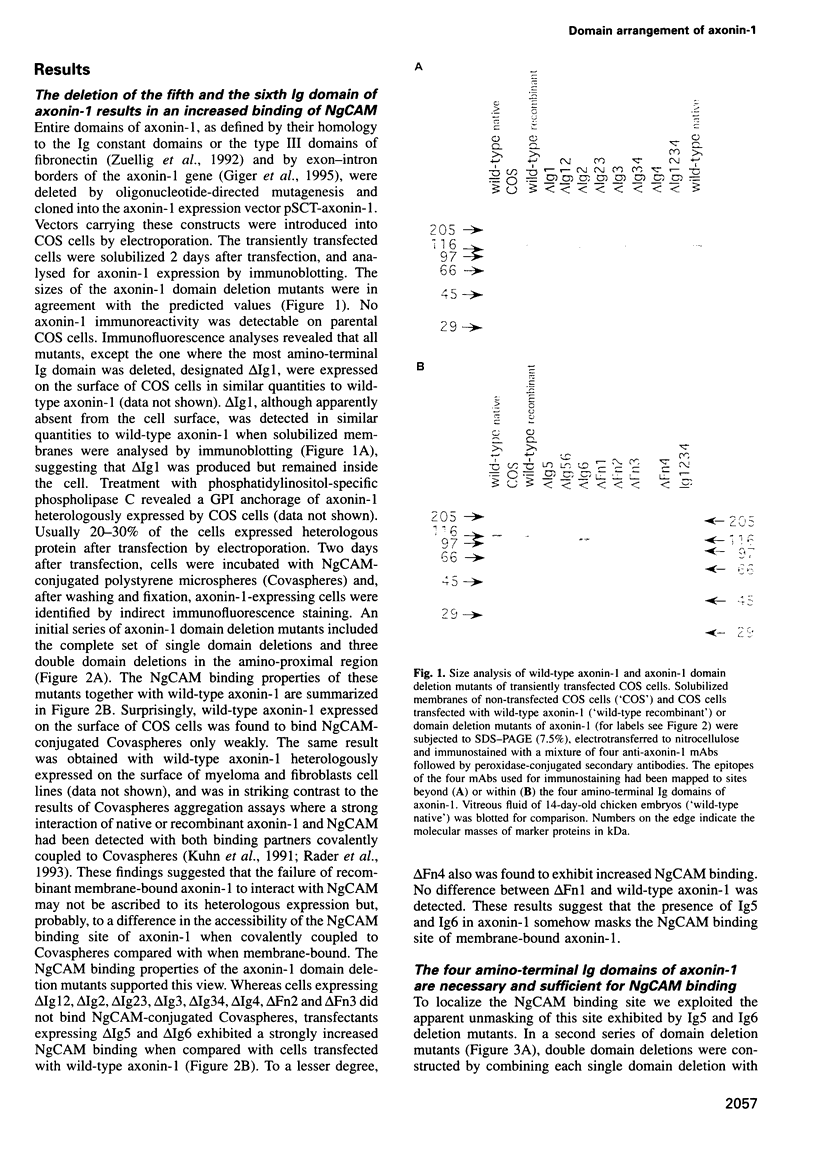
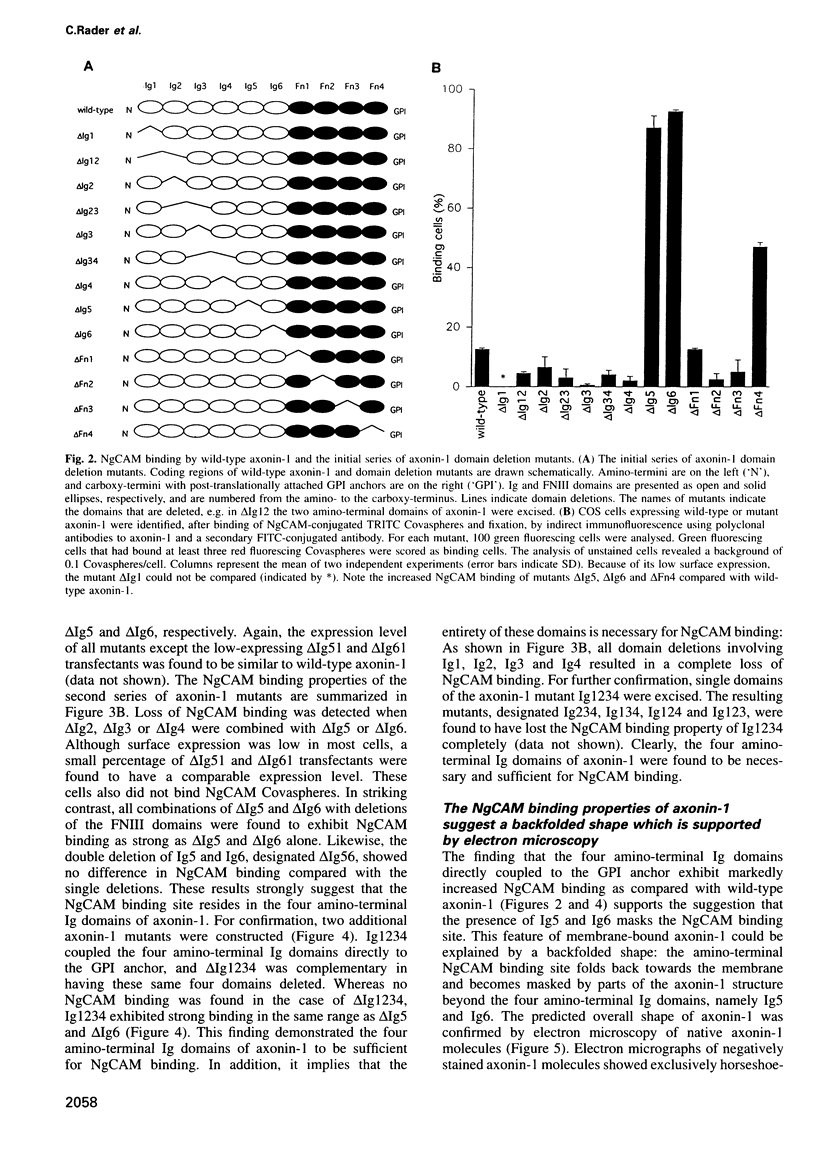
Abstract
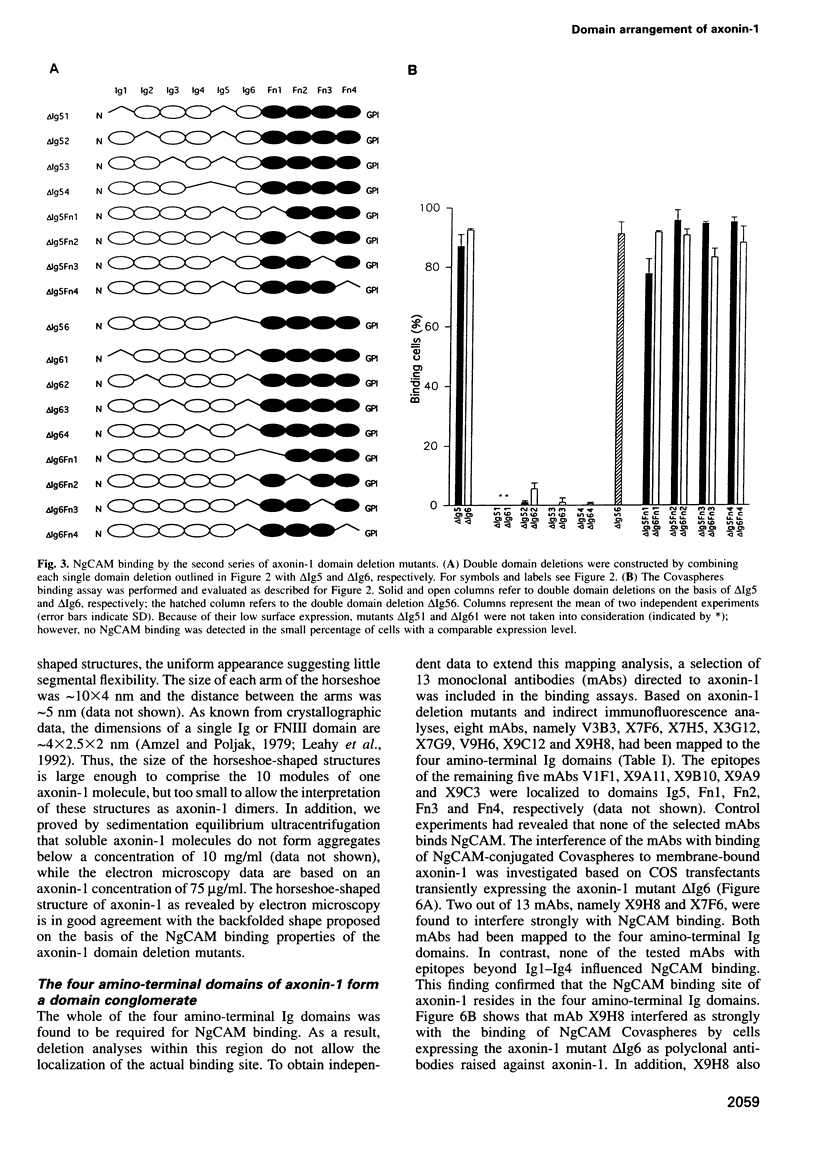
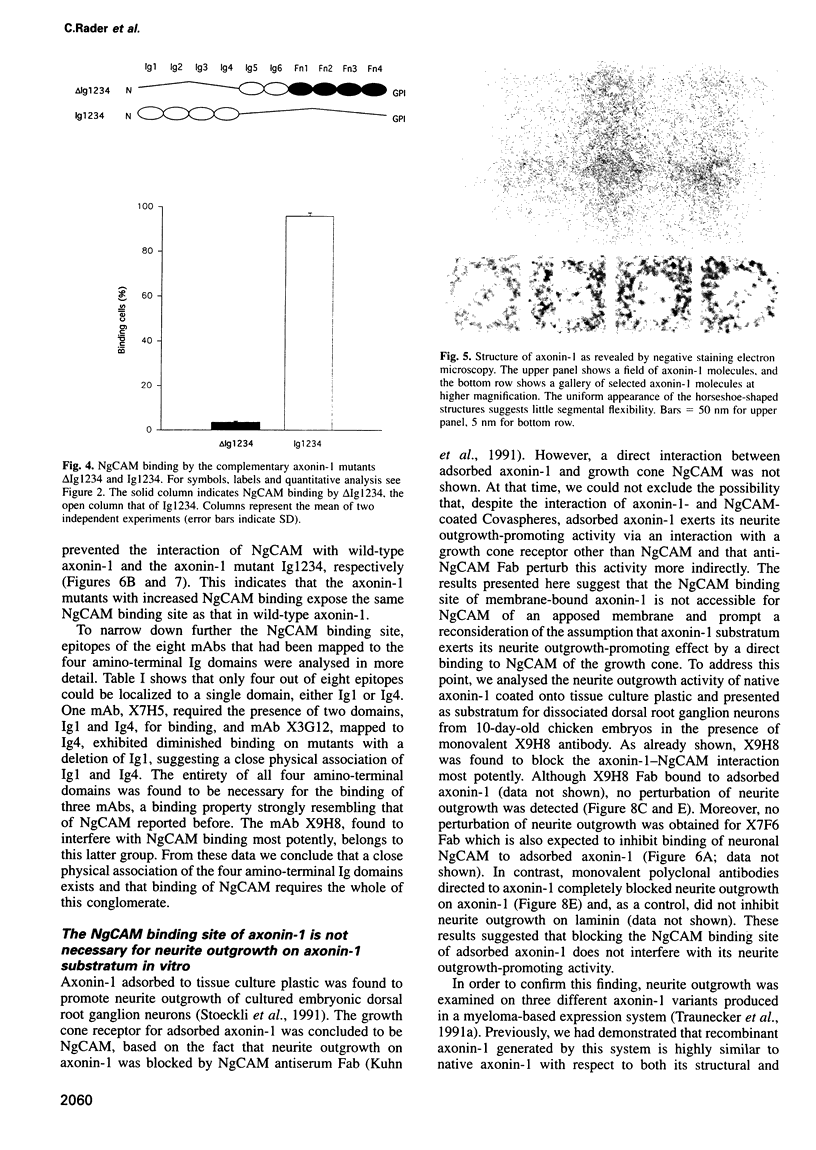
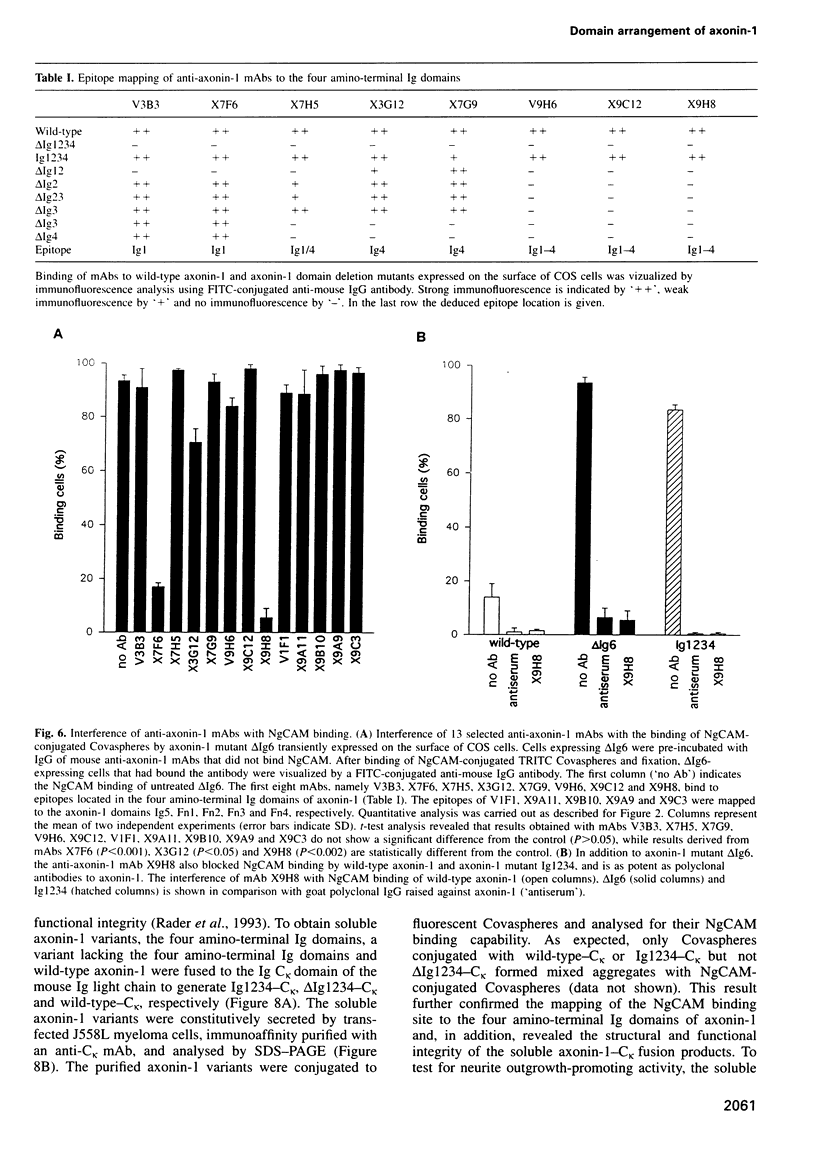
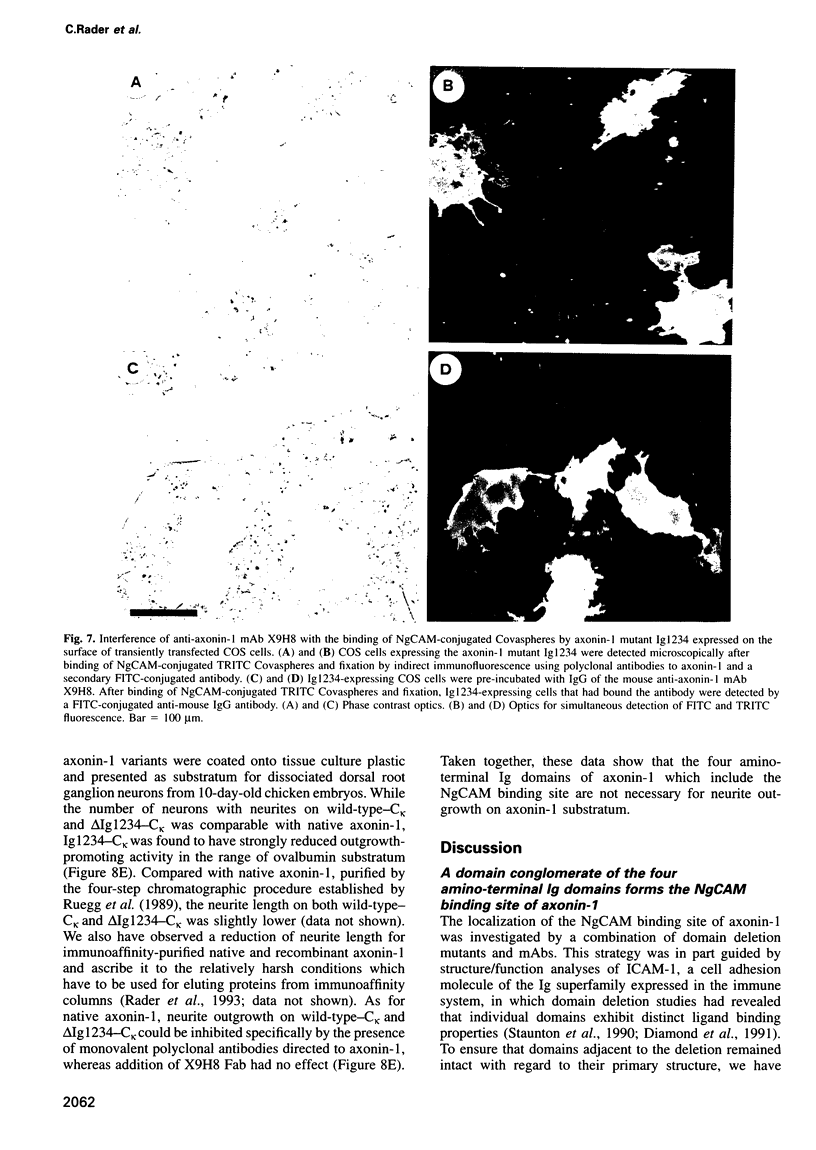
The neuronal cell adhesion molecule axonin-1 is composed of six immunoglobulin and four fibronectin type III domains. Axonin-1 promotes neurite outgrowth, when presented as a substratum for neurons in vitro, via a neuronal receptor that has been identified as the neuron-glia cell adhesion molecule, NgCAM, based on the blocking effect of polyclonal antibodies directed to NgCAM. Here we report the identification of axonin-1 domains involved in NgCAM binding. NgCAM-conjugated microspheres were tested for binding to COS cells expressing domain deletion mutants of axonin-1. In addition, monoclonal antibodies directed to axonin-1 were assessed for their ability to block the axonin-1-NgCAM interaction, and their epitopes were mapped using the domain deletion mutants. The results suggest that the four amino-terminal immunoglobulin domains of axonin-1 form a domain conglomerate which is necessary and sufficient for NgCAM binding. Surprisingly, NgCAM binding to membrane-bound axonin-1 was increased strongly by deletion of the fifth or sixth immunoglobulin domains of axonin-1. Based on these results and on negative staining electron microscopy, we propose a horseshoe-shaped domain arrangement of axonin-1 that obscures the NgCAM binding site. Neurite outgrowth studies with truncated forms of axonin-1 show that axonin-1 is a neurite outgrowth-promoting substratum in the absence of the NgCAM binding site.
Full text
PDF
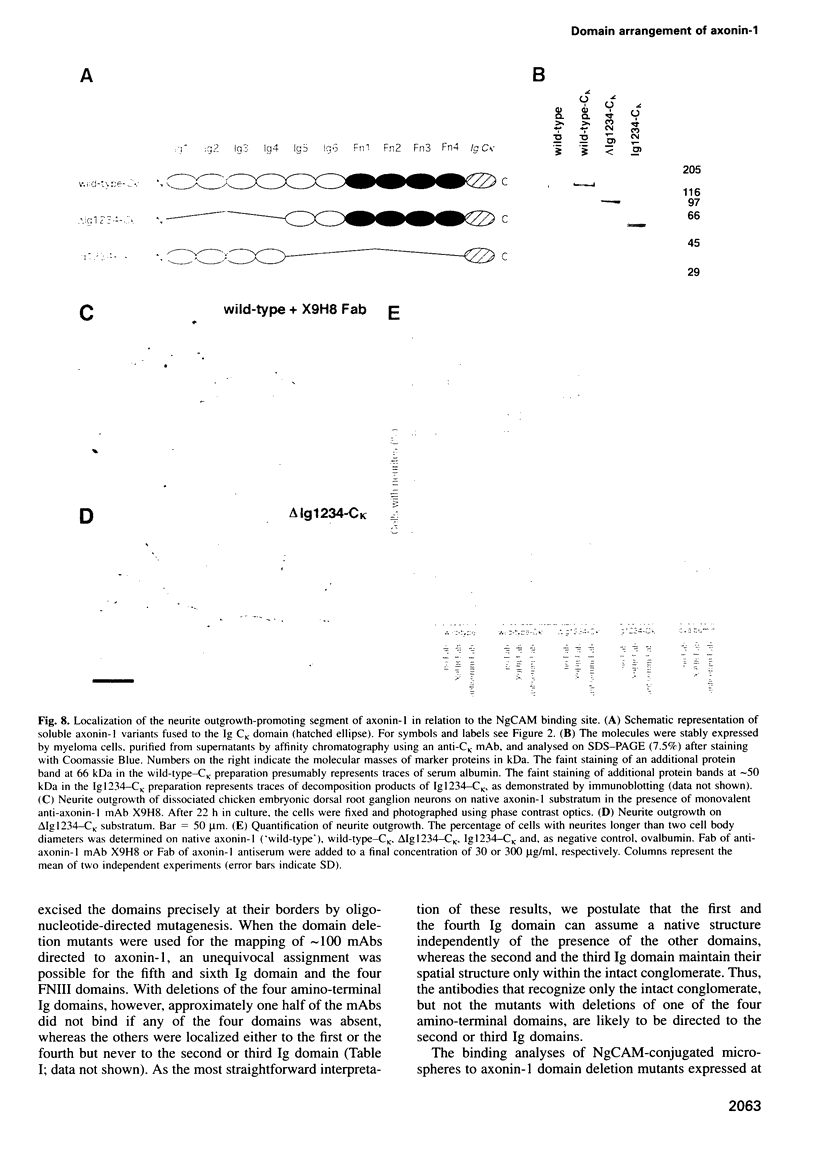
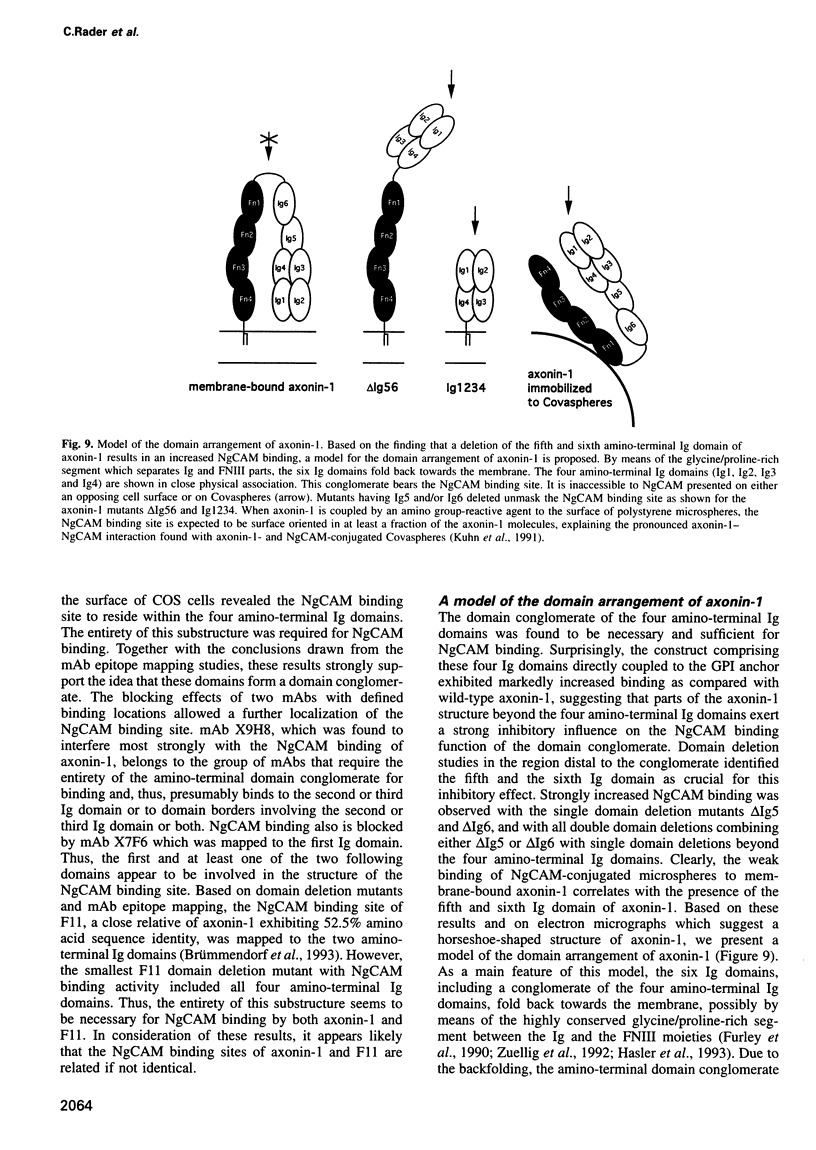




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Appel F., Holm J., Conscience J. F., von Bohlen und Halbach F., Faissner A., James P., Schachner M. Identification of the border between fibronectin type III homologous repeats 2 and 3 of the neural cell adhesion molecule L1 as a neurite outgrowth promoting and signal transducing domain. J Neurobiol. 1995 Nov;28(3):297–312. doi: 10.1002/neu.480280304. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Ziffer J. A., Edelman G. M., Cunningham B. A. Crystallographic studies of bovine beta2-microglobulin. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3345–3349. doi: 10.1073/pnas.74.8.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brümmendorf T., Hubert M., Treubert U., Leuschner R., Tárnok A., Rathjen F. G. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993 Apr;10(4):711–727. doi: 10.1016/0896-6273(93)90172-n. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T., Rathjen F. G. Axonal glycoproteins with immunoglobulin- and fibronectin type III-related domains in vertebrates: structural features, binding activities, and signal transduction. J Neurochem. 1993 Oct;61(4):1207–1219. doi: 10.1111/j.1471-4159.1993.tb13611.x. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T., Wolff J. M., Frank R., Rathjen F. G. Neural cell recognition molecule F11: homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989 Apr;2(4):1351–1361. doi: 10.1016/0896-6273(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Burgoon M. P., Grumet M., Mauro V., Edelman G. M., Cunningham B. A. Structure of the chicken neuron-glia cell adhesion molecule, Ng-CAM: origin of the polypeptides and relation to the Ig superfamily. J Cell Biol. 1991 Mar;112(5):1017–1029. doi: 10.1083/jcb.112.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M. A., Grady R. C., Mushinski J. F., Marcu K. B. PANG, a gene encoding a neuronal glycoprotein, is ectopically activated by intracisternal A-type particle long terminal repeats in murine plasmacytomas. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1337–1341. doi: 10.1073/pnas.91.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Felsenfeld D. P., Hynes M. A., Skoler K. M., Furley A. J., Jessell T. M. TAG-1 can mediate homophilic binding, but neurite outgrowth on TAG-1 requires an L1-like molecule and beta 1 integrins. Neuron. 1994 Mar;12(3):675–690. doi: 10.1016/0896-6273(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Furley A. J., Morton S. B., Manalo D., Karagogeos D., Dodd J., Jessell T. M. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990 Apr 6;61(1):157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Gennarini G., Cibelli G., Rougon G., Mattei M. G., Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989 Aug;109(2):775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger R. J., Vogt L., Zuellig R. A., Rader C., Henehan-Beatty A., Wolfer D. P., Sonderegger P. The gene of chicken axonin-1. Complete structure and analysis of the promoter. Eur J Biochem. 1995 Feb 1;227(3):617–628. doi: 10.1111/j.1432-1033.1995.tb20181.x. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Shatz C. J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993 Jan;72 (Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Grumet M., Mauro V., Burgoon M. P., Edelman G. M., Cunningham B. A. Structure of a new nervous system glycoprotein, Nr-CAM, and its relationship to subgroups of neural cell adhesion molecules. J Cell Biol. 1991 Jun;113(6):1399–1412. doi: 10.1083/jcb.113.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler T. H., Rader C., Stoeckli E. T., Zuellig R. A., Sonderegger P. cDNA cloning, structural features, and eucaryotic expression of human TAG-1/axonin-1. Eur J Biochem. 1993 Jan 15;211(1-2):329–339. doi: 10.1111/j.1432-1033.1993.tb19902.x. [DOI] [PubMed] [Google Scholar]
- Kayyem J. F., Roman J. M., de la Rosa E. J., Schwarz U., Dreyer W. J. Bravo/Nr-CAM is closely related to the cell adhesion molecules L1 and Ng-CAM and has a similar heterodimer structure. J Cell Biol. 1992 Sep;118(5):1259–1270. doi: 10.1083/jcb.118.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn T. B., Stoeckli E. T., Condrau M. A., Rathjen F. G., Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4). J Cell Biol. 1991 Nov;115(4):1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy D. J., Hendrickson W. A., Aukhil I., Erickson H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992 Nov 6;258(5084):987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader C., Stoeckli E. T., Ziegler U., Osterwalder T., Kunz B., Sonderegger P. Cell-cell adhesion by homophilic interaction of the neuronal recognition molecule axonin-1. Eur J Biochem. 1993 Jul 1;215(1):133–141. doi: 10.1111/j.1432-1033.1993.tb18015.x. [DOI] [PubMed] [Google Scholar]
- Ranscht B. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988 Oct;107(4):1561–1573. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen F. G., Wolff J. M., Frank R., Bonhoeffer F., Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987 Feb;104(2):343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rols M. P., Delteil C., Serin G., Teissié J. Temperature effects on electrotransfection of mammalian cells. Nucleic Acids Res. 1994 Feb 11;22(3):540–540. doi: 10.1093/nar/22.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg M. A., Stoeckli E. T., Kuhn T. B., Heller M., Zuellig R., Sonderegger P. Purification of axonin-1, a protein that is secreted from axons during neurogenesis. EMBO J. 1989 Jan;8(1):55–63. doi: 10.1002/j.1460-2075.1989.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Stoeckli E. T., Kuhn T. B., Duc C. O., Ruegg M. A., Sonderegger P. The axonally secreted protein axonin-1 is a potent substratum for neurite growth. J Cell Biol. 1991 Feb;112(3):449–455. doi: 10.1083/jcb.112.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli E. T., Landmesser L. T. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995 Jun;14(6):1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Suter D. M., Pollerberg G. E., Buchstaller A., Giger R. J., Dreyer W. J., Sonderegger P. Binding between the neural cell adhesion molecules axonin-1 and Nr-CAM/Bravo is involved in neuron-glia interaction. J Cell Biol. 1995 Nov;131(4):1067–1081. doi: 10.1083/jcb.131.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunecker A., Lanzavecchia A., Karjalainen K. Bispecific single chain molecules (Janusins) target cytotoxic lymphocytes on HIV infected cells. EMBO J. 1991 Dec;10(12):3655–3659. doi: 10.1002/j.1460-2075.1991.tb04932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunecker A., Oliveri F., Karjalainen K. Myeloma based expression system for production of large mammalian proteins. Trends Biotechnol. 1991 Apr;9(4):109–113. doi: 10.1016/0167-7799(91)90038-j. [DOI] [PubMed] [Google Scholar]
- Venstrom K., Reichardt L. Beta 8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol Biol Cell. 1995 Apr;6(4):419–431. doi: 10.1091/mbc.6.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielmetter J., Kayyem J. F., Roman J. M., Dreyer W. J. Neogenin, an avian cell surface protein expressed during terminal neuronal differentiation, is closely related to the human tumor suppressor molecule deleted in colorectal cancer. J Cell Biol. 1994 Dec;127(6 Pt 2):2009–2020. doi: 10.1083/jcb.127.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y., Kawasaki M., Tamada A., Nagata S., Kagamiyama H., Mori K. Overlapping and differential expression of BIG-2, BIG-1, TAG-1, and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. J Neurobiol. 1995 Sep;28(1):51–69. doi: 10.1002/neu.480280106. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y., Kawasaki M., Tani A., Tamada A., Nagata S., Kagamiyama H., Mori K. BIG-1: a new TAG-1/F3-related member of the immunoglobulin superfamily with neurite outgrowth-promoting activity. Neuron. 1994 Aug;13(2):415–426. doi: 10.1016/0896-6273(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Zuellig R. A., Rader C., Schroeder A., Kalousek M. B., Von Bohlen und Halbach F., Osterwalder T., Inan C., Stoeckli E. T., Affolter H. U., Fritz A. The axonally secreted cell adhesion molecule, axonin-1. Primary structure, immunoglobulin-like and fibronectin-type-III-like domains and glycosyl-phosphatidylinositol anchorage. Eur J Biochem. 1992 Mar 1;204(2):453–463. doi: 10.1111/j.1432-1033.1992.tb16655.x. [DOI] [PubMed] [Google Scholar]