2,6,7, direct observations and conclusions about these signals in intact neurons have been harder to realize, in part because they are not directly associated with membrane potential changes. With the advent of new technology and more sensitive Ca2+ indicators (BOX 1), these [Ca2+]i changes have begun to be characterized and have provoked renewed interest in the consequences of these changes. It is now clear that signalling mediated by Ca2+ release through the classic endoplasmic reticulum channels — ryanodine receptors (RyRs) and inositol trisphosphate receptors (IP3Rs) — exists in most neurons. These signals can combine with or complement the voltage- and ligand-gated Ca2+ transients (which together are often referred to as the ‘calcium toolkit’)8. The great range of neuronal cell types, and their complex arborizations, leads to diverse expression patterns of these signals and probably diverse functions, many of which have not yet been rigorously established.
Ca2+ waves
Propagating Ca2+ waves are the most dramatic expression of Ca2+ release from internal stores. They reflect regenerative Ca2+ release, where elevated cytoplasmic Ca2+ induces further Ca2+ release (calcium-induced calcium release (CICR)) through a nonlinear cooperative process. There are two types of wave — the most common are waves mediated through the opening of IP3Rs (FIG. 1). These waves were first described in nonneuronal cells, such as Xenopus laevis oocytes9,10 and HeLa cells11, in which their main properties were characterized. In oocytes, this [Ca2+]i increase provides a developmental signal, and in other cells, such as exocrine gland cells, they can transfer information from one side of the cell to another12. The other type of Ca2+ wave, which is much rarer, is mediated by the regenerative activation of RyRs. They have been observed in cardiac myocytes13, but it is not clear whether they occur under normal physiological conditions.
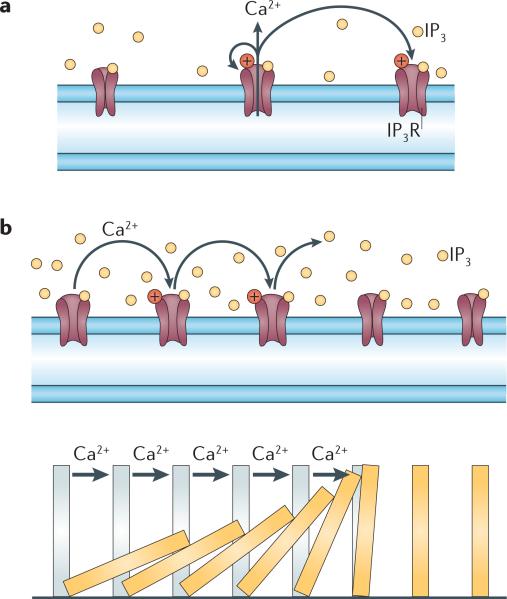
Figure 1. Model of regenerative Ca2+ release and wave propagation.
a | Opening the inositol trisphosphate receptor (IP3R) requires both IP3 and Ca2+. The Ca2+ released through the receptor can act on the same IP3R or other IP3Rs to cause calcium-induced calcium release (CICR). b | By acting on other receptors, Ca2+ release can propagate as far as IP3 is available. The analogy to ‘toppling dominos’ (lower panel) is appropriate. Figure is adapted from REF. 138 © (2010) The Japan Academy.
Ca2+ waves in neurons were discovered more recently. In these cells, with their spatially extended, intricate dendrites and axons, the properties of calcium waves and other [Ca2+]i changes take on interesting forms. Although detailed information about the spatial distribution of relevant channels and receptors involved in Ca2+ release in neurons is still lacking, it is clear that the molecular configurations are different in different regions of the cell and in different neuronal cell types6,14, which leads to different patterns of Ca2+ release.
Synaptically activated, IP3-mediated Ca2+ waves (FIG. 2) have been observed in pyramidal neurons in the rodent hippocampus (CA1 and CA3 regions), cortex (layer 2/3 (L2/3) and L5) and principal neurons in the amygdala15–20. Interestingly, they have also been observed in pyramidal neurons in the turtle cortex21. As turtles diverged from mammals over 300 million years ago and occupy a different ecological niche, this finding suggests that Ca2+ waves evolved early and are a robust and conserved property of pyramidal neurons and possibly other cell types.
Figure 2. Synaptically activated Ca2+ waves in a neocortical pyramidal neuron.
These waves can be easily evoked with repetitive focal synaptic stimulation in acute slices. In this experiment, a pyramidal neuron was loaded with the low-affinity indicator furaptra (300 μM) via a patch electrode on the soma and stimulated via a tungsten electrode (dashed arrow) at 100 Hz for 0.25 s (a and c). Two views of the resulting changes in intracellular calcium concentration ([Ca2+]i) are shown. Part b shows a pseudocolour line scan of the fluorescence changes along a selected series of pixels in a. The timescale for b is the same as for the optical and electrical traces shown below in d. Part d shows the time course of the fluorescence changes at the regions of interest (ROIs), which are indicated by coloured rectangles in c. The time courses and amplitudes of the delayed responses differ at the four ROIs. This figure illustrates that two waves initiated at different locations in dendrites (close to branch points) can be propagated along the main dendrite in both directions. In this cell, the waves did not propagate into the soma. ΔF/F, relative change in fluorescence. Figure courtesy of W.N.R.
The peak [Ca2+]i amplitude of these waves can be over 5 μM if measured with non-buffering low-affinity Ca2+ indicators16,22, which is much higher than the 0.15–0.3 μM signal that results from VGCC-mediated Ca2+ entry that is evoked by a backpropagating action potential (bAP) measured in the same dendritic region23,24. The synaptically evoked Ca2+ wave signal usually lasts much longer (0.5–1.5 s) than the brief Ca2+ transients (0.02–0.1 s) evoked by ligand-gated or spike-evoked Ca2+ signals25. The typical propagation velocity of these waves is ~100 μm/s when measured with low-affinity Ca2+ indicators26, whereas bAP-evoked Ca2+ signals propagate over this distance in 0.5 ms27. The large amplitude and long duration of the wave signals suggest that they should be effective activators of Ca2+-signalling pathways.
IP3-mediated Ca2+ release occurs in other neuron types but may be expressed in different ways. Ca2+ release, but not in the form of propagating waves, is prominent in cerebellar Purkinje neurons, especially in spines28,29, and has been observed in some interneurons30. The lack of propagating waves in these cells may be due to the high concentration of endogenous Ca2+ buffers31,32, which could interfere with the regenerative character of CICR, as injection of exogenous buffers, such as EGTA, can prevent wave propagation without preventing Ca2+ release17,33.
From a molecular perspective, Ca2+ wave generation in neurons conforms to the standard signalling cascade that has been described in many other cell types. An exogenous agonist (neurotransmitter) activates phospholipase Cβ (PLCβ), which generates IP3 and diacylglycerol (DAG); IP3 acts on the IP3R to release Ca2+ from the endoplasmic reticulum, and the released Ca2+ acts on nearby IP3Rs to release more Ca2+ (CICR). Because activation of the IP3R requires both Ca2+ and IP3 (REFS 34–36), the initial Ca2+ release by mobilized IP3 requires the presence of some Ca2+ in the cytoplasm. If the concentration of IP3 is high enough, the resting [Ca2+]i can be sufficient37; at lower IP3 levels additional Ca2+, usually acquired from Ca2+ entry through VGCCs, is required. Once CICR is initiated, regenerative propagation will continue provided that IP3 is available; the level of IP3 at rest is not sufficient. The requirement for co-activation by IP3 and Ca2+ makes regenerative Ca2+ release a coincidence detector for metabotropic glutamate receptor (mGluR) or muscarinic acetylcho-line receptor (mAChR) activation (which mobilizes IP3) and postsynaptic Ca2+ signalling (from bAPs or dendritic Ca2+ spikes) in pyramidal neurons17,22,38 and Purkinje neurons39. The timing window for this synergistic response varies from 100 to 500 ms17,38,39. The duration of the window is closely related to the lifetime of IP3 in the dendritic region where the wave is generated, which is determined by a combination of IP3 degradation, IP3 diffusion and IP3 unbinding from the receptor38,40. If diffusion is the dominant factor, as seems to be the case in pyramidal neuron dendrites, then the timing window will be smaller if the spatial extent of Ca2+ release is more restricted because diffusional dissipation is more rapid in this case.
Spread of Ca2+ waves
The region where Ca2+ waves extend determines which downstream signalling mechanisms are activated by the [Ca2+]i changes they generate. In many small cells the extent of propagation of this regenerative mechanism is not a crucial issue; the released Ca2+ effectively spreads over the entire cell. In bigger cells, especially neurons with complex morphology, the distribution of the components of this signalling apparatus within the cell are vitally important for determining whether Ca2+ release transforms into a propagating Ca2+ wave.
In pyramidal neurons in the hippocampus and cortex, Ca2+ waves are usually detected in the primary apical dendrite, and sometimes in the soma, with slight penetration (10–20 μm) into the oblique and basal dendrites; the limits of propagation into the distal dendrites are not as precisely determined, but waves are rarely detected beyond the point where the thick dendrites begin to branch16,26. Similar patterns are observed in projection neurons in the basolateral amygdala, although the waves in the fine dendrites are not as easy to follow41. This restricted range for wave propagation is interesting because the endoplasmic reticulum is thought to extend continuously throughout the dendrites of pyramidal neurons42 and Purkinje neurons43,44. Furthermore, it is exactly orthogonal to the location of branches with high concentrations of spines45 and, consequently, to the location of Ca2+ entry through NMDA receptors and regenerative NMDA spikes26,46 (see Supplementary information S1 (movie)). Although there are some spines on the apical dendrite and some Ca2+ release in the finer processes, the sharpness of the boundary between the territories of NMDA spikes and Ca2+ waves is remarkable47. By contrast, the spatial extent of [Ca2+]i changes as a result of bAPs and Ca2+ spikes is more diffuse and extends over both apical dendrites and fine branches.
The location and spatial extent of individual Ca2+ waves depend on the stimulation protocol. Waves detected following threshold synaptic stimulation can be as small as 5 μm, although this is not a well-determined limit. These threshold waves can be generated in different locations in the apical dendrites, usually close to the site of stimulation. Waves generated by ionophoresis or puffing of metabotropic agonists show a similar distribution, except that they easily activate waves in the soma if the agonist is released over that location. Bath application of agonists (trans-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD) or carbachol (CCh)) in low concentrations will robustly support Ca2+ waves if they are triggered by bAPs (although high concentrations of agonists might activate waves by themselves). These agonist-generated waves extend over the apical dendrites and soma, that is, over the full potential range of wave generation. Lastly, waves can be generated by uncaging IP3 that has been loaded into the cells. These waves usually extend over the area of uncaging if the flash is confined to the main apical dendrite and the soma; uncaging IP3 in the oblique or basal dendrites evokes only a weak Ca2+ response or no response at all.
These patterns suggest that the main factors determining the extent of wave generation are the distribution of IP3Rs in the cell and the locus and magnitude of IP3 generation. Waves can only spread over the area within which IP3 is generated and diffuses. This region is constrained when IP3 is generated by focal synaptic stimulation, focal ionophoresis of metabotropic agonists (such as t-ACPD48,49 or muscarine50) or by localized uncaging of IP3 (REFS 19,38). However, when IP3 is mobilized all over the cell following bath application of agonists22,50 or by uncaging of IP3 over the entire neuron51, the waves will spread as far as IP3Rs are available in sufficient densities to support regenerative CICR. The spatiotemporal patterns of Ca2+ release are the same whether t-ACPD or CCh is used in these experiments22, suggesting that it is not the distribution of different kinds of metabotropic receptors that is crucial.
Brief trains of glutamatergic52 or cholinergic50 synaptic stimulation (or both41) in hippocampal slices generate localized Ca2+ waves that are usually confined to the dendrites. The extent of propagation increases with increasing stimulus intensity, which recruits presynaptic fibres over a larger area. These waves rarely extend into the soma following low-intensity stimulation because there are few synaptic contacts there (FIG. 2). However, following strong and sustained stimulation, enough IP3 can be generated to allow waves to spread into the soma. In that case, an intense Ca2+ release response is often generated in the cell body and nucleus19,50,52, reflecting the high concentration of IP3Rs in the soma and nuclear membrane. In some cases, a local synaptically activated dendritic Ca2+ wave can be induced to propagate into the soma in the presence of bath-applied CCh, which supplies IP3 to the somatic region, allowing extended CICR52. There is also evidence that a higher concentration of stored Ca2+, as regulated by a previous history of spike firing, can promote propagation to the soma19.
These considerations affect the potential functions of Ca2+ waves. It has been suggested that Ca2+ waves could be one mechanism that carries information from a synapse in the dendrites to the nucleus, where the large [Ca2+]i increase could activate certain genes or transcription factors involved in synaptic plasticity17,19,42,50. However, the restriction of mobilized IP3 to sites close to the activated synapse makes it unlikely that these waves can spread to the soma and transport a message from the synapse, except under unusual circumstances. It has also been proposed that dendritically activated Ca2+ waves could spread to the soma in brainstem preBötzinger complex neurons and activate transient receptor potential subfamily M (TRPM) channels in that location to drive rhythmic respiratory patterns53. However, there is little evidence that waves spread this far in physiological conditions. Indeed, in the case of the brainstem neurons, recent experiments54 questioned whether Ca2+ waves exist in this system and argued that they are not involved in somatic activation.
Sites of wave initiation
Where waves initiate reveals information about the distribution of vital molecules and sources of synaptic input that activate metabotropic receptors. Synaptically activated Ca2+ waves preferentially initiate at branch points in the dendrites of pyramidal neurons, even when the stimulating electrode is not directly opposite a branch point16,26,48. With strong stimulation, which activates many presynaptic fibres synapsing on several branches, multiple sites of initiation are observed (FIG. 2). One possible explanation for this pattern is that IP3 is generated at the highest concentrations on oblique dendrites, near the sites of most synaptic contacts, and then diffuses towards the main dendrite, where it contacts a high density of IP3Rs and CICR is initiated. Indeed there are examples of waves initiating in the first few micrometres of the oblique dendrites and then becoming much larger when they reach the main dendrite16. However, there is also a preference (although not as strong) for waves to be initiated at branch points when metabotropic agonists are bath applied26. This pattern suggests that IP3Rs are more concentrated near branch points, as the IP3 generated in this kind of experiment is likely to be relatively uniform throughout the cell following diffusion from sites of mobilization, although other metabolic processes could modulate this distribution55. Consistent with this model of IP3R distribution, synaptically activated wave propagation is often weaker in the region between branch points (designated as ‘cold spots’)48. A speculative model, based on Ca2+ imaging, of the distribution of relevant molecules involved in wave initiation and propagation is shown in FIG. 3. There is more information from immunocytochemistry on cultured neurons about the receptors, channels and accessory proteins that might affect the initiation site55. However, much less is known about the distribution of these molecules in central neurons in intact preparations48,56,57.
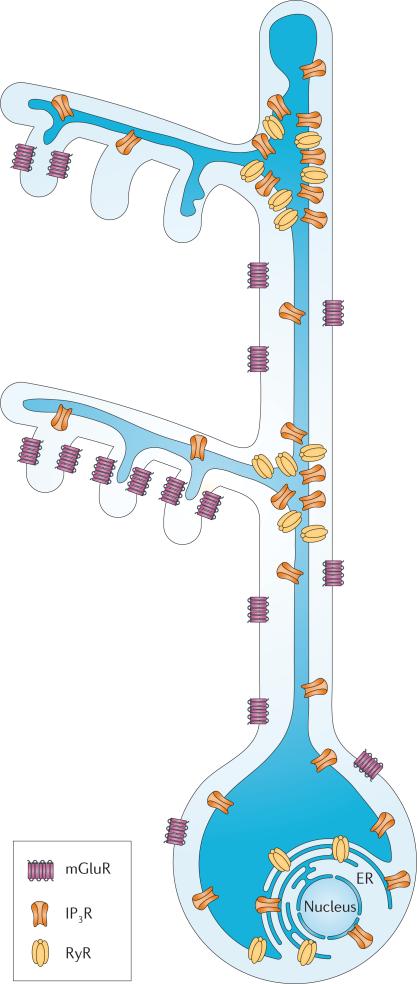
Figure 3. Model of the distribution of molecules that affect the generation of Ca2+ waves and sparks in a hippocampal pyramidal neuron.
The initiation of waves at branch points and the preference of events to occur at branch points suggest that inositol trisphosphate receptors (IP3Rs) and ryanodine receptors (RyRs) are concentrated at those sites. The propagation of waves to the soma and nucleus indicates that the receptors are located in those compartments. Metabotropic glutamate receptors (mGluRs) are located at the base of spines and at other extrasynaptic sites. The endoplasmic reticulum (ER) is continuous throughout the cell and connects to the nuclear membrane, but only invades some spines. Not all receptors are shown.
Following the generation of a Ca2+ wave, which releases massive amounts of Ca2+ from the endoplasmic reticulum, a new wave cannot be generated for around 20–60 s22,58, probably because endoplasmic reticulum stores are depleted at crucial sites. In pyramidal neurons, the main mechanism for refilling the stores (‘priming’) is by Ca2+ entry through VGCCs — Ca2+ moves into the cytoplasm and is then pumped into the endoplasmic reticulum15,19,51,59,60. Action potentials are the most effective priming mechanism59, but Ca2+ entry following subthreshold depolarization or even at resting potential is sometimes sufficient58,60. The importance of Ca2+ entry through store operated channels (SOCs) for refilling the endoplasmic reticulum in these neurons has not been demonstrated.
Function of Ca2+ waves
One obvious function of Ca2+ waves is that the large [Ca2+]i increase generated by these waves activates Ca2+-dependent membrane conductances. Indeed, many laboratories found that Ca2+-activated K+ channels of small conductance (SK channels) blocked by apamin are specifically opened by IP3-mediated waves in various neurons (CA1 pyramidal neurons61,62; cortical pyramidal neurons19,63,64; midbrain dopamine neurons65,66; and projection neurons in the basolateral amygdala20). There is little evidence that Ca2+ waves activate Ca2+-activated K+ channels of large conductance (BK channels). An interesting aspect to these experiments is that the waves are most effective in causing an SK channel-mediated after-hyperpolarization (AHP) in the neurons only if the wave is prominent in the soma. Dendritic Ca2+ waves that do not propagate into the soma, even if they evoke a larger dendritic [Ca2+]i increase than a somatic wave, cause a much weaker AHP response19,20,61–63,67. This result suggests that the density of SK channels is lower in the dendrites than in the soma, which is consistent with immunohistochemical observations68. However, the density in the dendrites cannot be too low because recent experiments showed that SK channels in spines, activated by Ca2+ entry through NMDA receptors, have an important role in regulating excitatory postsynaptic potential (EPSP) amplitude69,70. The (mostly) non-overlapping locations of weak synaptically activated Ca2+ waves and SK channel distribution suggests that wave-evoked AHPs are not prominent in pyramidal neurons, and therefore would not have a strong modulatory effect on firing patterns in physiological conditions.
A second potential consequence of the rise in [Ca2+]i from Ca2+ waves is that the released Ca2+ could directly inhibit Ca2+ entry through Ca2+ channels (calcium-dependent inactivation (CDI))71,72. In support of this hypothesis, synaptically activated Ca2+ waves locally suppress bAP-evoked [Ca2+]i increases in the dendritic regions of pyramidal neurons where the waves are largest47. As expected for this form of inhibition, other ways of causing large [Ca2+]i increases also suppress bAP-evoked Ca2+ signals in the dendrites, showing that it is the Ca2+ and not some other component of the signalling cascade that affects the bAP signal.
Most forms of synaptic plasticity require a rise in postsynaptic [Ca2+]i. Over the years, pharmacological evidence has implicated a role for Ca2+ release from stores in the induction of long-term potentiation (LTP) and/or long-term depression (LTD)73–79. Because Ca2+ waves generate a large and long-lasting [Ca2+]i increase in the dendrites, it is reasonable to suggest that these waves could induce plasticity. In addition, the synergistic action of mGluR activation and postsynaptic Ca2+ entry through VGCCs in generating Ca2+ release suggests that Ca2+ waves are a natural substrate for Hebbian plasticity mechanisms17,39. However, the current picture is confusing. There are few experiments in which post-synaptic Ca2+ release from stores has clearly been shown to be the main inducer of plasticity. Here we discuss a small number of examples that highlight the lack of clarity in this issue. For other reviews of this subject, see REFS 80–83.
There are several cases where synaptically activated Ca2+ release has been shown to induce LTP. Many of these studies are controversial, and there is no consensus on this issue. In one series of experiments, mGluR-evoked [Ca2+]i changes that were mediated through IP3Rs induced mossy fibre LTP in CA3 pyramidal neurons when other sources of postsynaptic [Ca2+]i rise were blocked84. Although these Ca2+ changes can be clearly seen and propagate as waves18, the conclusion that these waves are relevant to LTP induction has been challenged85. The best evidence that Ca2+ waves can induce a form of LTP comes from recent experiments on pyramidal neurons in slices86. The authors first induced LTP by the focal application of a muscarinic agonist to the dendrites, which also evoked a Ca2+ wave. Pharmacological dissection showed that the Ca2+ wave, and not some other signalling component, was the primary inducer of LTP. NMDA receptor activation was not required. This conclusion was supported by the demonstration that Ca2+ waves evoked by uncaging IP3 in the dendrites caused a similar enhancement of excitatory postsynaptic currents (EPSCs), which shows that muscarinic activation was not necessary. Interestingly, this form of LTP does not occlude homosynaptic LTP induced by repetitive stimulation of the Schaffer collaterals. The study did not examine whether the Ca2+ wave invaded or came close to the potentiated synapses. Also left unclear from these experiments is why the classic protocol for generating NMDA receptor-dependent LTP (tetanic stimulation of the Schaffer collaterals), which regularly evokes Ca2+ waves in the same parts of the dendrites17,61, does not seem to evoke this form of LTP.
Using imaging data from spines, one paper87 supports a role for nonlinear Ca2+ release in tetanus-induced NMDA receptor-dependent LTP. It claimed that most of the synaptically induced increase in Ca2+ in spines following a single synaptic stimulation was due to Ca2+ release from intracellular stores that can be blocked by ryanodine. The signal was local to spines and synchronous with the stimulus, without a delayed component typical of IP3-mediated Ca2+ release or a Ca2+ wave. Another study that examined tetanus-induced [Ca2+]i changes88 agrees that NMDA receptor-activated, ryanodine-sensitive Ca2+ release is prominent in spines. This signal is thought to be important in the induction of early LTP, but only following suprathreshold stimulation. A second NMDA receptor-dependent Ca2+ component that is prominent in dendrites was proposed to result from IP3-mediated Ca2+ release because of its sensitivity to xestospongin C. Both of these Ca2+ release signals were locked in time with the stimulus: results that are similar to those of Emptage et al.87. The possibility of Ca2+ wave generation was not examined because the Ca2+ measurements were made using two-photon microscope line scans that had no extended spatial resolution. Other groups have not found a rise in [Ca2+]i owing to activation of RyRs in or near spines89–91 and claim that the stimulus-locked rise in [Ca2+]i results from Ca2+ entry through NMDA receptor channels or VGCCs. This long-standing conflict is still not resolved. Some problems affecting the resolution may result from differences in preparations and/or recording techniques80,82 or differences in determining which form of LTP is induced88.
The evidence for IP3-mediated Ca2+ signalling in some forms of LTD is clearer but also not without controversy. The best example is LTD of the parallel fibre to Purkinje cell synapse, which is primarily induced by combined parallel fibre and climbing fibre activation92. Parallel fibre activation evokes mGluR- and IP3-mediated Ca2+ release in spines that do not spread as waves28,29 and that can be synergistically enhanced by co-activation of climbing fibre input39. The timing window for this synergism is consistent with the timing window for LTD generation. A specific role for IP3Rs in Purkinje cell spines was demonstrated in mutant mice that lack spine endoplasmic reticulum and that could not express LTD83,93. Recent experiments94 in which the [Ca2+]i increase was generated with controlled activation of caged Ca2+ suggest that it is the integrated [Ca2+]i increase in spines that evokes LTD and that IP3-mediated Ca2+ release is only one way of achieving the [Ca2+]i level required for LTD.
Experiments examining spike timing-dependent LTD in the L4 to L2/3 synapse in the cortex suggest that IP3-mediated Ca2+ release may have an important role in generating the endocannabinoids (eCBs) that are responsible for the depression95. This group found that LTD was blocked by intracellular heparin and thapsigargin, but not ryanodine, which is consistent with induction via the IP3 pathway. However, similar experiments by another group96 did not find that LTD was blocked by heparin. Furthermore, the same group96 found that direct two-photon imaging did not show a Ca2+ release component in the postsynaptic Ca2+ signal during the induction protocol. This issue has not been examined in more recent experiments, and the conflict remains unresolved.
An interesting example of Ca2+ release-dependent LTD in the hippocampal CA3 to CA1 pyramidal neuron synapse was demonstrated in organotypic slices using two-photon glutamate uncaging to activate individual spines91. Those authors found that the endoplasmic reticulum only invaded some dendritic spines. If glutamate was uncaged over those spines, a delayed [Ca2+]i rise, which resembled a Ca2+ wave (although the spatial extent of release was not determined), was observed in addition to a fast [Ca2+]i change time-locked to the stimulus. A delayed signal was not observed in spines without penetrating endoplasmic reticulum. The delayed signal was blocked by intracellular heparin. In some cases the delayed signal could be observed following synaptic stimulation. This is the only published example of Ca2+ release in pyramidal neurons following the activation of a single spine. Generation of this signal was correlated with an NMDA receptor-independent, synapse-specific form of LTD. Both the delayed signal and LTD were blocked by mGluR antagonists.
Localized Ca2+ release events
Spontaneous localized Ca2+ release events, often called ‘sparks’, were first discovered in cardiac myocytes97 and soon after in frog skeletal muscle fibres98 and other nonneuronal cell types. They were immediately suggested to be the building blocks of the large regenerative Ca2+ release that controls contraction. These events result from the opening of clusters of RyRs in the endoplasmic reticulum by local CICR. Although the events occur at rest with external Ca2+ removed, their frequency in myocytes is sensitive to changes in membrane potential, primarily as a result of Ca2+ entry through VGCCs in the plasma membrane. Ca2+ waves can be observed in some conditions13,99, but they rarely occur normally. In skeletal muscle, the connection between depolarization and increased spark frequency is more direct and is mediated through coupling between dihydropyridine (DHP) receptors and RyRs.
Similar events, mediated through IP3Rs, were first described in X. laevis oocytes100 and in HeLa cells11. These events (also known as ‘puffs’) have a number of similar features to sparks. They are localized, fast and occur stochastically. One difference is that they require IP3 in the cytoplasm, in addition to Ca2+, to open the IP3Rs. These events coalesce more easily into Ca2+ waves and will propagate throughout a cell as long as the levels of IP3 and the density of IP3Rs are high enough to activate regenerative CICR.
In general, Ca2+ sparks and puffs do not occur in the same cell types because the examined cells express either a great predominance of RyRs or IP3Rs. However, there are a few cases, for example, neonatal cardiomyocytes and oligodendrocyte progenitors13,101, in which interactions between these pathways have been observed. In some smooth muscle cells no interactions were noted, even though both events occurred in the same cells102. These and other properties of elementary events in non-neuronal cells were recently reviewed7.
Localized events in neurons that have spark-like and puff-like properties were first observed in differentiated PC12 cells and dissociated hippocampal neurons103. The organization of the receptor system underlying these events was not examined. Only a few spontaneous events were observed, but their frequency was greatly enhanced by application of caffeine or bradykinin. These events were significantly larger (50–100 μm) and slower (100–160 ms rise time, 95–160 ms decay time) than classical sparks or puffs, although some of these numbers may reflect buffering by the acetoxymethylester-loaded Ca2+ indicator and the speed of the confocal microscope used in the experiments. Interestingly, the events occurred more frequently at branch points, a property that was later observed in dendrites of intact pyramidal neurons in slices104. It is not clear whether these events in cultured cells are a good model for events in intact preparations. There is evidence that Ca2+ release is much less robust in cultured cells105.
Localized events in terminals and cell bodies
In several experiments, Ca2+ release in presynaptic terminals was suggested to have a role in both spontaneous and evoked synaptic transmission and plasticity106–109, although results in different preparations do not present a consistent picture. Localized events were first observed in a slice preparation in basket cell axons110,111. These transients, which may underlie some giant inhibitory postsynaptic currents (IPSCs) in Purkinje neurons, had a spatial extent of 5–10 μm and a duration of 0.2–2.0s (probably lengthened by indicator buffering). The amplitudes of the largest events were comparable to the amplitude of spike-evoked transients at the same locations. Interestingly, the frequency of these events was enhanced by low (10 μM) concentrations of ryanodine and suppressed at higher (100 μM) concentrations. These observations, together with strong immunostaining for RyRs in basket cell termini, suggest that these were RyR-mediated events. Similar spontaneous Ca2+ release events were observed in hippocampal presynaptic boutons in slice cultures112, although they were not studied in detail. Their role in synaptic function has been controversial113.
Localized ryanodine sensitive events called ‘syntillas’ were recorded in presynaptic terminals of magnocellular hypothalamic neurons114. Interestingly, their frequency could be enhanced by depolarization even in the absence of calcium influx, which differs from sparks in myocytes, where the function of depolarization is to promote Ca2+ entry through VGCCs. While searching for a function for these syntillas, the same group115 detected vesicular transmitter release, but did not find a close correlation with the syntillas.
By contrast, Ouyang et al.116 found RyR-mediated sparks just under the plasma membrane of the somata of cultured dorsal root ganglion neurons that correlated with the exocytosis of neurotransmitters. However, the correlation was stochastic, with about one vesicle released per ten detected sparks. The frequency of these small (~2 μm) and fast (~40–50 ms) spark-like events was sensitive to Ca2+ entry through VGCCs, but not to depolarization alone, and was enhanced by caffeine. Similar localized events were observed in the cell bodies of hippocampal pyramidal neurons117, but they were not characterized in detail.
Several groups found a ryanodine-sensitive component to voltage-dependent Ca2+ entry in cell bodies or presynaptic termini without describing a structure of localized events in this signal118–120. However, these measurements did not have the spatial or temporal resolution to resolve spark-like components in these signals.
Localized events in dendrites in intact preparations
Although Ca2+ signalling in dendrites has been studied intensely for more than two decades, this is the last place local release events were observed. One laboratory121 observed local Ca2+ release in the dendrites of developing chick retinal ganglion cells (at embryonic day 13 (E13)) that extended over a distance of approximately 10 μm, lasted about 10 s and occurred at a low frequency of around 0.1 Hz. These events, the frequency of which could be modulated by cholinergic synaptic transmission, stabilized the outgrowth of developing dendrites. Bonhoeffer's laboratory122 then found that similar large, long-lasting events (11 μm extent, 5 s duration) occurred in the dendrites of developing hippocampal pyramidal neurons in organotypic slice cultures prepared from postnatal day 0 (P0)–P2 newborn pups. These events resulted from Ca2+ release from stores and were closely correlated with the outgrowth of filopodia. Many were activated by GABAergic signalling and occurred close to sites of putative synapse formation123. The events detected close to synapses had shorter durations (0.5–0.7 s).
More recently, much faster and more spatially confined events, with different sensitivities to neurotransmitter antagonists, were observed in the dendrites of both hippocampal CA1 and CA3 rat pyramidal neurons and in L2/3 and L5 rat cortical pyramidal neurons in acute slices104. The events occur spontaneously at rest at moderate frequencies (1–2 Hz) at fixed locations in the dendrites. They occur at approximately the same frequency in cells from animals of all tested ages (P3–P80). However, their measured amplitudes are largest in younger animals (almost as large as the [Ca2+]i change from a bAP at the same location) and are approximately 20% of this level in older animals, although the peak amplitude at the exact site of release is not known124. This pattern suggests that they are most important during development, but may continue to have a signalling role in mature animals; however, there is no direct evidence for this connection. They have a spatial extent of 3–5 μm (FIG. 4; Supplementary information S2 (movie)). They occur most often at branch points, which suggests that RyRs might be concentrated at those sites (FIG. 3) and may contribute to wave initiation at the same locations. They have fast rise times (less than 10 ms) and recovery times (~100 ms), which are largely determined by the time for Ca2+ to diffuse away from a localized source of 3–4 μm extent124. They are unaffected by tetrodoxin (TTX) or any ionotropic transmitter inhibitors and are only weakly affected by mGluR inhibitors.
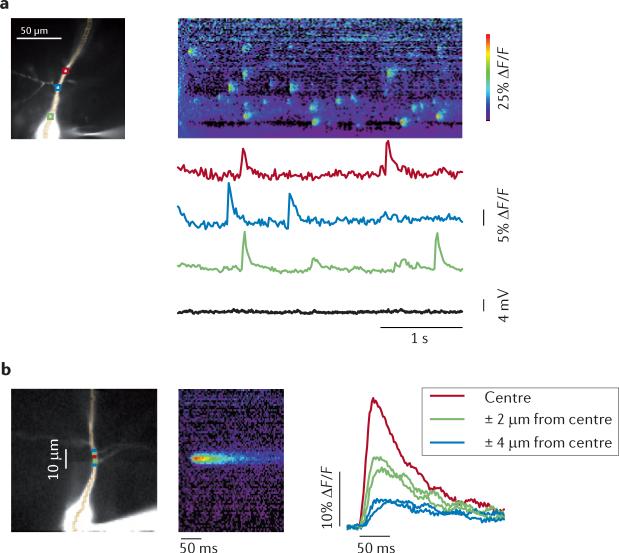
Figure 4. Spontaneous Ca2+ release events occur in localized regions of the dendrites of hippocampal pyramidal neurons.
a | The left image shows a CA1 pyramidal neuron in an acute rat hippocampal slice filled with 100 μM Oregon Green BAPTA1. Three regions of interest (ROIs) are marked with coloured boxes. The string of pixels along the dendrite indicates the location of the line scan image to the right. In normal artificial cerebral spinal fluid, spontaneous increases in intracellular calcium concentration ([Ca2+]i) were detected asynchronously at the three locations. There was no corresponding change in membrane potential. The pseudocolour line scan image (right panel) shows the increases in [Ca2+]i at all locations along the dendrite. b | The left image shows averaged records of event signals from nearby locations on a pyramidal cell dendrite. The data were recorded at 500 Hz, and spontaneous event signals from 11 events at the same location were aligned at the starting time of the rising phase of the fluorescence transient. The pseudocolour image shows the line scan of the averaged signal. The traces show the signal at the centre and neighbouring (± 2 μm and ± 4 μm from centre) locations. The data are consistent with Ca2+ release at the centre and Ca2+ diffusion to nearby locations. ΔF/F, relative change in fluorescence. Figure is adapted, with permission, from REF. 104. © (2009) Society for Neuroscience.
Similar to sparks in myocytes, the frequency of these events can be modulated by changes in membrane voltage around resting potential, which is caused largely by Ca2+ entry through L-type VGCCs. The increase in frequency continues at higher potentials, but there is no evidence that this signal becomes regenerative. Sensitivity in the subthreshold potential range means that normal voltage variations in these CNS neurons, such as changes between ‘up’ and ‘down’ states125, could strongly affect event frequency. Interestingly, event frequency can also be modulated by low concentrations of IP3, which is generated either by uncaging or by weak repetitive mGluR-mediated synaptic transmission. This dual modulation is unusual, but might resemble the crosstalk between RyR- and IP3R-mediated events described in oligodendrocytes101. It is not yet clear whether the IP3-sensitive events are the same or different from the spontaneous voltage-sensitive events. As sparks in myocytes are not modulated by mGluR signalling, these events may have some different molecular components from the localized events in myocytes.
The clearly different characteristics of these fast dendritic events (even those in very young animals) from the events described in developing retinal ganglion cells and pyramidal neurons suggests that they result from different signalling mechanisms. However, it is possible that differences in preparations (acute versus cultured slices) and Ca2+-imaging methods (acetoxymethylester-loaded versus injected indicators) are responsible for some of the divergence. Future experiments, possibly using genetically encoded Ca2+ indicators, may determine whether they have common origins.
The function of these localized events in the dendrites of mature neurons is unclear. One hypothesis is that they activate localized conductances or enzymes. In smooth muscle cells, sparks frequently generate spontaneous transient outward currents (STOCs)126–128. These currents result from the activation of BK channels and lead to muscle relaxation. In a few neurons, similar currents called spontaneous miniature outward currents (SMOCs) have been detected that activate either SK channels (in rat medial preoptic neurons129) or BK channels (in parasympathetic cardiac neurons130). These studies did not determine in which part of the neuron the currents were activated nor did they suggest a clear consequence of their generation. SMOCs have not been detected in pyramidal neurons.
Observing Ca2+ waves and localized events in vivo
The dendritic Ca2+ waves and localized release events described in this Review have not been observed in neurons in vivo, although no experiments have yet been specifically designed to look for them. It is reasonable to ask whether this absence is due to technical challenges or whether the conditions for evoking these signals do not normally pertain in the intact preparations or in current experimental protocols.
Large-amplitude Ca2+ waves have been observed in cortical L2/3 and L5 pyramidal neurons in slices16,19. Smaller Ca2+ signals from bAPs in the dendrites of these cells have been detected in vivo in many two-photon imaging experiments131,132. Therefore, exceeding detection threshold in vivo is not an issue for these large waves. A second possibility is that they are not as time-locked to the stimulus as signals from bAPs and EPSPs, which have been recorded following sensory stimulation in other experiments. In slices, the peak of Ca2+ waves often occur after a synaptic tetanus with variable latency; if the recording period in vivo is restricted, the waves might be missed. A third, and more likely possibility, is that the synaptic pattern that is effective in evoking waves in slices is not the pattern that cortical pyramidal neurons receive during sensory stimulation. In slices, Ca2+ waves are usually evoked synaptically with an extracellular stimulating electrode placed close to the dendrites of the examined pyramidal neuron. Cooperative activation of many fibres is often necessary to reach threshold for regenerative Ca2+ waves17. A reasonable hypothesis is that this cooperativity is achieved by combining the IP3 that is mobilized at several synapses to reach threshold concentration. This summation can occur more easily with electrode stimulation because it activates bundles of presynaptic fibres that make contact on spines that are probably close together on a single dendritic branch. Consistent with this idea, most experiments find that it is necessary to give repetitive synaptic stimulation to evoke waves133, as if the IP3 at the initiation site must be summated from several stimuli to reach threshold.
By contrast, the first in vivo imaging studies of synaptic summation following sensory stimulation134 suggest that activated synapses are widely distributed over the dendrites and are not activated in bundles on individual dendrites, a pattern that is less favourable for regenerative Ca2+ wave generation. Important exceptions to this perspective are the experiments of Holbro et al.91. In their studies on CA1 pyramidal neurons in organotypic cultures, they were able to evoke regenerative Ca2+ release signals in a subset of spines using two-photon glutamate uncaging. The locations of the activated spines (oblique or main dendrite) were not indicated. Because only single spines were activated, cooperative activation was not required. The spatial extent of Ca2+ release was not determined, but was probably small because IP3 was generated at only one spine. In that case, Ca2+ release (even if of large amplitude) would be difficult to detect without knowledge of the location of the activated synapse.
It is also possible that the chance of generating Ca2+ waves is affected by the modulatory state of the neuronal environment, as several G protein-coupled receptor transmitters enhance wave generation in slices22,50. Therefore, it may be preferable to look for these waves in awake animals, in which the levels of these compounds are higher135.
A different set of considerations affects the detection in dendrites of localized Ca2+ release events. These spark-like events occur spontaneously in pyramidal neurons in slices and do not require a specific stimulus. As many aspects of cellular Ca2+ signalling in slices are reproduced faithfully in vivo131, it is likely that these events also occur in neurons in the intact animal. The limitation in detection is probably due to the small size of the events (20–70% of the amplitude of a bAP signal in the dendrites), which might make them indistinguishable from noise in most in vivo two-photon Ca2+ measurements. Also, the stochastic nature of their generation makes them hard to observe in experiments that are designed to look for signals linked in time to a stimulus. As systematic in vivo Ca2+ measurements are just beginning, we may expect progress in detecting small events and waves in the near future.
Conclusions
Over the past decade, increasingly detailed information about Ca2+ release in neurons and other cell types has accumulated. Some of this information has been summarized in several excellent reviews2,6–8,14. In this Review, I have concentrated on the properties and functions of Ca2+ waves and localized release events in intact preparations, primarily in neurons in brain slices. In these preparations, some of the differences between neurons and other cell types and model cell preparations are clearly revealed. Among the interesting properties of Ca2+ waves are their large amplitude, their prominence in only subregions of dendrites and the fact that they can be generated by coincident activation of mGluR inputs and postsynaptic Ca2+ entry. Among the major remaining questions, two prominent issues are: what conditions evoke these waves during normal brain activity, and do these waves have a specific function? The spark-like events are just beginning to be examined. Although they were studied for many years in other cell types, they managed to stay under the radar in neurons until recently. Because they occur spontaneously in the slice preparation, it is very likely that they occur in vivo. It is also interesting that their frequency can be modulated by normal synaptic activity and membrane potential changes. It is not yet clear whether they have a specific function, such as releasing neurotransmitters or triggering developmental changes, or whether they are just the building blocks for larger [Ca2+]i changes.
In other preparations, particularly cardiac myocytes and X. laevis oocytes, much more is known about the detailed microstructure of these Ca2+ signals and the molecules that underlie them. Experiments using currently available techniques, applying the lessons learned from these other preparations, should supply much of the missing information about waves and sparks in neurons. Improved Ca2+ indicators and imaging instrumentation will extend the reach of these experiments. In addition, the power of mouse genetics can be used because both Ca2+ waves and spark-like events have been detected with similar properties in murine pyramidal neurons (S. Manita, K. Miyazaki and W.N.R., unpublished observations). A big step forward will occur when they can be studied in vivo.
Supplementary Material
NMDA spikes
Short, sharp increases in NMDA receptor current that result from regenerative mechanisms in which the nonlinear component is the voltage dependence of the NMDA receptor. These spikes usually occur in dendrites, leading to large increases in intracellular calcium concentration but relatively small changes in somatic membrane potential.
Coincidence detector
A term derived from electrical engineering that refers to the output of a circuit that is dependent on the simultaneous arrival of two (or more) inputs. The inositol trisphosphate receptor (IP3R) is a coincidence detector for Ca2+ and IP3.
Box 1 | Recording Ca2+ waves and sparks.
Ca2+ waves and sparks are detected using variations of standard Ca2+-imaging methods. As with many forms of imaging, better results are obtained using techniques that maximize the sensitivity of the measurement with high spatial and temporal resolution, especially as signal averaging is not very useful in examining these events. Not all of these goals can be achieved simultaneously. Oregon Green BAPTA1 is a good indicator for the detection of small intracellular calcium concentration ([Ca2+]i) changes in sparks. However, the large [Ca2+]i changes in Ca2+ waves saturate this high-affinity indicator. Low-affinity indicators, such as Oregon Green BAPTA5N or furaptra, are better choices if quantitative assessment of wave parameters is the goal. Confocal microscopy has good spatial and temporal resolution in the line scan mode and has been used in many experiments to examine localized Ca2+ events. However, this technique sometimes misses the extended spatial parameters of waves or the stochastic aspects of sparks. Charge-coupled device (CCD) cameras can have good temporal and spatial resolution but usually not both. They are a good choice for capturing the spatial aspects of these events. They have poor spatial resolution in thick specimens. Sometimes choosing a thin specimen, such as cultured neurons or dendrites in slices, can overcome these limitations. These issues have been discussed in several review articles7,136 and imaging handbooks137.
Hebbian plasticity
A form of neuronal plasticity in which a change in a property (often synaptic strength) results from the simultaneous activation (sometimes repetitively) of presynaptic and postsynaptic cells.
‘Up’ and ‘down’ states
Persistent depolarizations and hyperpolarizations in neurons that can differ by 10 to 20 mV. They are primarily observed in cortical neurons and are thought to be driven by network activity.
Acknowledgements
I thank members of my laboratory for many insightful discussions and J. Lisman and P. Sah for comments on an earlier version of this manuscript. W.N.R. is supported by a grant from the US National Institutes of Health (NS-16295).
Footnotes
FURTHER INFORMATION
William N. Ross.s homepage: http://www.nymc.edu/People/William.N.Ross/index.html
SUPPLEMENTARY INFORMATION
See online article: S1 (movie) | S2 (movie)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Competing interests statement
The author declares no competing financial interests.
References
- 1.Bloodgood BL, Sabatini BL. Ca2+ signalling in dendritic spines. Curr. Opin. Neurobiol. 2007;17:345–351. doi: 10.1016/j.conb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Neuronal calcium signalling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [An influential review that highlights the importance of diverse calcium signals in architecturally complex dendrites and axons] [DOI] [PubMed] [Google Scholar]
- 3.Augustine GJ, Santamaria F, Tanaka K. Local calcium signalling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 4.Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- 5.Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- 6.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Lederer WJ. Calcium sparks. Physiol. Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [A comprehensive review of calcium sparks in different cell types that emphasizes results from cardiac myocytes. A good starting point for exploring this field] [DOI] [PubMed] [Google Scholar]
- 8.Bootman MD, Lipp P, Berridge MJ. The organization and functions of local Ca2+ signals. J. Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- 9.Parker I, Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc. R. Soc. Lond. B. 1991;246:269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- 10.Lechleiter J, Girard S, Peralta E, Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991;252:123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- 11.Bootman M, Niggli E, Berridge M, Lipp P. Imaging the heirarchical Ca2+ signalling system in HeLa cells. J. Physiol. 1997;499:307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen OH, Tepikin AV. Polarized calcium signalling in exocrine gland cells. Annu. Rev. Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 13.Luo D, et al. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium. 2008;43:165–174. doi: 10.1016/j.ceca.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 15.Miller LD, Petrozzino JJ, Golarai G, Connor J. Ca2+ release from intracellular stores induced by afferent stimulation of CA3 pyramidal neurons in hippocampal slices. J. Neurophysiol. 1996;76:554–562. doi: 10.1152/jn.1996.76.1.554. [This is the first description of synaptically activated calcium release in neurons in a brain slice preparation] [DOI] [PubMed] [Google Scholar]
- 16.Larkum ME, Watanabe S, Nakamura T, Lasser-Ross N, Ross WN. Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons. J. Physiol. 2003;549:471–488. doi: 10.1113/jphysiol.2002.037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura T, Barbara JG, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–737. doi: 10.1016/s0896-6273(00)81125-3. [This is the first description of synaptically activated calcium waves in pyramidal neurons. The synergistic release of calcium stresses the role of the IP3 receptor as a coincidence detector for IP3 and calcium. Reference 39 shows similar coincidence detection in Purkinje neurons] [DOI] [PubMed] [Google Scholar]
- 18.Kapur A, Yeckel M, Johnston D. Hippocampal mossy fiber activity evokes Ca2+ release in CA3 pyramidal neurons via a metabotropic glutamate receptor pathway. Neuroscience. 2001;107:59–69. doi: 10.1016/s0306-4522(01)00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb. Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power JM, Sah P. Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J. Neurosci. 2008;28:3209–3220. doi: 10.1523/JNEUROSCI.4310-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larkum ME, Watanabe S, Lasser-Ross N, Rhodes P, Ross WN. Dendritic properties of turtle pyramidal neurons. J. Neurophysiol. 2008;99:683–694. doi: 10.1152/jn.01076.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, et al. Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons. J. Neurosci. 2000;20:8365–8376. doi: 10.1523/JNEUROSCI.20-22-08365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helmchen F, Imoto K, Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signalling in dendrites of pyramidal neurons. Biophys. J. 1996;70:1069–1081. doi: 10.1016/S0006-3495(96)79653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys. J. 2000;78:2655–2667. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca2+ ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Lasser-Ross N, Nakamura K, Ross WN. Spatial segregation and interaction of calcium signalling mechanisms in rat hippocampal CA1 pyramidal neurons. J. Physiol. 2002;543:465–480. doi: 10.1113/jphysiol.2002.020362. [An interesting paper that emphasizes that synaptically activated calcium entry through NMDA receptors and synaptically activated calcium waves occur in different dendritic regions] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- 28.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 29.Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [References 28 and 29 are the first descriptions of IP3-mediated calcium release in Purkinje cells. In these cells, calcium release does not propagate as a wave] [DOI] [PubMed] [Google Scholar]
- 30.Topolnik L, Chamberland S, Pelletier J-G, Ran I, Lacaille J-C. Activity-dependent compartmentalized regulation of dendritic Ca2+ signalling in hippocampal interneurons. J. Neurosci. 2009;29:4658–4663. doi: 10.1523/JNEUROSCI.0493-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozsa B, Zelles T, Vizi ES, Lendvai B. Distance-dependent scaling of calcium transients evoked by backpropagating spikes and synaptic activity in dendrites of hippocampal interneurons. J. Neurosci. 2004;24:661–670. doi: 10.1523/JNEUROSCI.3906-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartmann J, Konnerth A. Determinants of postsynaptic Ca2+ signalling in Purkinje neurons. Cell Calcium. 2005;37:459–466. doi: 10.1016/j.ceca.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Shuai J, Pearson JE, Parker I. Modeling Ca2+ feedback on a single inositol 1,4,5-trisphosphate receptor and its modulation by Ca2+ buffers. Biophys. J. 2008;95:3738–3752. doi: 10.1529/biophysj.108.137182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3-and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 35.Iino M, Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-trisphosphate-induced Ca2+ release. Nature. 1992;360:76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- 36.Parker I, Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc. Natl Acad. Sci. USA. 1990;87:260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foskett JK, White C, Cheung KH, Mak D-OD. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manita S, Ross WN. IP3 mobilization and diffusion determine the timing window of Ca2+ release by synaptic stimulation and a spike in rat CA1 pyramidal cells. Hippocampus. 2010;20:524–539. doi: 10.1002/hipo.20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SS, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nature Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 40.Sarkisov DV, Wang SS-H. Order-dependent coincidence detection in cerebellar Purkinje neurons at the inositol trisphosphate receptor. J. Neurosci. 2008;28:133–142. doi: 10.1523/JNEUROSCI.1729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Power JM, Sah P. Distribution of IP3-mediated calcium responses and their role in nuclear signalling in rat basolateral amygdala neurons. J. Physiol. 2007;580:835–857. doi: 10.1113/jphysiol.2006.125062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martone M, Zhang Y, Simpliciano V, Carragher B, Ellisman M. Three-dimensional visualization of the smooth endoplasmic reticulum in Purkinje cell dendrites. J. Neurosci. 1993;13:4636–4646. doi: 10.1523/JNEUROSCI.13-11-04636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terasaki M, Slater NT, Fein A, Schmidek A, Reese TS. Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc. Natl Acad. Sci. USA. 1994;91:7510–7514. doi: 10.1073/pnas.91.16.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Megías M, Emri Z, Freund TF, Gulyás AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 46.Major G, Polsky A, Denk W, Schiller J, Tank DW. Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. J. Neurophysiol. 2008;99:2584–2601. doi: 10.1152/jn.00011.2008. [DOI] [PubMed] [Google Scholar]
- 47.Manita S, Miyazaki K, Ross WN. Synaptically activated Ca2+ waves and NMDA spikes locally suppress voltage dependent Ca2+ signalling in rat pyramidal cell dendrites. J. Physiol. 2011;589:4903–4920. doi: 10.1113/jphysiol.2011.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzpatrick JS, et al. Inositol-1,4,5-trisphosphate receptor-mediated Ca2+ waves in pyramidal neuron dendrites propagate through hot spots and cold spots. J. Physiol. 2009;587:1439–1459. doi: 10.1113/jphysiol.2009.168930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaffe DB, Brown TH. Metabotropic glutamate receptor activation induces calcium waves within hippocampal dendrites. J. Neurophysiol. 1994;72:471–474. doi: 10.1152/jn.1994.72.1.471. [This is the first observation and analysis of calcium waves in neuronal dendrites. Activation by focally applying the mGluR agonist t-ACPDsuggested a role for IP3-mediated calcium release in generating the waves] [DOI] [PubMed] [Google Scholar]
- 50.Power JM, Sah P. Nuclear calcium signalling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J. Neurosci. 2002;22:3454–3462. doi: 10.1523/JNEUROSCI.22-09-03454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stutzmann GE, LaFerla FM, Parker I. Ca2+ signalling in mouse cortical neurons studied by two-photon imaging and photoreleased inositol triphosphate. J. Neurosci. 2003;23:758–765. doi: 10.1523/JNEUROSCI.23-03-00758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe S, Hong M, Lasser-Ross N, Ross WN. Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons. J. Physiol. 2006;575:455–468. doi: 10.1113/jphysiol.2006.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mironov SL. Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J. Physiol. 2008;586:2277–2291. doi: 10.1113/jphysiol.2007.149021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Negro CA, Hayes JA, Rekling JC. Dendritic calcium activity precedes inspiratory bursts in preBötzinger complex neurons. J. Neurosci. 2011;31:1017–1022. doi: 10.1523/JNEUROSCI.4731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacob SN, et al. Signalling microdomains regulate inositol 1,4,5-trisphosphate-mediated intracellular calcium transients in cultured neurons. J. Neurosci. 2005;25:2853–2864. doi: 10.1523/JNEUROSCI.4313-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharp AH, et al. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate-and ryanodine-sensitive Ca2+ release channels in rat brain. J. Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hertle DN, Yeckel MF. Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience. 2007;150:625–638. doi: 10.1016/j.neuroscience.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garaschuk O, Yaari Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurons. J. Physiol. 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong M, Ross WN. Priming of intracellular calcium stores in rat CA1 pyramidal neurons. J. Physiol. 2007;584:75–87. doi: 10.1113/jphysiol.2007.137661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Power JM, Sah P. Intracellular calcium store filling by an L-type calcium current in the basolateral amygdala at subthreshold membrane potentials. J. Physiol. 2005;562:439–453. doi: 10.1113/jphysiol.2004.076711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Hassar L, Hagenston AM, D'Angelo LB, Yeckel MF. Metabotropic glutamate receptors regulate hippocampal CA1 pyramidal neuron excitability via Ca2+ wave-dependent activation of SK and TRPC channels. J. Physiol. 2011;589:3211–3229. doi: 10.1113/jphysiol.2011.209783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong M, Manita S, Ross WN. Soc.Neurosci. San Diego, California: Nov 3–7, 2007. Calcium waves generated by uncaging IP3 or synaptic stimulation evoke an apamin-sensitive AHP in the perisomatic region of hippocampal CA1 pyramidal neurons. Abstr. 786.6. [Google Scholar]
- 63.Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J. Neurosci. 2005;25:10308–10320. doi: 10.1523/JNEUROSCI.2697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada S-I, Takechi H, Kanchiku I, Kita T, Kato N. Small-conductance Ca2+-dependent K+ channels are the target of spike-induced Ca2+ release in a feedback regulation of pyramidal cell excitability. J. Neurophysiol. 2004;91:2322–2329. doi: 10.1152/jn.01049.2003. [DOI] [PubMed] [Google Scholar]
- 65.Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- 66.Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J. Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signalling in neocortical neurons. J. Neurophysiol. 2007;97:2215–2229. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- 68.Sailer CA, Kaufmann WA, Marksteiner J, Knaus H-G. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol. Cell. Neurosci. 2004;26:458–469. doi: 10.1016/j.mcn.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Ngo-Anh TJ, et al. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nature Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- 70.Faber ESL, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nature Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- 71.Brehm P, Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978;202:1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- 72.Dunlap K. Calcium channels are models of self-control. J. Gen. Physiol. 2007;129:379–383. doi: 10.1085/jgp.200709786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harvey J, Collingridge GL. Thapsigargin blocks the induction of long-term potentiation in rat hippocampal slices. Neurosci. Lett. 1992;139:197–200. doi: 10.1016/0304-3940(92)90551-h. [DOI] [PubMed] [Google Scholar]
- 74.Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- 75.Reyes M, Stanton PK. Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J. Neurosci. 1996;16:5951–5960. doi: 10.1523/JNEUROSCI.16-19-05951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raymond CR, Redman SJ. Different calcium sources are narrowly tuned to the induction of different forms of LTP. J. Neurophysiol. 2002;88:249–255. doi: 10.1152/jn.2002.88.1.249. [DOI] [PubMed] [Google Scholar]
- 77.Behnisch T, Reymann KG. Thapsigargin blocks long-term potentiation induced by weak, but not strong tetanisation in rat hippocampal CA1 neurons. Neurosci. Lett. 1995;192:185–188. doi: 10.1016/0304-3940(95)11641-9. [DOI] [PubMed] [Google Scholar]
- 78.Taufiq AM, et al. Involvement of IP3 receptors in LTP and LTD induction in guinea pig hippocampal CA1 neurons. Learn. Mem. 2005;12:594–600. doi: 10.1101/lm.17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–879. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Svoboda K, Mainen ZF. Intracellular stores spill their guts. Neuron. 1999;22:427–430. doi: 10.1016/s0896-6273(00)80698-4. [DOI] [PubMed] [Google Scholar]
- 81.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu. Rev. Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sjöström PJ, Rancz EA, Roth A, Häusser M. Dendritic excitability and synaptic plasticity. Physiol. Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- 83.Rose CR, Konnerth A. Stores not just for storage: intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- 84.Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nature Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mellor J, Nicoll R. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nature Neurosci. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- 86.Fernández de Sevilla D, Núñez A, Borde M, Malinow R, Buño W. Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J. Neurosci. 2008;28:1469–1478. doi: 10.1523/JNEUROSCI.2723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Emptage N, Bliss TVP, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- 88.Raymond CR, Redman SJ. Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus. J. Physiol. 2006;570:97–111. doi: 10.1113/jphysiol.2005.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature. 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- 90.Kovalchuk Y, Eilers J, Lisman J, Konnerth A. NMDA receptor-mediated subthreshold Ca2+ signals in spines of hippocampal neurons. J. Neurosci. 2000;20:1791–1799. doi: 10.1523/JNEUROSCI.20-05-01791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holbro N, Grunditz A, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signalling and plasticity at hippocampal synapses. Proc. Natl Acad. Sci. USA. 2009;106:15055–15060. doi: 10.1073/pnas.0905110106. [An important paper showing that uncaging glutamate over single spines evokes delayed calcium release and LTD only in those cases where the endoplasmic reticulum penetrates the spine. Blockage by mGluR antagonists and heparin suggested that the calcium release was mediated by IP3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Konnerth A, Dreessen J, Augustine GJ. Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proc. Natl Acad. Sci. USA. 1992;89:7051–7055. doi: 10.1073/pnas.89.15.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miyata M, et al. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28:233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka K, et al. Ca2+ requirements for cerebellar long-term synaptic depression: role for a postsynaptic leaky integrator. Neuron. 2007;54:787–800. doi: 10.1016/j.neuron.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 95.Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J. Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nevian T, Sakmann B. Spine Ca2+ signalling in spike-timing-dependent plasticity. J. Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [The first description of stochastic, RyR-mediated, calcium sparks in myocytes. Together with reference 100, these papers initiated the field of localized calcium events in cells] [DOI] [PubMed] [Google Scholar]
- 98.Tsugorka A, Ríos E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995;269:1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- 99.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 100.Parker I, Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990;250:977–979. doi: 10.1126/science.2237441. [The first description of localized IP3-evoked calcium release puffs. The oocyte proved to be a favourable preparation for studying these signals as it contains few, if any, RyRs] [DOI] [PubMed] [Google Scholar]
- 101.Haak LL, et al. Sparks and puffs in oligodendrocyte progenitors: cross talk between ryanodine receptors and inositol trisphosphate receptors. J. Neurosci. 2001;21:3860–3870. doi: 10.1523/JNEUROSCI.21-11-03860.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J. Physiol. 2005;569:533–544. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koizumi S, et al. Characterization of elementary Ca2+ release signals in NGF-differentiated PC12 cells and hippocampal neurons. Neuron. 1999;22:125–137. doi: 10.1016/s0896-6273(00)80684-4. [DOI] [PubMed] [Google Scholar]
- 104.Manita S, Ross WN. Synaptic activation and membrane potential changes modulate the frequency of spontaneous elementary Ca2+ release events in the dendrites of pyramidal neurons. J. Neurosci. 2009;29:7833–7845. doi: 10.1523/JNEUROSCI.0573-09.2009. [This is the first description of localized calcium release events in dendrites. The two ways of modulating the frequency of these events are unusual] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Womack MD, Walker JW, Khodakhah K. Impaired calcium release in cerebellar Purkinje neurons maintained in culture. J. Gen. Physiol. 2000;115:339–346. doi: 10.1085/jgp.115.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simkus CRL, Stricker C. The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurons of rat barrel cortex. J. Physiol. 2002;545:521–535. doi: 10.1113/jphysiol.2002.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lelli A, et al. Presynaptic calcium stores modulate afferent release in vestibular hair cells. J. Neurosci. 2003;23:6894–6903. doi: 10.1523/JNEUROSCI.23-17-06894.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Unni VK, Zakharenko SS, Zablow L, DeCostanzo AJ, Siegelbaum SA. Calcium release from presynaptic ryanodine-sensitive stores is required for long-term depression at hippocampal CA3-CA3 pyramidal neuron synapses. J. Neurosci. 2004;24:9612–9622. doi: 10.1523/JNEUROSCI.5583-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- 110.Llano I, et al. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nature Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [This is the first observation of localized calcium release events in presynaptic terminals. The association of large IPSCs in Purkinje cells with these events suggested a possible function for these signals] [DOI] [PubMed] [Google Scholar]
- 111.Conti R, Tan YP, Llano I. Action potential-evoked and ryanodine-sensitive spontaneous Ca2+ transients at the presynaptic terminal of a developing CNS inhibitory synapse. J. Neurosci. 2004;24:6946–6957. doi: 10.1523/JNEUROSCI.1397-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 113.Carter AG, Vogt KE, Foster KA, Regehr WG. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J. Neurosci. 2002;22:21–28. doi: 10.1523/JNEUROSCI.22-01-00021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Crescenzo V, et al. Ca2+ syntillas, miniature Ca2+release events in terminals of hypothalamic neurons, are increased in frequency by depolarization in the absence of Ca2+ influx. J. Neurosci. 2004;24:1226–1235. doi: 10.1523/JNEUROSCI.4286-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.ZhuGe R, et al. Syntillas release Ca2+ at a site different from the microdomain where exocytosis occurs in mouse chromaffin cells. Biophys. J. 2006;90:2027–2037. doi: 10.1529/biophysj.105.071654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ouyang K, et al. Ca2+ sparks and secretion in dorsal root ganglion neurons. Proc. Natl Acad. Sci. USA. 2005;102:12259–12264. doi: 10.1073/pnas.0408494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berrout J, Isokawa M. Homeostatic and stimulus-induced coupling of the L-type Ca2+ channel to the ryanodine receptor in the hippocampal neuron in slices. Cell Calcium. 2009;46:30–38. doi: 10.1016/j.ceca.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng Y. Ryanodine-sensitive component of calcium transients evoked by nerve firing at presynaptic nerve terminals. J. Neurosci. 1996;16:6703–6712. doi: 10.1523/JNEUROSCI.16-21-06703.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Isokawa M, Alger BE. Ryanodine receptor regulates endogenous cannabinoid mobilization in the hippocampus. J. Neurophysiol. 2006;95:3001–3011. doi: 10.1152/jn.00975.2005. [DOI] [PubMed] [Google Scholar]
- 120.Friel D, Tsien RW. A caffeine-and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurons modulates effects of Ca2+ entry on [Ca2+]i. J. Physiol. 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lohmann C, Myhr KL, Wong ROL. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- 122.Lohmann C, Finski A, Bonhoeffer T. Local calcium transients regulate the spontaneous motility of dendritic filopodia. Nature Neurosci. 2005;8:305–312. doi: 10.1038/nn1406. [DOI] [PubMed] [Google Scholar]
- 123.Lohmann C, Bonhoeffer T. A role for local calcium signalling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 124.Miyazaki K, Manita S, Ross WN. Soc. Neurosci. San Diego, California: Nov 14, 2010. Developmental profile of localized spontaneous Ca2+ release events in the dendrites of rat hippocampal pyramidal neurons. Abstr. 246.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waters J, Larkum M, Sakmann B, Helmchen F. Supralinear Ca2+ influx into dendritic tufts of layer 2/3 neocortical pyramidal neurons in vitro and in vivo. J. Neurosci. 2003;23:8558–8567. doi: 10.1523/JNEUROSCI.23-24-08558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nelson MT, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 127.Pérez GJ, Bonev AD, Nelson MT. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am. J. Physio. Cell Physiol. 2001;281:C1769–C1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 128.Brenner R, et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 129.Klement G, et al. Spontaneous ryanodine-receptor-dependent Ca2+ activated K+ currents and hyperpolarizations in rat medial preoptic neurons. J. Neurophysiol. 2010;103:2900–2911. doi: 10.1152/jn.00566.2009. [DOI] [PubMed] [Google Scholar]
- 130.Merriam L, Scornik FS, Parsons RL. Ca2+-induced Ca2+ release activates spontaneous miniature outward currents (SMOCs) in parasympathetic cardiac neurons. J. Neurophysiol. 1999;82:540–550. doi: 10.1152/jn.1999.82.2.540. [DOI] [PubMed] [Google Scholar]
- 131.Larkum ME, Waters J, Sakmann B, Helmchen F. Dendritic spikes in apical dendrites of neocortical layer 2/3 pyramidal neurons. J. Neurosci. 2007;27:8999–9008. doi: 10.1523/JNEUROSCI.1717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Waters J, Helmchen F. Background synaptic activity is sparse in neocortex. J. Neurosci. 2006;26:8267–8277. doi: 10.1523/JNEUROSCI.2152-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou S, Ross WN. Threshold conditions for synaptically evoking Ca2+ waves in hippocampal pyramidal neurons. J. Neurophysiol. 2002;87:1799–1804. doi: 10.1152/jn.00601.2001. [DOI] [PubMed] [Google Scholar]
- 134.Jia H, Rochefort NL, Chen X, Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010;464:1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]
- 135.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol. Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 136.Dargan SL, Demuro A, Parker I. Imaging Ca2+ signals in Xenopus oocytes. Meth. Mol. Biol. 2006;322:103–119. doi: 10.1007/978-1-59745-000-3_8. [DOI] [PubMed] [Google Scholar]
- 137.Helmchen F, Konnerth A. Imaging in Neuroscience: a Laboratory Manual. Cold Spring Harbor Press; Cold Spring Harbor, New York: 2011. [Google Scholar]
- 138.Iino M. Spatiotemporal dynamics of Ca2+ signalling and its physiological roles. Proc. Jpn Acad. Ser. B. 2010;86:244–256. doi: 10.2183/pjab.86.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.