Abstract
The field of behavioral economics has made important inroads into the understanding of substance use disorders through the concept of reinforcer pathology. Reinforcer pathology refers to the joint effects of (a) the persistently high valuation of a reinforcer, broadly defined to include tangible commodities and experiences, and/or (b) the excessive preference for the immediate acquisition or consumption of a commodity despite long-term negative outcomes. From this perspective, reinforcer pathology results from the recursive interactions of endogenous person-level variables and exogenous environment-level factors. The current review describes the basic principles of behavioral economics that are central to reinforcer pathology, the processes that engender reinforcer pathology, and the approaches and procedures that can repair reinforcement pathologies. The overall goal of this review is to present a new understanding of substance use disorders as viewed by recent advances in behavioral economics.
Keywords: addiction, behavior economics, reinforcer pathology, elasticity of demand, discounting, treatment
INTRODUCTION
Effects vary with the conditions which bring them to pass, but laws do not vary. Physiological and pathological states are ruled by the same forces; they differ only because of the special conditions under which the vital laws manifest themselves.
Claude Bernard, An Introduction to the Study of Experimental Medicine
Claude Bernard, the father of experimental medicine, suggested that pathological and normal processes differ quantitatively, not qualitatively. Indeed, pathological processes often result from normal processes that go awry. For example, cell growth and division are processes necessary for continuation of multicellular organisms. However, if cell growth and division are not regulated, then the pathological process of cancer results. The same can be said for behavioral systems, as normal behavioral development and functioning require regularly seeking and obtaining appropriate reinforcers contingent on adaptive and prosocial behavior. For some individuals, these reinforcement processes can go awry when substances with a high potential for dependence are used, particularly in contexts where those substances are easily available and when alternative opportunities for reinforcement are lacking. The role of inordinate reinforcement in substance dependence has been recognized for some time. However, only recently has reinforcement pathology been explicitly recognized and defined (Bickel et al. 2011a, Carr et al. 2011). Reinforcement pathologies have previously been defined as the joint effects of (a) the persistently high valuation of a reinforcer, broadly defined to include tangible commodities and experiences, and/or (b) the excessive preference for the immediate acquisition or consumption of a commodity despite long-term negative outcomes (Bickel et al. 2011a, p. 407). Excessive consumption of psychoactive drugs and food as well as excessive gambling exemplify reinforcement pathology and constitute substantial public health problems in the United States. Reinforcement pathology, from this perspective, results from the recursive interactions between endogenous (e.g., physiologically mediated subjective response to drugs, steep devaluation of delayed reward) and exogenous factors (e.g., drug and alternative reinforcement price/availability).
The understanding of reinforcement pathology has been facilitated by behavioral economics, a discipline that hybridizes economics and psychology. As we use it here, behavioral economics refers to the application of economic concepts and approaches to the molar study of individuals’ choices and decisions. Extending from basic operant learning approaches, application of behavioral economics to substance use disorders began in the 1990s, with considerable success in relating the quantity of drug-taking behavior to substance price. Some years later, this research domain used controls and began to compare the trade-offs between immediate and delayed outcomes in the decision making of those with substance dependence. Over the intervening period, these approaches have been increasingly applied to an ever-widening range of phenomena. In this article, we explore the concept of reinforcement pathology by reviewing (a) the basic principles of behavioral economics that are central to reinforcer pathology, (b) the processes that engender reinforcer pathology, and (c) the approaches and procedures that can repair it.
PRINCIPLES OF BEHAVIORAL ECONOMICS
Reinforcement pathologies are, in part, the result of patterns of choices that have an additive influence on one’s subsequent options and choices. Said another way, the decision-making processes underlying the consumption of a pathological reinforcer change as prolonged use of the commodity increases. Clarifying the processes that undergird these choices allows a deeper understanding of reinforcement pathologies. An exhaustive review of the factors that influence choice is beyond the scope of this review. Instead, we focus on two primary principles of behavioral economics that permit the objective study and precise characterization of reinforcement pathology as we have defined it: demand and discounting.
Demand
Descriptively, individuals who exhibit reinforcement pathology value their substance of dependence more than other commodities. However, by saying “value more,” we are referring not only to the total amount consumed or the subjective hedonic value (e.g., drug liking or enjoyment), but also to the total level of resource allocated toward obtaining the substance and to the extent to which consumption is sensitive (or insensitive) to a change in price. Moreover, outside of controlled laboratory environments, price is defined broadly and could include monetary cost, effort, or time required to obtain a commodity or combination of commodities. This inclusive definition of price allows for the application of behavioral economics across many different types of experimental designs and can also be used to compare the behavior of different species. As a convention, price can be examined as a cost-benefit ratio, referred to as the unit price (see below for greater detail on this topic) where the cost (monetary, effort, time, or some combination) is divided by the unit of the commodity. In behavioral studies, unit price is often modeled with response requirement (e.g., with a fixed-ratio schedule of reinforcement) defined as the cost, divided by reinforcer magnitude (e.g., number of reinforcers delivered upon completion of the response requirement) defined as the benefit (Hursh 1980).
The effects of a changing price, or unit price, on purchasing behavior can be discerned by measuring elasticity of demand. Demand, the amount of a commodity purchased at a given price, is described as inelastic when it is relatively insensitive to a price change and elastic when it is relatively sensitive to a price change. Quantitatively, elasticity of demand is defined as the proportional change in consumption relative to the proportional change in price; a proportional change of consumption is either less than the corresponding proportional change in price (inelastic) or proportionally greater than the corresponding change in consumption (elastic) (Hursh 1980, 1984).
Demand can be further investigated by generating a demand curve, which shows consumption as a function of price. Indeed, the two forms of assessment (quantitative and graphical) share many common features; that is, the slope of a demand curve, when plotted in double logarithmic coordinates, is equivalent to a changing elasticity of demand. Demand analysis can distinguish the subjective value for two different commodities (see Figure 1). By quantifying two dimensions of reinforcer consumption, demand curve analysis shows that the reinforcing efficacy of a commodity is inherently dependent on the unit price at which it is assessed and that initial demand for two products cannot necessarily be used to predict preference at higher prices.
Figure 1.
Panel a shows demand curves for two hypothetical reinforcers. Although both reinforcers show the same intensity of demand (level of consumption when the commodity is available at very low prices), the reinforcer represented by open red squares shows higher elasticity (sensitivity to price) compared with the reinforcer represented by closed blue circles. Panel b shows demand curve (closed blue circles; left y-axis) and corresponding response output curve (open red circles; right y-axis). Also shown are the price at which maximal responding occurs (Pmax) and the corresponding maximal response output (Omax).
As also shown in Figure 1, two additional variables may be determined from demand curves: Pmax and Omax (Hursh & Winger 1995, Winger et al. 2006). Pmax is closely related to elasticity and is the price at which response output (e.g., money spent or responses emitted) reaches its maximum value. It is also the price at which demand shifts from inelastic to elastic. The corresponding maximal response output at Pmax is referred to as Omax. The price associated with peak responding (Pmax) is 300. The corresponding expected peak response output (Omax) is 10,000 responses. As described below, individual differences in demand for substances may provide clinically relevant indicators of addiction severity (MacKillop & Murphy 2007).
Unit price
Demand analysis quantifies the effect of price on consumption. As discussed above, price can be more than cost; it can be a cost-benefit ratio that is also referred to as unit price. Unit price has brought parsimony to otherwise disjointed findings in the literature, serving as a standardized rubric to compare studies that used differing prices and reinforcer magnitudes (see also Bickel et al. 2000). Re-analyses of previously published drug self-administration data, along with other more recent studies, have shown that the two components that contribute to unit price (response requirement and reinforcer magnitude) have functionally equivalent effects on drug self-administration (Bickel et al. 1990, DeGrandpre et al. 1993). For example, studies support the implication of unit price that doubling the response requirements and halving reinforcer magnitude yield comparable decreases in drug consumption. This provides parsimony by functionally integrating two processes previously considered independent into one functional process, thus yielding policy importance. Consider this point: The Food and Drug Administration (FDA) can regulate the amount of nicotine contained in cigarettes (but cannot eliminate nicotine altogether) but has no control over the price manufacturers charge for their product. As a method of decreasing demand and consumption for cigarettes, the advances made through the use of unit price in behavioral economic studies discussed above suggest that decreasing the amount of nicotine in a cigarette by half will function the same as if the price were doubled (DeGrandpre et al. 1993).
Reinforcer interaction
Demand for a commodity is important for the choices drug-dependent individuals will make to obtain the reinforcer that contributed to their pathology; indeed, the individual may at times select the substance over opportunities that may have afforded greater long-term advantages. This suggests the importance of also understanding how that specific commodity interacts with the vast number of other commodities that constitute the context within an individual’s life. Behavioral economics provides an easily operationalized and sophisticated framework to quantify the degree and type of interaction between different reinforcers. Behavioral economics specifies that commodities can interact in one of three ways that describes a continuum. At one end of the continuum, commodities can function as substitutes; that is, as price increases for a commodity such as butter and its consumption decreases, consumption increases for another commodity (the substitute) that has remained constant in price (e.g., margarine). The slope of increase in consumption of margarine as a function of the unit price for butter would indicate the degree of substitution (e.g., steeper increase indicates greater substitute). At the other end of the continuum, two reinforcers can interact as complements. For example, as the price of soup increases and its consumption decreases, so might the consumption of soup crackers also decrease despite the absence of a change in their price. Between these two extremes, commodities can be independent; that is, as the price of butter increases and its consumption decreases, the consumption of soup may remain unchanged.
Opportunity cost
According to behavioral economics, reinforcers can also interact in another way: “Opportunity cost” refers to situations where options are mutually exclusive; that is, selection of one renders the other choices as unavailable. Economics stipulates that the opportunity cost of a choice is equal to the best alternative not taken (Bickel et al. 1993). The concept of opportunity cost for drug reinforcement has been frequently demonstrated in both nonhuman animal and human studies; that is, alternative reinforcement decreases the consumption of abused substances by increasing the cost of substance use (e.g., Bickel et al. 1993, Higgins et al. 1994, Nader & Woolverton 1991). Said another way, providing the alternative reinforcer in explicit competition with substance use increases the opportunity cost of substance use (i.e., what could be gained from abstinence); therefore, substance use decreases. As described below, individuals with few alternatives to substance use are less likely to change their use successfully (Murphy et al. 2005, Vuchinich & Tucker 1996). Moreover, treatment approaches that attempt to increase levels of substance-free reinforcement---whether by enhancing social support or goal-directed/prosocial behaviors---are generally more effective than treatments that focus only on reducing substance use (Murphy et al. 2012).
Discounting and Related Processes
Discounting processes are another behavioral economic concept that has proven extremely relevant to the study of such reinforcer pathologies as addiction. The general discounting framework determines how much value a reinforcer loses as a function of a manipulated variable. Three primary variables that have been manipulated and are germane to this discussion are delay, probability, and social distance. Delay (or temporal) discounting examines the extent to which the value of a reinforcer decreases as a function of its temporal distance (Mazur 1987, Rachlin et al. 1991). Probability discounting determines the degree to which uncertainty of a reinforcer decreases the value of that reward (Rachlin et al. 1991). Social discounting determines the degree to which a person’s valuation of a reinforcer delivered to another person decreases as a function of the social distance between those two individuals (Jones & Rachlin 2006). Other parameters that have been manipulated in the context of discounting include the magnitude and type of the reward (e.g., money, drugs, health, or sex) (e.g., Baker et al. 2003, Johnson & Bruner 2012, Lawyer et al. 2010), whether the rewards are gains or losses (Thaler 1981), combinations of delays and probabilities (Yi et al. 2006), whether the rewards are received in the future or the past (Yi et al. 2006), the influence of delay on social decisions (Bickel et al. 2012b), and cross-commodity (e.g., money now versus drug later) interactions (e.g., Bickel et al. 2011b, Yoon et al. 2009).
Delay discounting
Of all the discounting processes, delay discounting was the first identified and has been the most extensively studied. Experimental research into delay discounting has its origins in Herrnstein’s matching law, which specified that allocation of responses in a choice situation is proportional to the relative rates of reinforcement among the options (Chung & Herrnstein 1967; Herrnstein 1961, 1970). This, in turn, suggested that subjective reinforcing value decreases, owing to delay, by dividing reinforcer magnitude by delay (Baum & Rachlin 1969).
Experimentally, delay discounting is commonly studied through assessments of one’s preferences between smaller immediate rewards and larger delayed rewards. In humans, money is the reward most typically used in this work owing to its generality as a reinforcer, relative ability to maintain value into the future, and amenability to precise quantification. Many studies have used hypothetical rewards, to which direct behavioral and neural comparisons of real and hypothetical rewards have shown comparable effects (Baker et al. 2003; Bickel et al. 2009; Johnson & Bickel 2002; Johnson et al. 2007; Lagorio & Madden 2005; Madden et al. 2003, 2004). Delay discounting is an intuitive concept, in that when offered a choice between receiving $100 now, versus receiving $100 after a 1-month delay, most individuals would prefer the immediate $100. However, by systematically adjusting choices, as in psychophysical experiments, this intuitive concept becomes a powerful tool for assessing individual differences and understanding fundamental processes in decision making. So, to continue with the example, when faced with the choice between receiving $75 now versus receiving $100 after a 1-month delay, some people may still prefer the immediate option, but others would prefer to wait for the larger delayed option. Likewise, if the immediate option is $50, an even larger proportion of individuals may prefer to wait for the delayed option. By assessing such choices in individuals across a variety of delays, delay-discounting procedures can specifically quantify the devaluation of rewards across delays, which in turn allows an assessment of a graphical representation of this behavior in the form of the discounting function (see Figure 2) and an index of discounting rate. In addition to strict assessment of delay discounting by tracking preferences between smaller-sooner and larger-later rewards, some studies falling within the domain of intertemporal choice have assessed the discounting of different commodities across time, for example, choices between drug reward now versus money reward later (e.g., Bickel et al. 2011b, Yoon et al. 2009).
Figure 2.

Hypothetical delay-discounting function. Reward value decreases in a hyperbolic fashion as delay until receipt of the reward increases.
Building from the framework provided by the matching law, Mazur (1987) determined the relationship between reinforcing value and delay approximated a hyperbola and proposed that the model represented by Equation 1 best described this relationship:
| (1) |
In this equation, V is the present value of the delayed reward; that is, the magnitude of immediate reward subjectively equivalent to a delayed larger reward. V is expressed here as a proportion of the later outcome. D is the delay from the choice until the occurrence of the outcome, and k is a free parameter that serves as an index for discounting rate. Figure 2 shows a hypothetical example of delay-discounting data, showing reward value plotted against delay until the reward, with the best-fitting function defined by Equation 1. Although some newer multiparameter mathematical models provide an improved fit and in turn provide further insights about the nature of the temporal (e.g., Green et al. 1994, Rachlin 2006, Yi et al. 2009), the hyperbolic model expressed in Equation 1 typically provides a good fit across studies that have used varied techniques, such as the use of real and hypothetical rewards. Furthermore, the single parameter k is more interpretable than the parameters from multiparameter models, which are inherently interdependent on one another. Finally, the hyperbolic model is able to account for an empirically demonstrated feature of delay discounting---the reversal of preferences from larger-later to smaller-sooner rewards as the time until receipt of the smaller-sooner reward draws closer (Frederick et al. 2002, Green et al. 1994) (see Figure 3).
Figure 3.
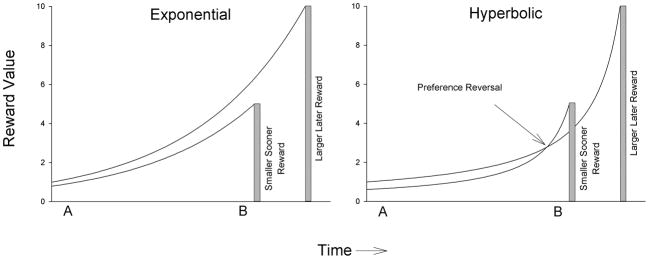
Panel a shows that assuming exponential discounting, relative reward (preference) between a smaller-sooner (blue) and larger-later (red) reward remains constant through time (e.g., at both Time A and Time B). Panel b shows that assuming hyperbolic discounting, preference reverses from a larger-later (red) reward (at Time A) to a smaller-sooner (blue) reward (Time B).
With exponential discounting, if a larger-later reward is preferred when both it and a smaller-sooner reward are distant in the future (as at Time A), then that larger-later reward is preferred even when the smaller-sooner reward is imminently available (as at Time B). Time does not result in a change in preference as the reward draws closer. In contrast, Figure 1 shows hyperbolic discounting and the resulting possibility of a preference reversal. At Time A when both a smaller-sooner and a larger-later reward are far into the future, the discounted-value curve of the larger-later reward is above that of the smaller-sooner reward. Thus, a person would indicate a preference for the objectively larger reward at this point. However, as predicted by the crossing of discounting curves allowed by the hyperbolic functions, at Time B when the smaller-sooner reward is imminently available, people reverse their previous preferences for the larger reward and instead select the smaller-sooner reward.
The choice dynamic implied by the hyperbolic discounting model is reflected in characteristic patterns of decisions made by many substance abusers. Substance abusers often have a fairly consistent stated preference for the larger delayed rewards associated with abstinence that often goes unrealized. Problem drinkers may wake up every morning with a desire to abstain to attain future positive health, education, or vocational outcomes, which they, at 8:00 AM with no immediate access to alcohol in the home, value more than the smaller more proximal rewards associated with drinking. However, when the availability of the smaller-sooner reward is imminent---for example, at the end of the workday when a coworker mentions that there will be a happy hour right after work---the value of drinking, now immediately available, may surpass the delayed value of abstinence resulting in a preference reversal and the decision to drink. This phenomenon may also explain the loss of control that often occurs within substance use episodes. Substance users often violate initial self-imposed limits when faced with the immediate opportunity to consume more of the substance. These self-control failures and instances of behavior that are seemingly at odds with “true preferences” or values are a key feature of relapse and addiction.
Closely related to delay discounting, delay of gratification refers to the observation that waiting for a consequence reduces reinforcement. This framework was pioneered in a series of studies in children by Mischel and colleagues (1972). Participants were asked to wait an unspecified period of time for the experimenter to return to the room before consuming a preferred food reward. In the meantime, the child could signal the experimenter to return to the room at any time, but the participant would receive a less-preferred food reward. Although delay-discounting and intertemporal choice tasks are similar, delay of gratification tasks differ in that receiving the preferred delayed reward requires not only a single choice, but rather continual resistance from opting for the sooner less-preferred reward. In long-term follow-up research, those preschool children who were able to wait for the preferred delayed reward scored higher on college entrance exams, thus suggesting the robustness of such tasks for indexing important future life consequences (Mischel et al. 1989). As reviewed in a subsequent section, a similar assessment of preference between sooner less-valuable and delayed more-valuable rewards in delay-discounting tasks is also associated with important real-life consequences such as propensity to addiction.
Excessive probability discounting
Probability discounting, or the devaluation of rewards due to their uncertainty, is assessed by tasks very similar to the delay-discounting task described above, except that the smaller and larger rewards differ by certainty rather than delay (smaller certain reward versus a larger uncertain reward, e.g., a choice between a 100% chance of receiving $50 or a 50% chance of receiving $100). In probability discounting procedures, subjects who gamble have shown less discounting due to uncertainty relative to control nongambling participants (Holt et al. 2003, Madden & Bickel 2009). That is, gamblers value uncertain rewards more highly than others. However, the association may be less robust in substance abuse disorders: Some studies have failed to find differences (Andrade & Petry 2012, Reynolds et al. 2003, Takahashi et al. 2009), but others have found that substance users showed greater probability discounting than nonusers (Reynolds et al. 2004; Yi et al. 2007, 2012). Thus, although gamblers may overvalue uncertain rewards, substance abusers may undervalue uncertain rewards. Many of the delayed rewards that may compete with substance use are also uncertain (e.g., future health, educational outcomes), relative to the consistent immediate rewards associated with substance use (e.g., euphoria, sedation, analgesia). Steep delay and probability discounting may reflect a maladaptive decision-making bias, and individuals who devalue delayed or uncertain outcomes may prefer drug use relative to many alternative behaviors (Rachlin et al. 1991). More research is needed to explore the complex relationship among substance use, gambling, and probability discounting.
Other forms of discounting
Social discounting tasks determine the extent to which a participant’s personal value of a reward declines as a function of one’s social distance to the reward’s recipient (Jones & Rachlin 2006). Social discounting tasks have participants make hypothetical choices between receiving a certain amount of money versus having a person at a certain social distance receive an amount of money (e.g., $5 given to you or $75 given the fifth-closest person to you in your life). Individuals typically value a reward given to a very close individual (e.g., a family member, romantic partner, or close friend) more than one given to a distant individual (e.g., a friendly acquaintance). As with delay and uncertainty sensitivity, individuals vary in their sensitivity in the extent to which a certain social distance reduces value to oneself. Social discounting shows remarkable similarities to delay and probability discounting in its mathematical form (Bradstreet et al. 2012; Kowal et al. 2007; Rachlin 2006; Yi et al. 2011a,b).
As with delay and probability discounting, initial research suggests social discounting may be associated with substance use and abuse. Bradstreet et al. (2012) showed that, among pregnant women who were smokers, those who relapsed to smoking after quitting showed a higher degree of social discounting (i.e., showed a greater decrease in personal reward value as a result of social distance to the reward recipient) than those who quit smoking and did not relapse. Yi et al. (2012) found higher rates of social discounting (as well as delay and probability discounting) in methamphetamine users compared with control participants. In another study, Bickel et al. (2012b) compared individual delay discounting (standard delay discounting) and social temporal discounting (individuals made intertemporal monetary choices for a group of which they are a member) in hazardous-to-harmful drinkers, smokers, and controls. Compared with controls, hazardous-to-harmful drinkers and smokers discounted more on individual discounting, but only smokers discounted delayed rewards more rapidly under the social condition.
Interactions of Demand and Discounting: Experimental Evidence
The comorbidity of excessive discounting and demand (for the commodity to which they are addicted) in individuals with substance use disorder raises the question of whether a relationship exists between these two concepts. An individual who discounts the future may be willing to expend an inordinate amount of resources to obtain a drug dose even at high prices and may consume large quantities when drug prices are low (Murphy & MacKillop 2006). Several studies have explicitly tested whether a directional relationship between demand and discounting exists, but the results from this incipient line of inquiry have been largely inconclusive. For example, in one study in rats, subjects that had higher discounting rates (i.e., those that preferred smaller-sooner over later-delayed food reinforcement) showed lower elasticity for nicotine self-injections (that is, greater continued consumption of nicotine despite increases in the work required to receive the injection) (Diergaarde et al. 2012). However, delay discounting was unrelated to demand elasticity for alcohol self-administration. Another study found that rats with higher delay discounting for a food reward also exhibited greater demand for cocaine, but not for food (Koffarnus & Woods 2013). In humans, a study in smokers found that delay discounting for money was affected by nicotine deprivation, but demand for cigarettes was not affected (Field et al. 2006). Among heavy drinkers, a greater severity of alcohol use disorder was associated with both greater delay discounting of money rewards and greater alcohol demand (MacKillop et al. 2010a). Moreover, higher alcohol demand was associated with greater discounting. However, compared with smokers without schizophrenia, those with schizophrenia have greater demand for cigarettes but not greater discounting (MacKillop & Tidey 2011). The number of studies relating demand and discounting is currently limited, and definitive results will require additional study. Given the importance of these constructs, the relationships between demand and discounting may have a great impact on the understanding of addiction.
PROCESSES ENGENDERING REINFORCER PATHOLOGY
The preceding sections detail key features of substance use disorders from a behavioral economic perspective in phenomenological and operational terms. In this section, we discuss the putative processes that give rise to the overvaluation and overconsumption of psychoactive substances. We also discuss the empirical evidence supporting this perspective. Importantly, in addition to emphasizing the processes of demand and discounting, a behavioral economic perspective is also highly consilient with mechanisms that have been identified from different psychological and neurobiological perspectives. Where sharp differences are present, they result from the framing and theoretical conceptualization of the same empirical phenomena. Most contemporary perspectives emphasize endogenous (person-level) factors in the development and maintenance of addictive behavior, such as alterations to biological circuits by chronic drug exposure, maladaptive cognitive templates, or intense experiential cravings. What is unique about a behavioral economic perspective is that it attempts to synthesize the concurrent interplay of processes that are operative within the individual and those in the environment. In the following, we use a behavioral economic perspective to discuss both person-level factors and environmental factors in addictive behavior. In addition, we use the same lens to understand neurobiological and genetic perspectives on the etiology of addictive disorders.
Person-Level Factors: Elevated Demand and Delay Discounting
In terms of intraindividual processes, high levels of drug demand and delay discounting (or immediate preference in other forms of intertemporal choice) are theorized to be recursive etiological markers in the development of substance use disorders. Drug demand and delay discounting putatively capture important variability of an individual’s valuation of drugs as reinforcers and preference for smaller-sooner rewards relative to larger-delayed rewards, respectively. As recursive markers, they are theorized to reflect both current preferences and, in turn, predict subsequent preferences. This theoretically applies across the chronology of use, from predicting the acquisition and escalation of substance use to forecasting substance use maintenance or success in treatment. The empirical evidence supporting each domain is discussed in turn.
Persistently elevated drug demand as an etiological process
In studies of drug demand, consistent evidence supports a significant association between indices of demand and substance dependence. For example, indices of alcohol demand have been significantly associated with severity of alcohol misuse in studies using young adult samples (Murphy & MacKillop 2006; Murphy et al. 2009, 2013; Wahlstrom et al. 2012) and community samples (Acker et al. 2012, Gray & MacKillop 2013, MacKillop et al. 2010a). Moreover, higher levels of alcohol demand predict poor response to brief alcohol interventions in young adults (Bernstein et al. 2013, MacKillop & Murphy 2007). Specifically, high alcohol demand was associated with a differentially lower intervention response.
Recent studies have also examined indices of alcohol demand in mechanistic relationships with established person-level variables. For example, high alcohol demand moderates the relationship between impulsive personality traits and alcohol misuse, such that higher Omax was associated with a stronger relationship between urgency (i.e., acting out under conditions of negative affect) and alcohol-related problems (Smith et al. 2010). Similarly robust relationships have been observed for tobacco demand and nicotine dependence. In a wide diversity of populations, higher levels of tobacco demand are consistently associated with greater nicotine dependence (Chase et al. 2013, Few et al. 2012, Liao et al. 2013, MacKillop & Tidey 2011, MacKillop et al. 2008, Murphy et al. 2011). Also consistent with a role for drug demand in substance dependence, compared with smokers receiving a placebo, those who were administered the efficacious smoking-cessation medication varenicline showed significant increases in demand elasticity for cigarettes (McClure et al. 2013).
In addition, a substantial body of work connects demand for food to obesity, which, in our framework, is also a reinforcement pathology much like substance use disorders. Early studies showed elevated food reinforcement among obese individuals (Jacobs & Wagner 1984, Johnson 1974), and these findings have been largely supported in more recent work using validated assessments of demand. For example, food incentive value has been positively associated with body mass index and obesity in both children and adults (Epstein et al. 2007, Saelens & Epstein 1996, Temple et al. 2008). Laboratory studies also support the link between food demand and eating behavior. Food demand is positively associated with greater food consumption in the laboratory (Epstein et al. 2004, 2008, 2011; Rollins et al. 2010; Saelens & Epstein 1996; Temple et al. 2008). Indeed, food demand is a better predictor of consumption than are food hedonic subjective ratings (Epstein et al. 2004, Temple et al. 2008). Finally, in a recent weight-loss intervention trial in overweight children, food demand predicted treatment success and moderated the effects of an enriched environment (i.e., availability of alternative nonfood reinforcers) (Best et al. 2012). Specifically, an enriched environment was not associated with a positive outcome for children exhibiting high food demand.
A significant limitation to the existing literature on demand as an etiological process is that the vast majority of studies are cross sectional. The prospective clinical studies to date (Bernstein et al. 2013, Best et al. 2012, MacKillop & Murphy 2007) suggest that high demand is a maintaining factor that reduces the benefits of interventions, but additional research is needed to establish elevated demand as an etiological process relevant to substance dependence and to identify predictors of elevated demand. Two recent longitudinal preclinical studies revealed escalating demand for cocaine and opiates over the course of increased experience (Christensen et al. 2008, Wade-Galuska et al. 2011), which is supportive of the etiological hypothesis but is by no means definitive. As such, a recursive causal role remains primarily a hypothesis, and the need for longitudinal studies, especially in human populations, is very high.
Delay discounting as an etiological process
Returning to impulsive intertemporal choice, the evidence supporting a causal etiological role is relatively robust. As with demand, a consistent body of evidence significantly associates steep delay discounting with substance use disorders. Numerous categorical studies have contrasted addiction-related groups with control groups and found significantly greater discounting for substances such as alcohol (Bjork et al. 2004, Claus et al. 2011, Mitchell et al. 2005, Petry 2001, Vuchinich & Simpson 1998, Yankelevitz et al. 2012), cocaine (Coffey et al. 2003, Heil et al. 2006), tobacco (Baker et al. 2003, Bickel et al. 1999b, Heyman & Gibb 2006, Johnson et al. 2007, Mitchell 1999, Reynolds 2006b), opioids (Kirby & Petry 2004, Kirby et al. 1999, Madden et al. 1997), and methamphetamine (Hoffman et al. 2006, 2008; Monterosso et al. 2007). One study examining marijuana-dependent individuals and controls did not find a significant difference, but it showed a trend toward greater discounting in dependent individuals; this effect, however, had a smaller effect size than those found in many of the previous studies with other drugs (Johnson et al. 2010). A recent meta-analysis of these categorical studies found evidence of a medium effect-size difference across studies (Cohen’s d = 0.58) (MacKillop et al. 2011), which was moderated by clinical severity. Specifically, significantly greater discounting (d = 0.61) was found in those studies using participants that met clinical criteria compared with those using nonclinical samples (d = 0.45). Moreover, smoking rates are robustly related to delay discounting (Alessi & Petry 2003; Cheng et al. 2012; Epstein et al. 2003; Johnson et al. 2007; MacKillop et al. 2010a; Ohmura et al. 2005; Reynolds 2004, 2006a; Sweitzer et al. 2008). In addition, increased discounting is a negative prognostic factor in treatment for nicotine dependence (MacKillop & Kahler 2009, Sheffer et al. 2012), stimulant dependence (Washio et al. 2011), and marijuana dependence (Stanger et al. 2012). In a series of longitudinal studies, a naturalistic index of discounting based on relative discretionary expenditures toward alcohol relative to savings predicts the natural resolution of alcohol misuse among middle-aged adults (Tucker et al. 2002, 2006, 2008). In addition to substance use, studies have also suggested that greater delay discounting is associated with important clinical problems associated with substance use, including HIV risk via injection equipment sharing among heroin abusers (Odum et al. 2000) and condom use among cocaine abusers (Johnson & Bruner 2012).
Complementing these findings is a large preclinical literature examining the role of discounting in the etiology and maintenance of addiction. This work has revealed the fundamental neurobiological circuits that subserve delay-discounting decision making (for a review, see Cardinal 2006). Furthermore, these studies demonstrate the independence of the neurobiological processes that underlie delay discounting from those underlying response-disinhibition measures of “impulsivity” (Winstanley et al. 2006). More importantly, these studies have found that individual differences in discounting are predictive of initiation and progression of drug self-administration in rodent models (Anker et al. 2009; Marusich & Bardo 2009; Perry et al. 2005, 2007). This finding is important because it establishes that high levels of discounting, prior to any drug exposure, set the stage for the development of addictive behavior. In addition, chronic exposure to addictive drugs significantly increases delay-discounting rates (Dallery & Locey 2005, Koffarnus & Woods 2013, Mendez et al. 2010, Roesch et al. 2007, Simon et al. 2007).
Complementing these findings are a number of longitudinal studies showing that greater delay discounting predicts the development of addictive behavior. In a study of early adolescents over a 5-year period, discounting significantly predicted increases in smoking over time (Audrain-McGovern et al. 2009a). Furthermore, discounting remained generally stable as a characteristic, and alternative path modeling did not support the opposite relationship, of greater smoking behavior leading to greater discounting. In a twin study, greater delay discounting predicted the development of substance use and other externalizing behaviors from ages 12 to 14 (Anokhin et al. 2011). Most recently, a 4-year longitudinal study examining determinants of alcohol misuse showed that working memory predicted the development of alcohol misuse, and this relationship was partially mediated by discounting (Khurana et al. 2013). Although more longitudinal studies are needed, these provide converging evidence that greater delay discounting plays a causal role in the development of addictive behavior. Together, the preclinical findings and longitudinal findings support the view of steep delay discounting as a recursive etiological factor in the development of addictive behavior, serving as both a predisposing risk process and a process further exacerbated by excessive substance use.
Factors that Dynamically Affect Demand and Discounting
The processes that give rise to elevated demand and discounting are typically not the focus of direct experimental examination. However, a small number of studies imposed experimental manipulations on these variables to understand how in vivo changes in both variables may take place. For example, environmental drug cues significantly increased demand for alcohol and tobacco (Acker & Mackillop 2013; MacKillop et al. 2010b, 2012b), suggesting that drug-related associative conditioning leads to dynamic increases in demand. Similarly, acute withdrawal from nicotine significantly increased tobacco demand (MacKillop et al. 2012b) and also made increased discounting even more precipitous among smokers and opiate-dependent individuals (Field et al. 2006, Giordano et al. 2002, Mitchell 2004, Yi & Landes 2012). In a preclinical study, a parallel relationship was reported in which morphine withdrawal increased demand for a substitute opiate (Wade-Galuska et al. 2011). These studies contextualize demand and discounting with two well-established processes in substance dependence: reactivity to drug-related environmental cues and withdrawal following the development of physiological dependence. As such, they suggest that associative conditioning and acute withdrawal have direct effects on these domains and, in turn, on choice behavior. Accordingly, these factors acutely affect substance use decision making via behavioral economic indices. Furthermore, these individual experiences are predicted to summate over time, partially contributing to the high levels of demand and discounting that are stably observed in affected individuals. This relatively incipient line of research holds promise for characterizing microlevel events that give rise to persistent dysregulation of decision making.
Environmental Factors: Availability of Alternative Reinforcers (Opportunity Cost)
The preceding domains are theorized to represent only one major class of influences, the motivational factors within the individual. However, because of its foundations in economics and behavioral psychology, a behavioral economics perspective gives equal weight to the larger environmental context within which addictive behavior takes place and, more specifically, the alternative reinforcers to which a person has access. As noted above, a behavioral economics perspective is a molar account of addiction, focusing on aggregates of behavior, not instances, and both the endogenous conditions of the organism and exogenous characteristics of their environment. Put simply, a person’s internal motivational state and the alternative reinforcers available in the environmental context are theorized to jointly determine the decision to use or not to use a drug. From this perspective, the yin and yang of addiction are high levels of endogenous factors (high demand and impulsive discounting) and low levels of exogenous factors (alternative reinforcers). Furthermore, a recursive etiological process is proposed to be operative for alternative reinforcers over the course of the development of a substance use disorder. For example, an individual may begin drinking recreationally and as part of a diverse repertoire of positively and negatively reinforcing behaviors. But, as use escalates and negative consequences mount (e.g., dissolution of a relationship, loss of a job, legal difficulties), the availability of these alternative reinforcers diminish, which commensurately increases the reinforcing value of alcohol (Rachlin 1997). This recursive etiological feedforward loop illustrates the classic vicious cycle of addiction (e.g., the “primrose path” model of addiction formulated by Herrnstein & Prelec 1992).
In terms of empirical research, considerable experimental and descriptive evidence supports the importance of alternative reinforcers (Cosgrove & Carroll 2003, Cosgrove et al. 2002). In preclinical animal models, the highest rates of substance use are present in contexts with the least availability of substance-free reinforcements, such a palatable food, exercise, enriched housing, and social access (Alexander et al. 1978, Carroll et al. 2009, Cosgrove & Carroll 2003, Cosgrove et al. 2002, Hadaway et al. 1979). Similar results have been found in human laboratory and clinical studies (e.g., Bickel et al. 1995, Vuchinich & Tucker 1988). Recent functional magnetic resonance imaging (fMRI) studies also indicate that chronic substance abusers report diminished neural activation to nondrug rewards (Lubman et al. 2009). Using measures that characterize the proportionate reinforcement received from a variety of sources, substance misusers report less reinforcement from nondrug activities compared with control participants (Correia et al. 1998, 2003; Van Etten et al. 1998). In continuous relationships, alcohol-free reinforcement is significantly negatively associated with alcohol consumption and alcohol-related problems (Murphy et al. 2005). In one longitudinal study, the presence of alternative reinforcers negatively predicted the development of smoking over a four-year period (participants ages 18–22) (Audrain-McGovern et al. 2011). Furthermore, an indirect relationship was evident, such that higher levels of baseline depression predicted reduced alternative reinforcement over time and, as a result, increases in smoking. Another longitudinal study with heroin-dependent adults found that subjective valence ratings of pleasant (drug-free) pictures negatively predicted future heroin use after controlling for baseline craving and heroin use (Lubman et al. 2009).
Clinical studies also point to the importance of alternative reinforcers (Higgins et al. 2004). For example, among young adult smokers, those who increased substitute reinforcers across treatment had approximately double the rate of treatment success (Audrain-McGovern et al. 2009b). Similarly, proportionate alcohol-free reinforcement has been associated with poorer response to a brief alcohol intervention in young adults (Murphy et al. 2005) and young adult drinkers who were asked to increase engagement in exercise and creative activities spontaneously reduced their drinking compared with control participants (Correia et al. 2005). Successful treatment is associated with the development of more sources of alternative reinforcement (Rogers et al. 2008). More broadly, several of the behavioral economic treatments operate by enhancing alternative reinforcers (discussed below) (Murphy et al. 2012).
Neurobiological Substrates
Dramatic advances have taken place in our understanding of the neurobiology of addictive behavior, including the role of genetic factors in liability for addictive disorders. Although a comprehensive review is beyond the scope of the current article, this section briefly summarizes the literature through the lens of behavioral economics. In its distilled form, the neurobiology of addictive behavior is theorized to reflect the result of functional and structural changes to the brain resulting from long-lasting neuroadaptations that are induced by chronic exposure. Three key neural regions and circuits are disrupted by this process, including the prefrontal cortex, which is responsible for diverse forms of executive function such as decision making; subcortical motivational circuits, which include structures such as ventral striatum and nucleus accumbens; and what are referred to as “antireward” circuits, which include the stress systems that subserve the acute and protracted aversive states that follow from intoxication into acute and protracted withdrawal (for reviews, see Kalivas & Volkow 2005, Koob & Le Moal 2008, Volkow et al. 2012). Together, these changes confer distinct vulnerabilities for continued addictive behavior, including altered decision making, powerful motivational drives, reduced sensitivity to substance-free rewards, and a semichronic aversive state (Kalivas & Volkow 2005, Koob & Le Moal 2008, Lubman et al. 2009, Volkow et al. 2012).
These neuroadaptive changes are highly compatible with a behavioral economic perspective. In the context of drug demand, exaggerated drive for a drug is an obvious substrate for persistently high incentive value. However, attenuated executive functioning would also be expected to increase demand by reducing cognitive capacity to fully consider the associated costs, as would a protracted aversive state, which would putatively drive up the value of the drug on the basis of its negatively reinforcing capacity to alleviate that state. Reduced sensitivity to drug-free rewards would similarly increase the demand for substances. With regard to impulsive discounting, the prefrontal and subcortical circuits are largely those that have been implicated with discounting decision making in fMRI research (Bickel et al. 2009, McClure et al. 2004), suggesting that commensurate impairment in discounting may be a behavioral manifestation of these neuroadaptive changes. With regard to the antireward system, the role of stress in discounting has been only modestly studied, and results are mixed (Diller et al. 2011, Fields et al. 2009, Torregrossa et al. 2012, White et al. 2008). However, purely from conjecture, given the laboratory evidence on withdrawal effects discussed above, the chronic augmentation of the antireward system may be a further plausible mechanism for increasing discounting. Finally, from the perspective of alternative reinforcers, diminished executive functioning should reduce the probability a person would investigate, plan for, and engage in new activities. Considered in totality, neuroscience and behavioral economics can largely be seen as highly compatible, and the disconnect between them is simply a side effect of their occupying different scientific levels of analysis.
Similarly, in behavioral genetics, major advances have established the substantial role of genetic factors in the liability for addictive disorders (for reviews, see Goldman et al. 2005, Kendler et al. 2012, MacKillop et al. 2010c). However, substantial evidence suggests that there is no single “addiction gene” or small number of critical variants. Rather, the current evidence suggests that liability is conferred via many genetic loci, each exerting a small effect. Where consistent evidence is available, it pertains to genes responsible for a drug’s pharmacokinetics (metabolic processing) (e.g., Luczak et al. 2006) and pharmacodynamics (CNS actions) (e.g., Bierut 2010, MacKillop et al. 2010c).
Where behavioral genetics and behavioral economics may be reciprocally informative is in using behavioral-economic characteristics as intermediate phenotypes or endophenotypes (Gottesman & Gould 2003). These terms refer either to characteristics that are at least mechanistically informative in terms of genetic relationships (intermediate phenotypes) or to characteristics that meet a number of criteria demonstrating independence and links to both genetic variables and clinical phenotypes (endophenotypes). Both classes of variables are currently receiving a great deal of interest because, relative to clinical phenotypes, they offer more stable, focal, reliable phenotypes that are, putatively, more closely related to genetic variation. As such, associations with specific variants are expected to be larger in magnitude, more reliable, and more interpretable from a mechanistic standpoint. Thus, accumulating evidence suggests that greater delay discounting may be a useful endophenotype (MacKillop 2013). Specifically, evidence supports the notion that variation in discounting is heritable (Anokhin et al. 2011, Huskinson et al. 2012), reliable (Beck & Triplett 2009, Ohmura et al. 2006, Takahashi et al. 2007), associated with the level of familial addictive behavior (Acheson et al. 2011), and linked to specific polymorphisms (Boettiger et al. 2007, Eisenberg et al. 2007, Paloyelis et al. 2010), albeit with some inconsistency in the latter findings. Furthermore, indices of demand or other behavioral economic indices may also be useful for clarifying genetic influences on addictive behavior.
THE REPAIR OF REINFORCER PATHOLOGIES
Constraint of Unhealthy Choice
In the preceding sections, we have outlined the behavioral economic processes associated with reinforcement pathology. We have also discussed how they may function in the development and maintenance of substance use disorders. In the following sections, we examine the evidence and the implications of behavioral economics for the repair of reinforcement pathologies.
Direct environmental constraints: reducing the availability and increasing the price of unhealthy choice commodities
As noted above, behavioral economics provides a molar account of behavior in that it relates aggregates of behavior (e.g., patterns of substance use and substance-free activities over time) to aggregates of reinforcement or price contingencies as measured over some interval (Tucker et al. 2002, Vuchinich 1995). This perspective does not attempt to predict the outcome of a decision to use (or not to use) drugs on a particular day or moment, which would be the goal of a molecular account of behavior (Vuchinich & Heather 2003). As predicted by the law of demand and described above, consumption of most commodities, including psychoactive substances, is inversely related to price or response requirement (Bickel et al. 2012a, DeGrandpre & Bickel 1996, Higgins et al. 2004, Hursh & Roma 2013, MacKillop et al. 2012a, Murphy & MacKillop 2006). As also discussed above, price is defined broadly and may include the monetary cost of purchasing a drug as well as time or effort costs associated with obtaining a drug (e.g., operant response requirement or, in a natural environment, the time/effort required to purchase a drug). Although difficult to quantify, the potential legal and health costs as well as the negative impact of substance use on access to other rewards are also “costs” that influence an individual’s substance use decisions. Increases in any of these costs can decrease substance use, and conversely, decreases in these costs can increase substance use (Hursh & Roma 2013). These observations are consistent with the broader econometric studies comparing the relationships between differing tax levels and licit drug consumption (Chaloupka et al. 2012, Wagenaar et al. 2010).
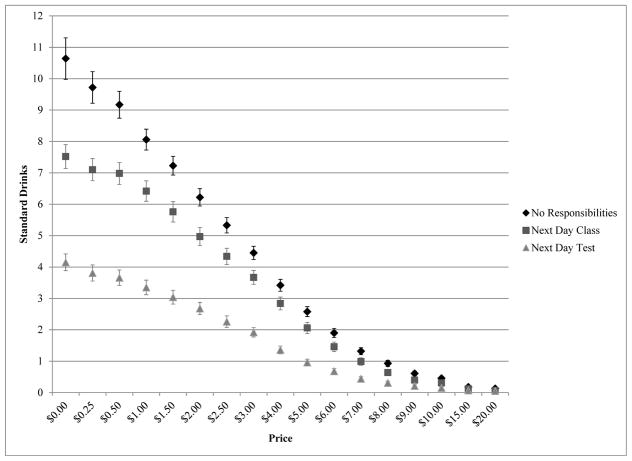
At a behavioral level, the impact of both price and lost opportunity cost is illustrated in Figure 4, which plots hypothetical drink purchases as a function of price and next-day responsibility (Skidmore & Murphy 2011). The sample included 207 college-student binge drinkers who were instructed to make drink purchases across a range of prices and preceding three responsibility conditions for the next day: no class, morning class without a test, and morning class with a test. Consumption was reduced markedly as a function of both price and the availability of a competing next-day alternative reinforcer that would presumably be negatively impacted by heavy drinking (see also Gentile et al. 2012). The results of this study are consistent with behavioral economics theory and suggest that substance abuse prevention and intervention approaches are most likely to be successful when they attempt to increase direct constraints on substance use while simultaneously increasing the availability of particular classes of alternative reinforcers that are incompatible with substance use.
Figure 4.
Mean (±1 standard error of the mean) hypothetical consumption values across 17 different prices for alcohol purchases associated with three next-day responsibility conditions: (diamonds) no next-day responsibilities, (squares) next-day class, and (triangles) next-day test. From Skidmore JR, Murphy JG. 2011. The effect of drink price and next-day responsibilities on college student drinking: a behavioral economic analysis. Psychol. Addict. Behav. 25:57–68. Reprinted with permission from the American Psychological Association.
Moreover, a variety of effective public-health-level prevention approaches are consistent with behavioral economics research (Hursh & Roma 2013). These include enforcing age limits on cigarette and alcohol purchases, maintaining or increasing taxes and/or legal sanctions (related to alcohol/tobacco or illicit substance use, respectively), reducing the density of outlets that sell regulated substances (alcohol, tobacco, and, in some areas, marijuana), carefully regulating the prescribing practices related to commonly abused prescription medications (opioids, benzodiazepines, stimulants), and enhancing the availability of prosocial activities that may increase the opportunity costs of substance abuse (e.g., access to education, employment, recreational activities) (DeFulio & Silverman 2011, Gentile et al. 2012, Weitzman & Kawachi 2000). Interventions that attempt to increase constraints on drug choices are discussed next.
Contingency management as constraint
Research on the relationship between price and consumption has been successfully translated into a number of effective intervention strategies: Contingency management (CM) may be the most direct exemplar. CM approaches attempt to change the user’s environment such that (a) substance use and abstinence are readily detected, (b) abstinence is readily reinforced, (c) substance use results in a loss of reinforcement, and (d) the density of reinforcement derived from substance-free sources is increased to compete with the reinforcing effects of substance use (Higgins et al. 1991). Thus, one of the active mechanisms of CM is the increased costs (loss of reinforcement) associated with the detection of substance use. CM protocols typically strive to achieve continuous abstinence through the use of escalating schedules of reinforcement. For example, a substance user on an escalating CM schedule may receive a voucher worth $5 for day one of verified abstinence, but that amount would increase with each consecutive day of abstinence such that after seven days a single episode of substance use may “cost” the user $50 in addition to the actual cost of the substance. Thus, the opportunity cost of substance use, as defined above, is increased over time. Importantly, because substance abusers generally undervalue delayed outcomes (i.e., show steep delay discounting), effective CM approaches require that drug abstinence be objectively verified frequently so that the “cost” of substance use (lost voucher) is incurred soon after use.
CM procedures have been effectively applied to reduce the use of a number of substances (Hartzler et al. 2012, Stitzer et al. 2007), including cigarettes (Alessi et al. 2004, Yi et al. 2008), alcohol (Alessi & Petry 2013, Koffarnus et al. 2011, Petry et al. 2000), cocaine (Higgins et al. 1993, Petry et al. 2013), marijuana (Carroll et al. 2012), and opiates (Bickel & Marsch 1999, Bickel et al. 1999a, DeFulio & Silverman 2011, Silverman et al. 1996). CM treatment is also associated with increases in quality of life in substance abusers (Petry et al. 2007), and cost-effectiveness studies suggest that these interventions result in a net economic gain owing to their relative efficacy and the expense associated with treatment failures in substance-abusing populations (e.g., inpatient treatment, incarceration, increased health care utilization) (Olmstead et al. 2012, Sindelar et al. 2007). CM interventions have also been modified and are efficacious when (a) relatively low-value vouchers are delivered probabilistically, thereby reducing the total voucher costs incurred by treatment facilities (Olmstead & Petry 2009), and (b) when access to paid employment, rather than vouchers for goods or services, is used to reinforce abstinence (DeFulio & Silverman 2011). Both of these adaptations have the potential to increase the dissemination of CM.
Other Behavioral Approaches to Reduce Consumption by Increasing Constraints on Drug Choice
Several other substance abuse intervention approaches may work in part by increasing constraints on substance use, either by reducing the availability of a substance or increasing the real or perceived cost of alcohol or other substance use. The community reinforcement approach (CRA) focuses on assisting individuals to develop alternative reinforcement that is mutually incompatible with substance use. CRA has been used to treat clients who abuse alcohol (Smith et al. 1998), marijuana (Diamond et al. 2002), cocaine (Carroll et al. 1994), opiates (Bickel et al. 1997), and cigarettes (Roozen et al. 2006). Behavioral couples therapy (BCT) also attempts to increase the cost of substance use though the use of daily sobriety contracts, through ingestion of disulfiram (when indicated), and by encouraging significant others to withhold reinforcement if the substance-abusing partner drinks or uses other substances. A recent meta-analysis in the treatment of alcohol and other substance use disorders found that BCT was superior to a variety of control treatments, including cognitive behavioral therapy that did not include a significant other (Powers et al. 2008).
Brief motivational interventions (BMIs) have emerged as an effective strategy for reducing substance misuse among a variety of populations (Miller & Rollnick 2012). Although BMIs were not originally developed from behavioral economics theories, they attempt to increase an individual’s motivation to change in part by fostering an individual’s awareness of the costs and consequences of substance use. This is often accomplished via a discussion of the pros and cons of substance use (i.e., decisional balance exercise) as well as personalized feedback that highlights tangible costs (e.g., money spent on alcohol and other substances, calories and weight gain related to drinking, estimated life years lost from cigarette smoking). All these strategies encourage clients to consider the total costs of substance use, relative to the overall benefits, and are designed to motivate self-regulation and more informed decision making (Miller & Rollnick 2012). Another feature of BMIs that is consistent with, although not explicitly derived from, behavioral economics theory is that the outcomes of temporally extended patterns of choices related to substance use are aggregated into meaningful units such as substance use occasions per week, substance-related problems experienced over time, or money spent on substances over the course of a year.
From a behavioral economics perspective, removing drugs and associated paraphernalia (e.g., pipes, needles, ashtrays, etc.) from the home not only removes powerful triggers, which can increase craving and demand for substances (MacKillop et al. 2012b, MacKillop et al. 2010b), but also increases the effort costs associated with obtaining new drugs. Various forms of substance-use monitoring (e.g., by clinicians, parents, or significant others) via the increasing availability of low-cost point-of-contact drug-testing kits can also increase the costs of substance use. Parental monitoring is a particularly effective element of substance abuse treatment for adolescents (Komro et al. 2001).
Finally, although a full review is beyond the current scope of this article, a number of medications are FDA approved for the treatment of substance use disorders (e.g., O’Brien 1997), including pharmacotherapies for treating nicotine dependence, opiate use disorders, and alcohol use disorders. These treatment modalities are highly consistent with a behavioral economics perspective. The most common pharmacotherapy strategy is direct or indirect alleviation of withdrawal (e.g., nicotine patch/gum/lozenge/spray, methadone, buprenorphine), which can be thought of as a strategy for reducing demand for a particular drug. These medications may also be characterized as behavioral economics substitutes for the drug being abused. Alternatively, disulfiram for AUDs also putatively reduces demand, but it does so by directly imposing a response cost via the sickness that results when consumed with alcohol. Naltrexone pharmacotherapy for AUDs is somewhat distinct in that it putatively attenuates craving, blunts alcohol’s positively reinforcing stimulant effects, and potentiates its unpleasant sedative effects (for a review, see Ray et al. 2010). As such, in behavioral economics terms, it reduces demand via multifarious positive and negative reinforcement mechanisms.
Although relatively little research has focused on this area, there is clear conceptual overlap between known pharmacotherapy mechanisms and behavioral economics mechanisms as well as a small amount of empirical data. One recent study illustrates the promise of these intersecting lines of research. In a sample of Asian Americans, Bujarski et al. (2012) investigated the effects of naltrexone on alcohol demand, both in general and following intravenous alcohol administration to raise participants’ blood alcohol level to 0.06 g/dl. In addition, on the basis of evidence that possession of the G allele of a single nucleotide polymorphism in the OPRM1 gene (rs1799971) is associated with greater sensitivity to alcohol’s subjective effects (Ray & Hutchison 2004, 2007) and naltrexone’s clinical effects (Anton et al. 2008, Kim et al. 2009, Oslin et al. 2003), participants were genotyped for this locus. In Bujarski et al. (2012), compared with a placebo, naltrexone significantly reduced intensity of demand, Omax, and breakpoint, thus supporting the hypothesis that one of its mechanisms is the attenuation of the incentive value of alcohol across the demand curve. In addition, possession of the high-alcohol-sensitivity G allele was also associated with greater intensity of demand, suggesting that behavioral economics indices may be promising intermediate phenotypes. Although this study had a relatively small sample size and was exploratory in nature, it nonetheless illustrates the potential of integrating behavioral economics into addiction pharmacotherapy and pharmacogenetic research.
Reduced Constraint of Healthy Choice
Restructuring environments to enhance access to healthy choices
As noted above, experimental studies have shown that high rates of substance use most likely occur in contexts devoid of substance-free sources of reinforcement (Alexander et al. 1978, Carroll et al. 2009, Hadaway et al. 1979), that substance abuse is associated with diminished dopamine response to naturally occurring substance-free rewards (Koob 2006, Volkow et al. 2003), and that substance use will generally decrease if access to alternative reinforcers is increased (Higgins et al. 2004). These basic research findings have led to a variety of interventions that seek to increase the availability of healthy alternatives to substance use. These approaches vary with respect to the intensity of the intervention; that is, chronic substance abusers may require more step-by-step assistance in accessing drug-free rewards, whereas the enhancement of substance-free alternatives via brief interventions are viable in less-severe populations.
Community reinforcement, contingency management, and behavioral couples therapy: approaches enhancing access to substance-free reinforcement contingent on abstinence
Many of the aforementioned intervention approaches that attempt to increase constraints on substance use also seek to increase the availability of drug-free sources of reinforcement. This is a central element of CRA and CM approaches, which include counseling designed to improve employment prospects, marital and family relationships, and nondrinking social and recreational interactions. Vouchers earned from abstinence can be exchanged for a variety of items that are designed to increase substance-free reinforcement, including materials related to hobbies/recreational activities, sporting equipment, tickets to cultural/entertainment events, and educational supplies or tuition. A meta-analytic review concluded that CRA is more effective than usual care and that the addition of incentives for abstinence leads to improved outcomes (Roozen et al. 2003). The Therapeutic Workplace, developed by Silverman and colleagues (Silverman et al. 2001, 2002) is another example of a CM treatment approach that directly targets engagement in drug-free activities. In one series of studies, substance-abusing pregnant and postpartum women were able to earn a livable wage by working and/or acquiring job skills related to data entry. Access to work was made contingent on providing drug-free urine samples collected three times per week. Participants who provided the drug-free urine samples and completed a 3-h work shift received a payment voucher. A 3-year outcome study demonstrated the effectiveness of the Therapeutic Workplace with pregnant and postpartum women (Silverman et al. 2002). Subsequent research suggests that long-term Therapeutic Workplace effects may dissipate following termination of the program (DeFulio & Silverman 2011). This research suggests the importance of transitioning chronic substance abusers to stable employment settings that require regular drug tests and have consequences that are proportional to use severity (e.g., not a catastrophic consequence such as termination from employment for a single positive drug test) (e.g., Kleiman 2009).
A study with opioid-dependent patients demonstrated that CM can also be effective when vouchers are used to directly reinforce engagement in substance-free activities that are consistent with treatment goals (Iguchi et al. 1997). Participants were randomly assigned to one of three treatment conditions: urinalysis-based reinforcement (UA), reinforcement that was conditional on treatment-plan success (TP), and standard care. Participants in the UA and TP conditions could earn vouchers, described as treatment-assistance coupons, redeemable for expenses linked to a treatment plan (e.g., clothing for job interviews). Participants in the UA condition earned vouchers for opioid-negative urine specimens. Vouchers were awarded to TP participants for meeting objectively defined, and clearly verifiable, treatment-plan tasks. For example, vouchers could be earned for completing job applications or training. In each case, treatment-plan tasks were individually tailored for each client, and vouchers were used to shape behavior toward long-range treatment goals. Even though only UA participants were directly reinforced for abstaining, TP participants were more than twice as likely to produce negative urine specimens, highlighting the importance of reinforcing drug-free alternatives. These results support the notion that clients who are brought into contact with drug-free reinforcers occurring naturally in their environment are likely to maintain behavioral changes after removal of the CM contingencies (Higgins et al. 2004).
Similarly, brief behavioral-activation approaches, designed to reduce depression by increasing engagement in social and goal-directed activities, are effective as a supplemental or stand-alone intervention for substance use (Daughters et al. 2010, Reynolds et al. 2010). Finally, BCT explicitly seeks to involve the couple in rewarding drug-free activities and to increase the rate of reinforcement derived from behaviors aimed at improving the couple’s day-to-day interactions (Fals-Stewart et al. 2004, 2005; Powers et al. 2008).
Direct behavioral economics enhancement of a brief motivational intervention for alcohol misuse in young adults
As noted above, BMIs target nontreatment-seeking individuals and employ motivational interviewing to enhance motivation for change. These interventions are more efficacious than no- or minimal-treatment control conditions, and their effect sizes are generally small to moderate (Lundahl et al. 2010, Miller & Rollnick 2012). However, individuals who have few alternatives to drinking or elevated demand or impulsivity may not respond to standard BMIs (Ewing et al. 2009; MacKillop & Murphy 2007; Murphy et al. 2005, 2012; Stein et al. 2011). Importantly, BMIs have recently been modified to take into account behavioral economics principles via the addition of substance-free activity sessions (SFASs) (Murphy et al. 2012). This represents a novel approach of directly importing behavioral economics concepts into treatments that come from entirely distinct perspectives.
SFASs, which target substance-free activities and temporal discounting, were initially developed for heavy-drinking college students and targets increasing engagement in academic and other constructive campus/community activities that are associated with delayed rewards and generally negatively associated with drinking (Murphy et al. 2005). A single 50-min SFAS follows a standard alcohol-focused BMI session and encourages students to identify their college and career goals. Personalized feedback and information is then provided (a) to enhance motivation to pursue the stated goals by highlighting the value of the associated delayed reward (e.g., a discussion of the students reasons for pursuing a particular major or career, feedback on the future salary implications of obtaining a college degree and the income implications of college grade-point average) and (b) to provide a specific, personally tailored roadmap on how to achieve future goals (e.g., information on specific educational and experiential requirements for gaining entry into a given profession, including grade-point-average requirements, and specific campus and local internships and organizations that provide relevant experiences). SFASs include personalized feedback on current patterns of time allocation across a variety of activity categories that may be relevant to important goals and values (e.g., time spent studying, drinking, exercising, volunteering). Thus, the overarching goal of SFASs is to increase the salience of delayed outcomes and the extent to which the behavior leading to those rewards or punishers is viewed as part of a coherent molar pattern (rather than an isolated choice) to reduce impulsive response patterns (Hofmeyr et al. 2011, Siegel & Rachlin 1995).
A randomized controlled trial indicated that a two-session (alcohol BMI + SFAS) intervention resulted in larger reductions in alcohol problems (db = 0.52, p = 0.01) (see Figure 5) relative to a two-session (alcohol BMI + relaxation) active control condition in a sample of 82 binge-drinking college students. Moderation analyses indicated that BMI + SFAS was also associated with significantly greater reductions in heavy drinking among those participants reporting at baseline low levels of substance-free reinforcement or high levels of depression. The focus on increasing goal-directed substance-free activities may have been beneficial to these participants. Importantly, the SFASs increased relevant mechanisms such as future time orientation, self-regulation, and participation in evening studying/academic activities (Murphy et al. 2012).
Figure 5.
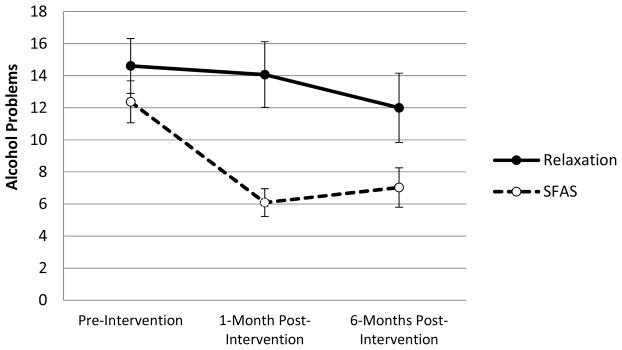
Changes in past-month number of alcohol problems from baseline to follow-up by intervention condition. Error bars reflect ±1 standard error of the mean. Abbreviation: SFAS, substance-free activity session. From Murphy JG, Dennhardt AA, Skidmore JR, Borsari B, Barnett NP, et al. 2012. A randomized controlled trial of a behavioral economic supplement to brief motivational interventions for college drinking. J. Consult. Clin. Psychol. 80:876–86. Reprinted with permission from the American Psychological Association.
Improved Impulse Control and Executive Function
As discussed above, substance use disorders are associated with decreased executive functioning and decreased impulse control. Therefore, clinical researchers have been interested in training procedures to improve executive function or impulse control as a way of treating the underlying decision-making patterns associated with impulse-control disorders including substance abuse. Various procedures have been developed to change an individual’s discount rate (for a review, see Koffarnus et al. 2013). However, most of these manipulations alter discount rate only temporarily, often only in the presence of some impermanent stimulus. Manipulations that influence human intertemporal choice behavior in a more lasting way include fading procedures, behavioral contracts, and pharmacological interventions.
Fading procedures
Fading procedures can increase an individual’s choice of larger, delayed rewards, thereby decreasing discount rate. First observed in laboratory animals (Mazur & Logue 1978), these procedures often start with a choice between two reinforcers of different sizes that are both available after the same delay. After choice of the larger reinforcer is established, delay to the smaller reinforcer is gradually decreased with an adjustment schedule that maintains choice of the larger, more delayed reinforcer. Over time, subjects learn to choose delayed reinforcers under conditions where they previously chose only the smaller-sooner option. This approach has been adapted for use in human populations, primarily with children and adults with developmental disabilities or traumatic brain injury (for a review, see Odum 2011). To date, there are no published reports assessing whether fading procedures can effectively increase self-control in substance-dependent individuals or populations vulnerable for developing substance dependence, although this may be a promising approach given the low self-control demonstrated in these groups.
Contracts including deposit contracts
In the context of substance abuse treatment, a contract typically refers to an agreement made with a counselor, friends, family, and/or oneself to avoid substance use by arranging an undesirable consequence should substance use occur. For example, the substance user may supply a relatively large up-front sum of money to be held by a third party, which is forfeited should substance use occur. Such a deposit contract is effective at controlling behaviors involving self-control including substance use and diet and exercise (Bowers et al. 1987; Dallery et al. 2008; Elliott & Tighe 1968; John et al. 2011; Lando 1977; Paxton 1980, 1981, 1983; Singh & Leung 1988; Volpp et al. 2008; Winett 1973). As discussed above, the average substance-dependent individual has an elevated discount rate, reducing the likelihood of self-controlled choices. Owing to the hyperbolic form of discounting curves, however, people with an elevated discount rate are still likely to make self-controlled choices when faced with two options that are both significantly delayed. A contract works as a precommitment strategy (Rachlin & Green 1972) by moving the decision point for engaging in substance use from the time that drugs are present and available for ingestion to a point in time days or weeks prior to ingestion. As a result, people avoid the preference reversal that often leads substance abusers to choose the immediate reward of substance use over the delayed rewards associated with abstinence. By agreeing ahead of time to forfeit a large sum of money or to experience another negative consequence after substance use, the substance user changes the consequences when drugs are available for use. Instead of the typical choice between engaging in substance use and possibly experiencing delayed negative consequences, the substance user is faced with the choice between engaging in substance use and definitely experiencing relatively immediate negative financial or other agreed-upon consequences.
Pharmacological approaches to impulse control
The drugs most commonly researched and used clinically to improve self-control are the stimulants amphetamine and methylphenidate. These drugs are primarily used to treat attention deficit hyperactivity disorder (ADHD), which is characterized by elevated discount rate (Schweitzer & Sulzer-Azaroff 1995; Solanto et al. 2001; Sonuga-Barke et al. 1992, 1996). In healthy controls, acute amphetamine decreases discount rate (de Wit et al. 2002). However, likely owing to their high abuse liability, these drugs are not typically used to treat self-control in substance abuse (Jasinski et al. 2008, Kollins et al. 2001). As an alternative, the nonstimulant drug atomoxetine is FDA approved for the treatment of ADHD and increases self-control in animal models (Robinson et al. 2008, Sun et al. 2012). Although such efficacy has not been evaluated in humans, should atomoxetine’s effect extend to this population, it has some potential as a self-control treatment because it has very low abuse liability in people with and without substance dependence (Heil et al. 2002, Jasinski et al. 2008).
Executive Function Training
Some evidence suggests that self-control and intertemporal choice behavior are associated with an individual’s executive function capacity (Bobova et al. 2009, Shamosh et al. 2008). As a result, some researchers have become interested in manipulating discount rate with interventions that affect executive functioning. Behavioral executive function training regimens have shown utility in improving the executive functioning and associated behaviors of groups with deficits in this area, such as individuals with schizophrenia (Krabbendam & Aleman 2003, Twamley et al. 2003).
Two experiments have assessed the effects of working-memory training in substance users. In one study, in-treatment stimulant-dependent adults were divided into two groups, and a computerized working-memory training regimen with known efficacy was compared with a similar control training regimen wherein participants were supplied with correct answers in the tasks to prevent them from exercising their working memory (Bickel et al. 2011c). In this study, discount rate was substantially reduced in the active group. Another study examined working-memory training in two groups of problem drinkers (Houben et al. 2011). Active working-memory training requiring recall of sequences of visual stimuli that progressively increased in length was compared with a control condition wherein static three-stimuli sequences were recalled. Compared with the control group, participants in the active condition showed increased working-memory capacity, and as a result of this intervention, they reported less alcohol use immediately after training and at a 1-month follow-up. Together, these experiments suggest that working-memory training may be an effective way of treating substance use, perhaps by modifying discount rate. However, this hypothesis remains speculative without further study.
CONCLUSIONS
The problems of substance dependence have largely remained the same. However, the scientific tools, both technological and theoretical, we use to investigate and understand this disorder change and evolve over time (Bickel et al. 2013). These new tools allow us to see dependence disorders in a different light and allow new questions to be addressed. Behavioral economics, in our view, should be properly considered a new theoretical tool, as it has provided new conceptual categories, independent variables, and dependent measures as well as an ever-growing array of data that suggests its insights are robust and fruitful. These tools were initially adapted from behavioral theory and microeconomics and applied in both animal and human laboratories. Over time, the behavioral economics of substance use disorders has been applied to more molecular analyses involving genetics as well as neuroimaging and the important molar issue of therapeutic applications to addiction. As a result, a new conceptual model of what may be an important feature underlying the phenomena of substance use disorders has developed. This model is what we refer to as reinforcer pathologies, that is, the persistent high valuation of a substance of dependence and the excessive discounting of delayed rewards. Much of the data reviewed above support the role of these processes. Substantial evidence suggests that these two factors are related to substance dependence, are predictive of treatment outcomes, and can rightfully be the target of clinical intervention.
One clear future direction is to better understand the interaction of these two components of reinforcer pathologies at any level of analysis. Presumably, a group either of adolescents at risk for substance dependence in a preventive model or of currently substance-dependent individuals entering treatment could be divided into a two-by-two matrix crossing individuals ranking high and low on measures of drug demand and temporal discounting (see Table 1). We predict, but at this time do not have data to test our hypothesis, that the lower demand and lower discounting group and higher demand and higher discounting groups would have the least and greatest likelihood of moving into substance dependence and show the greatest and least response to treatment, respectively. Such evidence would directly stimulate the development of specific treatment components that could be used to address these challenges and perhaps recognize a functional but nuanced difference among individuals with substance dependence. Such evidence and recognition may permit individuals to be matched to treatment on the basis of the functional phenotype they exhibit. For example, in a study described above, heavy-drinking young adults with low levels of substance-free reinforcement did better in a treatment that specifically addressed this deficit (Murphy et al. 2012). The development of treatments that selectively attenuate excessive valuation and discounting are still in the early stages, but progress has been made particularly in improving excessive temporal discounting.
Table 1.
Potential phenotypes of the two processes that define reinforcer pathology
| Low demand | High demand | |
|---|---|---|
|
|
||
| Limited discounting | No pathology | No pathology |
|
|
||
| Excessive discounting | No pathology | Reinforcer pathology |
|
|
||
Of equal importance is the development of behavioral economics procedures for use in treatments for substance dependence. As reviewed above, a variety of efforts have been and are being developed to integrate behavioral economics concepts into existing comprehensive treatments. These examples show that this conceptual model provides different theoretically driven options for treatment development. In particular, some of these approaches, such as emphasizing behavioral economics variables during brief motivational interviewing and the role of implicit or explicit incentives to increase the effort or the opportunity cost of continued substance use, hold promise of being developed at scale.
In sum, we present behavioral economics as a theoretical perspective that allows researchers to see substance dependence in a new light---a view of reinforcer pathologies that has pragmatic value in both understanding the disorder and approaching treatment. Furthermore, behavioral economics provides a suite of methods and tools to examine addictive behavior in systematic, precise, and novel ways. Despite considerable progress, the opportunities ahead in exploring the space made available by this approach abound and continue to have great potential in improving our understanding of addictive behavior.
Acknowledgments
The authors acknowledge the remarkable service and many contributions made by Dr. Susan Nolen-Hoeksema. This article is dedicated to her memory. This work was supported by NIH grants RO1DA024080, RO1DA030241, 1U19CA157345, R01DA034755, R01DA032363, and R21DA032717. The authors also thank Dr. George Wilson and Patsy Marshall for their assistance in the preparation of this review.
Footnotes
DISCLOSURE STATEMENT
W.K.B. is a principal in HealthSim, LLC, which specializes in the research and development of prevention science products.
Contributor Information
Warren K. Bickel, Email: wkbickel@vtc.vt.edu.
Matthew W. Johnson, Email: mwj@jhu.edu.
Mikhail N. Koffarnus, Email: mickyk@vtc.vt.edu.
James MacKillop, Email: jmackill@uga.edu.
James G. Murphy, Email: jgmurphy@memphis.edu.
LITERATURE CITED
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res. 2011;35:1607–13. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acker J, Amlung M, Stojek M, Murphy JG, MacKillop J. Individual variation in behavioral economic indices of the relative value of alcohol: incremental validity in relation to impulsivity, craving, and intellectual functioning. J Exp Psychopathol. 2012;3:423–36. [Google Scholar]
- Acker J, Mackillop J. Behavioral economic analysis of cue-elicited craving for tobacco: a virtual reality study. Nicotine Tob Res. 2013;15:1409–16. doi: 10.1093/ntr/nts341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Badger GJ, Higgins ST. An experimental examination of the initial weeks of abstinence in cigarette smokers. Exp Clin Psychopharmacol. 2004;12:276–87. doi: 10.1037/1064-1297.12.4.276. [DOI] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Process. 2003;64:345–54. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction. 2013;108:900–9. doi: 10.1111/add.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology. 1978;58:175–79. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Andrade LF, Petry NM. Delay and probability discounting in pathological gamblers with and without a history of substance use problems. Psychopharmacology. 2012;219:491–99. doi: 10.1007/s00213-011-2508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–48. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet. 2011;41:175–83. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, et al. An evaluation of μ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–44. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009a;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Rodgers K, Cuevas J, Wileyto EP. Young adult smoking: What factors differentiate ex-smokers, smoking cessation treatment seekers and nontreatment seekers? Addict Behav. 2009b;34:1036–41. doi: 10.1016/j.addbeh.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction. 2011;106:178–87. doi: 10.1111/j.1360-0443.2010.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–92. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Baum WM, Rachlin HC. Choice as time allocation. J Exp Anal Behav. 1969;12:861–74. doi: 10.1901/jeab.1969.12-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck RC, Triplett MF. Test-retest reliability of a group-administered paper-pencil measure of delay discounting. Exp Clin Psychopharmacol. 2009;17:345–55. doi: 10.1037/a0017078. [DOI] [PubMed] [Google Scholar]
- Bernstein M, Murphy JG, MacKillop J, Colby SM. Alcohol demand indices predict outcomes among heavy-drinking young adults receiving a brief intervention. Presented at Annu. Meet. Coll. Probl. Drug Depend., 75th; San Diego. 2013. [Google Scholar]
- Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, et al. Behavioral economic predictors of overweight children’s weight loss. J Consult Clin Psychol. 2012;80:1086–96. doi: 10.1037/a0029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Crean JP, Badger GJ. Buprenorphine dosing every 1, 2, or 3 days in opioid-dependent patients. Psychopharmacology. 1999a;146:111–18. doi: 10.1007/s002130051096. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Higgins ST, Badger GJ, Esch RA. Effects of adding behavioral treatment to opioid detoxification with buprenorphine. J Consult Clin Psychol. 1997;65:803–10. doi: 10.1037//0022-006x.65.5.803. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. Behavioral economics: a novel experimental approach to the study of drug dependence. Drug Alcohol Depend. 1993;33:173–92. doi: 10.1016/0376-8716(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I Functional equivalence of response requirement and drug dose. Life Sci. 1990;47:1501–10. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR, Badger GJ. Effects of simulated employment and recreation on drug taking: a behavioral economic analysis. Exp Clin Psychopharmacol. 1995;3:467–76. [Google Scholar]
- Bickel WK, Jarmolowicz DP, MacKillop J, Epstein LH, Carr K, et al. The behavioral economics of reinforcement pathologies. In: Shaffer HJ, editor. Addiction Syndrome Handbook. Washington, DC: Am. Psychol. Assoc; 2012a. [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Franck CT, Carrin C, Gatchalian KM. Altruism in time: social temporal discounting differentiates smokers from problem drinkers. Psychopharmacology. 2012b;224:109–20. doi: 10.1007/s00213-012-2745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM. The behavioral economics and neuroeconomics of reinforcer pathologies: implications for etiology and treatment of addiction. Curr Psychiatry Rep. 2011a;13:406–15. doi: 10.1007/s11920-011-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Landes RD, Christensen DR, Jackson L, Jones BA, et al. Single- and cross-commodity discounting among cocaine addicts: the commodity and its temporal location determine discounting rate. Psychopharmacology. 2011b;217:177–87. doi: 10.1007/s00213-011-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Combined behavioral and pharmacological treatment during opioid detoxification. a review. In: Higgins ST, Silverman K, editors. Motivation Behavior Change Among Illicit-Drug Abusers: Contemporary Research on Contingency-Management Interventions. Washington, DC: Am. Psychol. Assoc; 1999. pp. 265–79. [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Mueller ET, Jarmolowicz DP. What is addiction? In: McCrady B, Epstein EE, editors. Addictions: A Comprehensive Guidebook. 2 New York: Oxford Univ. Press; 2013. pp. 3–16. [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999b;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJC. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 2009;29:8839–46. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the Future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011c;69:260–65. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol Sci. 2010;31:46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2 like traits. Alcohol. 2004;34:133–50. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158 (Val/Val) genotype. J Neurosci. 2007;27:14383–91. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers TG, Winett RA, Frederiksen LW. Nicotine fading, behavioral contracting, and extended treatment: effects on smoking cessation. Addict Behav. 1987;12:181–84. doi: 10.1016/0306-4603(87)90024-4. [DOI] [PubMed] [Google Scholar]
- Bradstreet MP, Higgins ST, Heil SH, Badger GJ, Skelly JM, et al. Social discounting and cigarette smoking during pregnancy. J Behav Decis Mak. 2012;25:502–11. doi: 10.1002/bdm.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, MacKillop J, Ray LA. A behavioral economic approach to understanding alcoholism pharmacotherapy mechanisms: evidence for naltrexone in Asian Americans. Exp Clin Psychopharmacol. 2012;20:181–90. doi: 10.1037/a0027379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Carr KA, Daniel TO, Lin H, Epstein LH. Reinforcement pathology and obesity. Curr Drug Abuse Rev. 2011;4:190–96. doi: 10.2174/1874473711104030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction. 2012;107:1650–59. doi: 10.1111/j.1360-0443.2012.03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–97. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(Suppl 1):S70–78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Chaloupka FJ, Yurekli A, Fong GT. Tobacco taxes as a tobacco control strategy. Tob Control. 2012;21:172–80. doi: 10.1136/tobaccocontrol-2011-050417. [DOI] [PubMed] [Google Scholar]
- Chase HW, Mackillop J, Hogarth L. Isolating behavioural economic indices of demand in relation to nicotine dependence. Psychopharmacology. 2013;226:371–80. doi: 10.1007/s00213-012-2911-x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Lu Y, Han X, Gonzalez-Vallejo C, Sui N. Temporal discounting in heroin-dependent patients: no sign effect, weaker magnitude effect, and the relationship with inhibitory control. Exp Clin Psychopharmacol. 2012;20:400–9. doi: 10.1037/a0029657. [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Roma PG, Riley AL. Demand for cocaine and food over time. Pharmacol Biochem Behav. 2008;91:209–16. doi: 10.1016/j.pbb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Herrnstein RJ. Choice and delay of reinforcement. J Exp Anal Behav. 1967;10:67–74. doi: 10.1901/jeab.1967.10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus E, Kiehl KA, Hutchison K. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin Exp Res. 2011;35:1209–19. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Correia CJ, Benson TA, Carey KB. Decreased substance use following increases in alternative behaviors: a preliminary investigation. Addict Behav. 2005;30:19–27. doi: 10.1016/j.addbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Correia CJ, Carey KB, Simons J, Borsari BE. Relationships between binge drinking and substance-free reinforcement in a sample of college students: a preliminary investigation. Addict Behav. 2003;28:361–68. doi: 10.1016/s0306-4603(01)00229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia CJ, Simons J, Carey KB, Borsari BE. Predicting drug use: application of behavioral theories of choice. Addict Behav. 1998;23:705–9. [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Effects of a non-drug reinforcer, saccharin, on oral self-administration of phencyclidine in male and female rhesus monkeys. Psychopharmacology. 2003;170:9–16. doi: 10.1007/s00213-003-1487-x. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–71. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Dallery J, Meredith S, Glenn IM. A deposit contract method to deliver abstinence reinforcement for cigarette smoking. J Appl Behav Anal. 2008;41:609–15. doi: 10.1901/jaba.2008.41-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Magidson JF, Schuster RM, Safren SA. ACT HEALTHY: a combined cognitive-behavioral depression and medication adherence treatment for HIV-infected substance users. Cogn Behav Pract. 2010;17:309–21. doi: 10.1016/j.cbpra.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–25. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- DeFulio A, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: post-intervention outcomes. Addiction. 2011;106:960–67. doi: 10.1111/j.1360-0443.2011.03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK. Drug dependence as consumer demand. In: Green L, Kagel JH, editors. Advances in Behavioral Economics. Vol. 3. Westport, CT: Ablex; 1996. pp. 1–35. [Google Scholar]
- DeGrandpre RJ, Bickel WK, Hughes JR, Layng MP, Badger G. Unit price as a useful metric in analyzing effects of reinforcer magnitude. J Exp Anal Behav. 1993;60:641–66. doi: 10.1901/jeab.1993.60-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Godley SH, Liddle HA, Sampl S, Webb C, et al. Five outpatient treatment models for adolescent marijuana use: a description of the cannabis youth treatment interventions. Addiction. 2002;97(Suppl 1):70–83. doi: 10.1046/j.1360-0443.97.s01.3.x. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer AN, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addict Biol. 2012;17:576–87. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Diller JW, Patros CH, Prentice PR. Temporal discounting and heart rate reactivity to stress. Behav Process. 2011;87:306–9. doi: 10.1016/j.beproc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, MacKillop J, Modi M, Beauchemin J, Dang D, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Tighe T. Breaking the cigarette habit: effects of a technique involving threatened loss of money. Psychol Rec. 1968;18:503–13. [Google Scholar]
- Epstein LH, Carr KA, Lin H, Fletcher KD. Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr. 2011;94:12–18. doi: 10.3945/ajcn.110.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Temple JL, Cavanaugh MD. Food reinforcement and impulsivity in overweight children and their parents. Eat Behav. 2008;9:319–27. doi: 10.1016/j.eatbeh.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Richards JB, Saad FG, Paluch RA, Roemmich JN, Lerman C. Comparison between two measures of delay discounting in smokers. Exp Clin Psychopharmacol. 2003;11:131–38. doi: 10.1037/1064-1297.11.2.131. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121:877–86. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy J, Hawk LW, Jr, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol Behav. 2004;81:511–17. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Klostermann K, Yates BT, O’Farrell TJ, Birchler GR. Brief relationship therapy for alcoholism: a randomized clinical trial examining clinical efficacy and cost-effectiveness. Psychol Addict Behav. 2005;19:363–71. doi: 10.1037/0893-164X.19.4.363. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Birchler GR. Behavioral couples therapy for substance abuse: rationale, methods, and findings. Sci Pract Perspect. 2004;2:30–41. doi: 10.1151/spp042230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, LaChance HA, Bryan A, Hutchison KE. Do genetic and individual risk factors moderate the efficacy of motivational enhancement therapy? Drinking outcomes with an emerging adult sample. Addict Biol. 2009;14:356–65. doi: 10.1111/j.1369-1600.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Few LR, Acker J, Murphy C, MacKillop J. Temporal stability of a cigarette purchase task. Nicotine Tob Res. 2012;14:761–65. doi: 10.1093/ntr/ntr222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology. 2006;186:255–63. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fields S, Leraas K, Collins C, Reynolds B. Delay discounting as a mediator of the relationship between perceived stress and cigarette smoking status in adolescents. Behav Pharmacol. 2009;20:455–60. doi: 10.1097/FBP.0b013e328330dcff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, O’Donoghue T. Time discounting and time preference: a critical review. J Econ Lit. 2002;40:351–401. [Google Scholar]
- Gentile ND, Librizzi EH, Martinetti MP. Academic constraints on alcohol consumption in college students: a behavioral economic analysis. Exp Clin Psychopharmacol. 2012;20:390–99. doi: 10.1037/a0029665. [DOI] [PubMed] [Google Scholar]
- Giordano L, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–82. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gray JA, MacKillop J. Interrelationships among individual differences in alcohol demand, impulsivity, and alcohol misuse. Psychol Addict Behav. 2013 doi: 10.1037/a0032766. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fristoe N, Myerson J. Temporal discounting and preference reversals in choice between delayed outcomes. Psychon Bull Rev. 1994;1:383–89. doi: 10.3758/BF03213979. [DOI] [PubMed] [Google Scholar]
- Hadaway PF, Alexander BK, Coambs RB, Beyerstein B. The effect of housing and gender on preference for morphine-sucrose solutions in rats. Psychopharmacology. 1979;66:87–91. doi: 10.1007/BF00431995. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Lash SJ, Roll JM. Contingency management in substance abuse treatment: a structured review of the evidence for its transportability. Drug Alcohol Depend. 2012;122:1–10. doi: 10.1016/j.drugalcdep.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend. 2002;67:149–56. doi: 10.1016/s0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–94. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. J Exp Anal Behav. 1961;4:267–72. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. On the law of effect. J Exp Anal Behav. 1970;13:243–66. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ, Prelec D. A theory of addiction. In: Loewenstein G, Elster J, editors. Choice Over Time. New York: Russell Sage Found; 1992. pp. 331–60. [Google Scholar]
- Heyman GM, Gibb SP. Delay discounting in college cigarette chippers. Behav Pharmacol. 2006;17:669–79. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–87. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger G. Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry. 1993;150:763–69. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–24. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6:1015–20. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–70. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201:183–93. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyr A, Ainslie G, Charlton R, Ross D. The relationship between addiction and reward bundling: an experiment comparing smokers and non-smokers. Addiction. 2011;106:402–9. doi: 10.1111/j.1360-0443.2010.03166.x. [DOI] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J. Is discounting impulsive? Evidence from temporal and probability discounting in gambling and non-gambling college students. Behav Process. 2003;64:355–67. doi: 10.1016/s0376-6357(03)00141-4. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW, Jansen A. Getting a grip on drinking behavior: training working memory to reduce alcohol abuse. Psychol Sci. 2011;22:968–75. doi: 10.1177/0956797611412392. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Economic concepts for the analysis of behavior. J Exp Anal Behav. 1980;34:219–38. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics. J Exp Anal Behav. 1984;42:435–52. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Behav. 2013;99:98–124. doi: 10.1002/jeab.7. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–84. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Krebs CA, Anderson KG. Strain differences in delay discounting between Lewis and Fischer 344 rats at baseline and following acute and chronic administration of d-amphetamine. Pharmacol Biochem Behav. 2012;101:403–16. doi: 10.1016/j.pbb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi MY, Belding MA, Morral AR, Lamb RJ, Husband SD. Reinforcing operants other than abstinence in drug abuse treatment: an effective alternative for reducing drug use. J Consult Clin Psychol. 1997;65:421–28. doi: 10.1037//0022-006x.65.3.421. [DOI] [PubMed] [Google Scholar]
- Jacobs SB, Wagner MK. Obese and nonobese individuals: behavioral and personality characteristics. Addict Behav. 1984;9:223–26. doi: 10.1016/0306-4603(84)90062-5. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Faries DE, Moore RJ, Schuh LM, Allen AJ. Abuse liability assessment of atomoxetine in a drug-abusing population. Drug Alcohol Depend. 2008;95:140–46. doi: 10.1016/j.drugalcdep.2008.01.008. [DOI] [PubMed] [Google Scholar]
- John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med. 2011;26:621–26. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–46. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Exp Clin Psychopharmacol. 2007;15:187–94. doi: 10.1037/1064-1297.15.2.187. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Exp Clin Psychopharmacol. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bruner NR. The sexual discounting task: HIV risk behavior and the discounting of delayed sexual rewards in cocaine dependence. Drug Alcohol Depend. 2012;123:15–21. doi: 10.1016/j.drugalcdep.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WG. Effect of cue prominence and subject weight on human food-directed performance. J Personal Soc Psychol. 1974;29:843–48. doi: 10.1037/h0036390. [DOI] [PubMed] [Google Scholar]
- Jones B, Rachlin H. Social discounting. Psychol Sci. 2006;17:283–86. doi: 10.1111/j.1467-9280.2006.01699.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, et al. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nat Neurosci. 2012;15:181–89. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction. 2013;108:506–15. doi: 10.1111/add.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, et al. A micro opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology. 2009;201:611–18. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kleiman MAR. When Brute Force Fails: How To Have Less Crime and Less Punishment. Princeton, NJ: Princeton Univ. Press; 2009. [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 2013;99:32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Wong CJ, Diemer K, Needham M, Hampton J, et al. A randomized clinical trial of a Therapeutic Workplace for chronically unemployed, homeless, alcohol-dependent adults. Alcohol Alcohol. 2011;46:561–69. doi: 10.1093/alcalc/agr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH. Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addict Biol. 2013;18:8–18. doi: 10.1111/j.1369-1600.2011.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68:611–27. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Komro KA, Perry CL, Williams CL, Stigler MH, Farbakhsh K, Veblen-Mortenson S. How did Project Northland reduce alcohol use among young adolescents? Analysis of mediating variables. Health Educ Res. 2001;16:59–70. doi: 10.1093/her/16.1.59. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kowal BP, Yi R, Erisman AC, Bickel WK. A comparison of two algorithms in computerized temporal discounting procedures. Behav Process. 2007;75:231–36. doi: 10.1016/j.beproc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Aleman A. Cognitive rehabilitation in schizophrenia: a quantitative analysis of controlled studies. Psychopharmacology. 2003;169:376–82. doi: 10.1007/s00213-002-1326-5. [DOI] [PubMed] [Google Scholar]
- Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behav Process. 2005;69:173. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lando HA. Successful treatment of smokers with a broad-spectrum behavioral approach. J Consult Clin Psychol. 1977;45:361–66. doi: 10.1037//0022-006x.45.3.361. [DOI] [PubMed] [Google Scholar]
- Lawyer SR, Williams SA, Prihodova T, Rollins JD, Lester AC. Probability and delay discounting of hypothetical sexual outcomes. Behav Process. 2010;84:687–92. doi: 10.1016/j.beproc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Liao W, Luo X, Le CT, Chu H, Epstein LH, et al. Analysis of cigarette purchase task instrument data with a left-censored mixed effects model. Exp Clin Psychopharmacol. 2013;21:124–32. doi: 10.1037/a0031610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, et al. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatry. 2009;66:205–12. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Glatt SJ, Wall TL. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull. 2006;132:607–21. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of motivational interviewing: twenty-five years of empirical studies. Res Soc Work Pract. 2010;20:137–60. [Google Scholar]
- MacKillop J. Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J Exp Anal Behav. 2013;99:14–31. doi: 10.1002/jeab.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216:305–21. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Wier LM, David SP, Ray LA, et al. The neuroeconomics of nicotine dependence: a preliminary functional magnetic resonance imaging study of delay discounting of monetary and cigarette rewards in smokers. Psychiatry Res. 2012a;202:20–29. doi: 10.1016/j.pscychresns.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Brown CL, Stojek MK, Murphy CM, Sweet L, Niaura RS. Behavioral economic analysis of withdrawal- and cue-elicited craving for tobacco: an initial investigation. Nicotine Tob Res. 2012b;14:1426–34. doi: 10.1093/ntr/nts006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Monti PM, et al. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Abnorm Psychol. 2010a;119:106–14. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG. A behavioral economic measure of demand for alcohol predicts brief intervention outcomes. Drug Alcohol Depend. 2007;89:227–33. doi: 10.1016/j.drugalcdep.2007.01.002. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG, Ray LA, Eisenberg DTA, Lisman SA, et al. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol. 2008;16:57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- MacKillop J, O’Hagen S, Lisman SA, Murphy JG, Ray LA, et al. Behavioral economic analysis of cue-elicited craving for alcohol. Addiction. 2010b;105:1599–607. doi: 10.1111/j.1360-0443.2010.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Obasi E, Amlung MT, McGeary JE, Knopik VS. The role of genetics in nicotine dependence: mapping the pathways from genome to syndrome. Curr Cardiovasc Risk Rep. 2010c;4:446–53. doi: 10.1007/s12170-010-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology. 2011;216:91–99. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol. 2003;11:139–45. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK, editors. Impulsivity: The Behavioral and Neurological Science of Discounting. Washington, DC: Am. Psychol. Assoc; 2009. [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–62. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Raiff BR, Laforio CH, Begotka AM, Mueller AM, et al. Delay discounting of potentially real and hypothetical rewards: Il. Between- and within-subject comparisons. Exp Clin Psychopharmacol. 2004;12:251–56. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–54. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Behavior. Hillsdale NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mazur JE, Logue AW. Choice in a “self-control” paradigm: effects of a fading procedure. J Exp Anal Behav. 1978;30:11–17. doi: 10.1901/jeab.1978.30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine Tob Res. 2013;15:139–48. doi: 10.1093/ntr/nts101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, et al. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci. 2010;124:470–77. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Meeting in the middle: motivational interviewing and self-determination theory. Int J Behav Nutr Phys Act. 2012;9:25. doi: 10.1186/1479-5868-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Ebbesen EB, Zeiss AR. Cognitive and attentional mechanisms in delay of gratification. J Pers Soc Psychol. 1972;21:204–18. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–38. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–69. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and nonsmokers. Psychopharmacology. 1999;146:455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Effects of short-term nicotine deprivation on decision-making: delay, uncertainty, and effort discounting. Nicotine Tob Res. 2004;6:819–28. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–93. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, Correia CJ, Colby SM, Vuchinich RE. Using behavioral theories of choice to predict drinking outcomes following a brief intervention. Exp Clin Psychopharmacol. 2005;13:93–101. doi: 10.1037/1064-1297.13.2.93. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Dennhardt AA, Skidmore JR, Borsari B, Barnett NP, et al. A randomized controlled trial of a behavioral economic supplement to brief motivational interventions for college drinking. J Consult Clin Psychol. 2012;80:876–86. doi: 10.1037/a0028763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J. Relative reinforcing efficacy of alcohol among college student drinkers. Exp Clin Psychopharmacol. 2006;14:219–27. doi: 10.1037/1064-1297.14.2.219. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA. Reliability and validity of a demand curve measure of alcohol reinforcement. Exp Clin Psychopharmacol. 2009;17:396–404. doi: 10.1037/a0017684. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Tidey JW, Brazil LA, Colby SM. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend. 2011;113:207–14. doi: 10.1016/j.drugalcdep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, Yurasek AM, Dennhardt AA, Skidmore JR, McDevitt-Murphy ME, et al. Symptoms of depression and PTSD are associated with elevated alcohol demand. Drug Alcohol Depend. 2013;127:129–36. doi: 10.1016/j.drugalcdep.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology. 1991;105:169–74. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: trait variable? Behav Process. 2011;87:1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Badger GJ, Bickel WK. Needle sharing in opioid-dependent outpatients: psychological processes underlying risk. Drug Alcohol Depend. 2000;60:259–66. doi: 10.1016/s0376-8716(00)00111-3. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology. 2005;182:508–15. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N, Wehr P. Three-month stability of delay and probability discounting measures. Exp Clin Psychopharmacol. 2006;14:318–28. doi: 10.1037/1064-1297.14.3.318. [DOI] [PubMed] [Google Scholar]
- Olmstead TA, Cohen JP, Petry NM. Health-care service utilization in substance abusers receiving contingency management and standard care treatments. Addiction. 2012;107:1462–70. doi: 10.1111/j.1360-0443.2012.03831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead TA, Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine- or opioid-dependent outpatients. Drug Alcohol Depend. 2009;102:108–15. doi: 10.1016/j.drugalcdep.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, et al. A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–26. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton R. The effects of a deposit contract as a component in a behavioural programme for stopping smoking. Behav Res Ther. 1980;18:45–50. doi: 10.1016/0005-7967(80)90068-6. [DOI] [PubMed] [Google Scholar]
- Paxton R. Deposit contracts with smokers: varying frequency and amount of repayments. Behav Res Ther. 1981;19:117–23. doi: 10.1016/0005-7967(81)90035-8. [DOI] [PubMed] [Google Scholar]
- Paxton R. Prolonging the effects of deposit contracts with smokers. Behav Res Ther. 1983;21:425–33. doi: 10.1016/0005-7967(83)90012-8. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86:822–37. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol. 2007;75:983–91. doi: 10.1037/0022-006X.75.6.983. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Rash CJ. Contingency management treatments decrease psychiatric symptoms. J Consult Clin Psychol. 2013;81:926–31. doi: 10.1037/a0032499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–57. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Powers MB, Vedel E, Emmelkamp PM. Behavioral couples therapy (BCT) for alcohol and drug use disorders: a meta-analysis. Clin Psychol Rev. 2008;28:952–62. doi: 10.1016/j.cpr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Rachlin H. Four teleological theories of addiction. Psychon Bull Rev. 1997;4:462–73. [Google Scholar]
- Rachlin H. Notes on discounting. J Exp Anal Behav. 2006;85:425–35. doi: 10.1901/jeab.2006.85-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice, and self-control. J Exp Anal Behav. 1972;17:15–72. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–44. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9:13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the μ-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–95. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–77. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Reynolds B. Do high rates of cigarette consumption increase delay discounting? A cross-sectional comparison of adolescent smokers and young-adult smokers and nonsmokers. Behav Process. 2004;67:545–49. doi: 10.1016/j.beproc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006a;17:651–67. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B. The Experiential Discounting Task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behav Pharmacol. 2006b;17:133–42. doi: 10.1097/01.fbp.0000190684.77360.c0. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Karraker K, Horn K, Richards JB. Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behav Process. 2003;64:333–44. doi: 10.1016/s0376-6357(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Process. 2004;30:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Reynolds EK, Magidson JF, Bornovalova MA, Gwadz M, Ewart CK, et al. Application of the social action theory to understand factors associated with risky sexual behavior among individuals in residential substance abuse treatment. Psychol Addict Behav. 2010;24:311–21. doi: 10.1037/a0018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, et al. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–37. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci. 2007;27:245–50. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RE, Higgins ST, Silverman K, Thomas CS, Badger GJ, et al. Abstinence-contingent reinforcement and engagement in non-drug-related activities among illicit drug abusers. Psychol Addict Behav. 2008;22:544–50. doi: 10.1037/0893-164X.22.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–25. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen HG, Kerkhof AJ, van den Brink W. Experiences with an outpatient relapse program (community reinforcement approach) combined with naltrexone in the treatment of opioid-dependence: effect on addictive behaviors and the predictive value of psychiatric comorbidity. Eur Addict Res. 2003;9:53–58. doi: 10.1159/000068808. [DOI] [PubMed] [Google Scholar]
- Roozen HG, Van Beers SE, Weevers HJ, Breteler MH, Willemsen MC, et al. Effects on smoking cessation: naltrexone combined with a cognitive behavioral treatment based on the community reinforcement approach. Subst Use Misuse. 2006;41:45–60. doi: 10.1080/10826080500318665. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Samuelson P. A note on measurement of utility. Rev Econ Stud. 1937;4:155–61. [Google Scholar]
- Schweitzer JB, Sulzer-Azaroff B. Self-control in boys with attention deficit hyperactivity disorder: effects of added stimulation and time. J Child Psychol Psychiatry. 1995;36:671–86. doi: 10.1111/j.1469-7610.1995.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19:904–11. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Sheffer C, MacKillop J, McGeary J, Landes RD, Carter L, et al. Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. Am J Addict. 2012;21:221–32. doi: 10.1111/j.1521-0391.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E, Rachlin H. Soft commitment: self-control achieved by response persistence. J Exp Anal Behav. 1995;64:117–28. doi: 10.1901/jeab.1995.64-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID. Sustained cocaine abstinence in methadone maintenance patients through voucher based reinforcement therapy. Arch Gen Psychiatry. 1996;53:409–15. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Robles E, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: six-month abstinence outcomes. Exp Clin Psychopharmacol. 2001;9:14–23. doi: 10.1037/1064-1297.9.1.14. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Wong CJ, Hampton J, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: three-year abstinence outcomes. Exp Clin Psychopharmacol. 2002;10:228–40. doi: 10.1037//1064-1297.10.3.228. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–49. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindelar J, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction. 2007;102:309–16. doi: 10.1111/j.1360-0443.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Singh NN, Leung JP. Smoking cessation through cigarette-fading, self-recording, and contracting: treatment, maintenance and long-term followup. Addict Behav. 1988;13:101–5. doi: 10.1016/0306-4603(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Skidmore JR, Murphy JG. The effect of drink price and next-day responsibilities on college student drinking: a behavioral economic analysis. Psychol Addict Behav. 2011;25:57–68. doi: 10.1037/a0021118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Martens MP, Murphy JG, Buscemi J, Yurasek AM, Skidmore J. Reinforcing efficacy moderates the relationship between impulsivity-related traits and alcohol use. Exp Clin Psychopharmacol. 2010;18:521–29. doi: 10.1037/a0021585. [DOI] [PubMed] [Google Scholar]
- Smith JE, Meyers RJ, Delaney HD. The community reinforcement approach with homeless alcohol-dependent individuals. J Consult Clin Psychol. 1998;66:541–48. doi: 10.1037//0022-006x.66.3.541. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–28. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion: I. The effect of delay on choice. J Child Psychol Psychiatry Allied Discipl. 1992;33:387–98. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Williams E, Hall M, Saxton T. Hyperactivity and delay aversion. III: The effect on cognitive style of imposing delay after errors. J Child Psychol Psychiatry Allied Discipl. 1996;37:189–94. doi: 10.1111/j.1469-7610.1996.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, et al. Delay discounting predicts adolescent substance abuse treatment outcome. Exp Clin Psychopharmacol. 2012;20:205–12. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LA, Lebeau R, Colby SM, Barnett NP, Golembeske C, Monti PM. Motivational interviewing for incarcerated adolescents: effects of depressive symptoms on reducing alcohol and marijuana use after release. J Stud Alcohol Drugs. 2011;72:497–506. doi: 10.15288/jsad.2011.72.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Petry N, Peirce J, Kirby K, Killeen T, et al. Effectiveness of abstinence-based incentives: interaction with intake stimulant test results. J Consult Clin Psychol. 2007;75:805–11. doi: 10.1037/0022-006X.75.5.805. [DOI] [PubMed] [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: association with the Fagerström Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine Tob Res. 2008;10:1571–75. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Furukawa A, Miyakawa T, Maesato H, Higuchi S. Two-month stability of hyperbolic discount rates for delayed monetary gains in abstinent inpatient alcoholics. Neuroendocrinol Lett. 2007;28:131–36. [PubMed] [Google Scholar]
- Takahashi T, Ohmura Y, Oono H, Radford M. Alcohol use and discounting of delayed and probabilistic gain and loss. Neuroendocrinol Lett. 2009;30:749–52. [PubMed] [Google Scholar]
- Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008;87:1121–27. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler R. Some empirical evidence on dynamic inconsistency. Econ Lett. 1981;8:201–7. [Google Scholar]
- Torregrossa MM, Xie M, Taylor JR. Chronic corticosterone exposure during adolescence reduces impulsive action but increases impulsive choice and sensitivity to yohimbine in male Sprague-Dawley rats. Neuropsychopharmacology. 2012;37:1656–70. doi: 10.1038/npp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Foushee HR, Black BC. Behavioral economic analysis of natural resolution of drinking problems using IVR self-monitoring. Exp Clin Psychopharmacol. 2008;16:332–40. doi: 10.1037/a0012834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Black BC, Rippens PD. Significance of a behavioral economic index of reward value in predicting drinking problem resolution. J Consult Clin Psychol. 2006;74:317–26. doi: 10.1037/0022-006X.74.2.317. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Rippens PD. Predicting natural resolution of alcohol-related problems: a prospective behavioral economic analysis. Exp Clin Psychopharmacol. 2002;10:248–57. doi: 10.1037//1064-1297.10.3.248. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophr Bull. 2003;29:359–82. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Higgins ST, Budney AJ, Badger GJ. Comparison of the frequency and enjoyability of pleasant events in cocaine abusers versus non-abusers using a standardized behavioral inventory. Addiction. 1998;93:1669–80. doi: 10.1046/j.1360-0443.1998.931116695.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–36. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–68. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300:2631–37. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuchinich RE. Alcohol abuse as molar choice: an update of a 1982 proposal. Psychol Addict Behav. 1995;9:223–35. [Google Scholar]
- Vuchinich RE, Heather BN, editors. Choice, Behavioral Economics, and Addiction. Oxford, UK: Elsevier; 2003. [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Tucker JA. Contributions from behavioral theories of choice to an analysis of alcohol abuse. J Abnorm Psychol. 1988;97:181–95. doi: 10.1037//0021-843x.97.2.181. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Tucker JA. Alcoholic relapse, life events, and behavioral theories of choice: A prospective analysis. Exp Clin Psychopharmacol. 1996;4:19–28. [Google Scholar]
- Wade-Galuska T, Galuska CM, Winger G. Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. J Exp Anal Behav. 2011;95:75–89. doi: 10.1901/jeab.2011.95-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar AC, Tobler AL, Komro KA. Effects of alcohol tax and price policies on morbidity and mortality: a systematic review. Am J Public Health. 2010;100:2270–78. doi: 10.2105/AJPH.2009.186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom LC, McChargue DE, Mackillop J. DRD2/ANKK1 TaqI A genotype moderates the relationship between alexithymia and the relative value of alcohol among male college binge drinkers. Pharmacol Biochem Behav. 2012;102:471–76. doi: 10.1016/j.pbb.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Washio Y, Higgins ST, Heil SH, McKerchar TL, Badger GJ, et al. Delay discounting is associated with treatment response among cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2011;19:243–48. doi: 10.1037/a0023617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ER, Kawachi I. Giving means receiving: the protective effect of social capital on binge drinking on college campuses. Am J Public Health. 2000;90:1936–39. doi: 10.2105/ajph.90.12.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MJ, Morris CP, Lawford BR, Young RM. Behavioral phenotypes of impulsivity related to the ANKK1 gene are independent of an acute stressor. Behav Brain Funct. 2008;4:54. doi: 10.1186/1744-9081-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winett RA. Parameters of deposit contracts in the modification of smoking. Psychol Rec. 1973;23:49–60. [Google Scholar]
- Winger G, Galuska CM, Hursh SR, Woods JH. Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. J Pharmacol Exp Ther. 2006;318:223–29. doi: 10.1124/jpet.105.100461. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–95. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankelevitz RL, Mitchell SH, Zhang Y. Gender differences in factors associated with alcohol drinking: delay discounting and perception of others’ drinking. Drug Alcohol Depend. 2012;123:273–76. doi: 10.1016/j.drugalcdep.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Carter AE, Landes RD. Restricted psychological horizon in active methamphetamine users: future, past, probability, and social discounting. Behav Pharmacol. 2012;23:358–66. doi: 10.1097/FBP.0b013e3283564e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Charlton S, Porter C, Carter AE, Bickel WK. Future altruism: social discounting of delayed rewards. Behav Process. 2011a;86:160–63. doi: 10.1016/j.beproc.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Chase WD, Bickel WK. Probability discounting among cigarette smokers and nonsmokers: molecular analysis discerns group differences. Behav Pharmacol. 2007;18:633–39. doi: 10.1097/FBP.0b013e3282effbd3. [DOI] [PubMed] [Google Scholar]
- Yi R, de la Piedad X, Bickel WK. The combined effects of delay and probability in discounting. Behav Process. 2006;73:149–55. doi: 10.1016/j.beproc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Yi R, Johnson MW, Giordano LA, Landes RD, Badger GJ, Bickel WK. The effects of reduced cigarette smoking on discounting future rewards: an initial evaluation. Psychol Rec. 2008;58:163–74. doi: 10.1007/bf03395609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, King LF, Carter AE, Landes RD, Bickel WK. Intertemporal decision-making for a group. Psychol Rec. 2011b;60:3. doi: 10.1007/bf03395733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Landes RD. Temporal and probability discounting by cigarette smokers following acute smoking abstinence. Nicotine Tob Res. 2012;14:547–58. doi: 10.1093/ntr/ntr252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Landes RD, Bickel WK. Novel models of intertemporal valuation: past and future outcomes. J Neurosci Psychol Econ. 2009;2:102–11. doi: 10.1037/a0017571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Bradstreet MP, Badger GJ, Thomas CS. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology. 2009;205:305–18. doi: 10.1007/s00213-009-1541-4. [DOI] [PubMed] [Google Scholar]