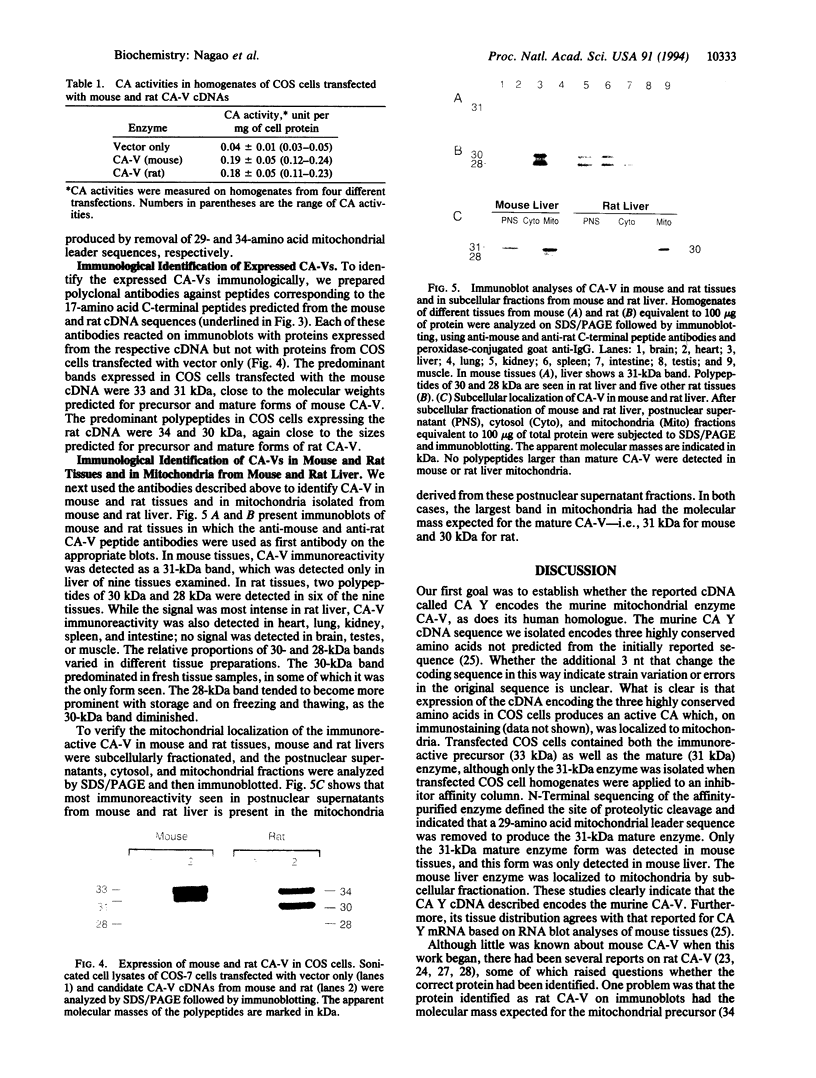
Abstract
When the human cDNA, isolated on the basis of homology to the murine carbonic anhydrase (CA) "Y" was expressed in COS cells, the human CA was targeted to and processed in mitochondria, as expected for CA-V. However, tissue distribution reported for the corresponding mouse CA Y mRNA was much more limited than that reported for the distribution of CA-V immunostaining in rat tissues. To determine whether the murine cDNA actually encodes a mitochondrial CA activity and to compare the tissue distribution of the homologous murine and rat gene products, we used reverse transcription-PCR to reisolate the murine CA-V candidate cDNA and used the murine cDNA probe to isolate the homologous rat cDNA. We compared the two cDNA sequences, the activities they expressed after transfection of COS cells, and the sites of N-terminal processing of expressed products. In addition, we used antibodies to the C-terminal peptides predicted from each cDNA to compare distribution of CA-V in mouse and rat tissues and to identify CA-Vs in mitochondria isolated from mouse and rat liver. From these studies, we conclude that both mouse and rat CA-V candidate cDNAs encode active CAs that are targeted to and processed in mitochondria and that there are real differences in tissue distribution of CA-V between mouse and rat. However, the findings that are M(r) of CA-V in rat tissues is smaller than that previously reported and that the tissue distribution also differs lead us to conclude that the antibody used in prior reports most likely misidentified another antigen in rat tissues as CA-V.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldred P., Fu P., Barrett G., Penschow J. D., Wright R. D., Coghlan J. P., Fernley R. T. Human secreted carbonic anhydrase: cDNA cloning, nucleotide sequence, and hybridization histochemistry. Biochemistry. 1991 Jan 15;30(2):569–575. doi: 10.1021/bi00216a035. [DOI] [PubMed] [Google Scholar]
- Amor-Gueret M., Levi-Strauss M. Nucleotide and derived amino-acid sequence of a cDNA encoding a new mouse carbonic anhydrase. Nucleic Acids Res. 1990 Mar 25;18(6):1646–1646. doi: 10.1093/nar/18.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N. D., Dodgson S. J., Quant P. A. Expression of hepatic mitochondrial carbonic anhydrase V. Biochim Biophys Acta. 1990 Dec 6;1036(3):237–241. doi: 10.1016/0304-4165(90)90040-4. [DOI] [PubMed] [Google Scholar]
- Coulson R. A., Herbert J. D. A role for carbonic anhydrase in intermediary metabolism. Ann N Y Acad Sci. 1984;429:505–515. doi: 10.1111/j.1749-6632.1984.tb12379.x. [DOI] [PubMed] [Google Scholar]
- DATTA P. K., SHEPARD T. H., 2nd Intracellular localization of carbonic anhydrase in rat liver and kidney tissues. Arch Biochem Biophys. 1959 Mar;81(1):124–129. doi: 10.1016/0003-9861(59)90182-1. [DOI] [PubMed] [Google Scholar]
- Deutsch H. F. Carbonic anhydrases. Int J Biochem. 1987;19(2):101–113. doi: 10.1016/0020-711x(87)90320-x. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Cherian K. Mitochondrial carbonic anhydrase is involved in rat renal glucose synthesis. Am J Physiol. 1989 Dec;257(6 Pt 1):E791–E796. doi: 10.1152/ajpendo.1989.257.6.E791. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd, Storey B. T., Mela L. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5562–5566. doi: 10.1073/pnas.77.9.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson S. J. Inhibition of mitochondrial carbonic anhydrase and ureagenesis: a discrepancy examined. J Appl Physiol (1985) 1987 Nov;63(5):2134–2141. doi: 10.1152/jappl.1987.63.5.2134. [DOI] [PubMed] [Google Scholar]
- Feldstein J. B., Silverman D. N. Purification and characterization of carbonic anhydrase from the saliva of the rat. J Biol Chem. 1984 May 10;259(9):5447–5453. [PubMed] [Google Scholar]
- Hendrick J. P., Hodges P. E., Rosenberg L. E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAREN T. H. A simplified micromethod for the determination of carbonic anhydrase and its inhibitors. J Pharmacol Exp Ther. 1960 Sep;130:26–29. [PubMed] [Google Scholar]
- Marsolais C., Huot S., David F., Garneau M., Brunengraber H. Compartmentation of 14CO2 in the perfused rat liver. J Biol Chem. 1987 Feb 25;262(6):2604–2607. [PubMed] [Google Scholar]
- Miyazaki J., Takaki S., Araki K., Tashiro F., Tominaga A., Takatsu K., Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989 Jul 15;79(2):269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Murakami H., Sly W. S. Purification and characterization of human salivary carbonic anhydrase. J Biol Chem. 1987 Jan 25;262(3):1382–1388. [PubMed] [Google Scholar]
- Nagao Y., Platero J. S., Waheed A., Sly W. S. Human mitochondrial carbonic anhydrase: cDNA cloning, expression, subcellular localization, and mapping to chromosome 16. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7623–7627. doi: 10.1073/pnas.90.16.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R. CO2 metabolism in the liver. Arch Biochem Biophys. 1983 Apr 15;222(2):442–448. doi: 10.1016/0003-9861(83)90543-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Hewett-Emmett D., Whyte M. P., Yu Y. S., Tashian R. E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A. 1983 May;80(9):2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram V., Rumbolo P., Grubb J., Strisciuglio P., Sly W. S. Carbonic anhydrase II deficiency: diagnosis and carrier detection using differential enzyme inhibition and inactivation. Am J Hum Genet. 1986 Feb;38(2):125–136. [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., Van Etten R. A., Clayton D. A. Isolation of mammalian mitochondrial DNA and RNA and cloning of the mitochondrial genome. Methods Enzymol. 1983;97:426–434. doi: 10.1016/0076-6879(83)97153-7. [DOI] [PubMed] [Google Scholar]
- Tashian R. E. Genetics of the mammalian carbonic anhydrases. Adv Genet. 1992;30:321–356. doi: 10.1016/s0065-2660(08)60323-5. [DOI] [PubMed] [Google Scholar]
- Tashian R. E. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989 Jun;10(6):186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- Tu C. K., Paranawithana S. R., Jewell D. A., Tanhauser S. M., LoGrasso P. V., Wynns G. C., Laipis P. J., Silverman D. N. Buffer enhancement of proton transfer in catalysis by human carbonic anhydrase III. Biochemistry. 1990 Jul 10;29(27):6400–6405. doi: 10.1021/bi00479a009. [DOI] [PubMed] [Google Scholar]
- Vänänen H. K., Carter N. D., Dodgson S. J. Immunocytochemical localization of mitochondrial carbonic anhydrase in rat tissues. J Histochem Cytochem. 1991 Apr;39(4):451–459. doi: 10.1177/39.4.1900871. [DOI] [PubMed] [Google Scholar]
- Waheed A., Zhu X. L., Sly W. S. Membrane-associated carbonic anhydrase from rat lung. Purification, characterization, tissue distribution, and comparison with carbonic anhydrase IVs of other mammals. J Biol Chem. 1992 Feb 15;267(5):3308–3311. [PubMed] [Google Scholar]
- Wanders R. J., Van Roermund C. W., Meijer A. J. Analysis of the control of citrulline synthesis in isolated rat-liver mitochondria. Eur J Biochem. 1984 Jul 16;142(2):247–254. doi: 10.1111/j.1432-1033.1984.tb08278.x. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Briggle T. V. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem. 1982 Oct 25;257(20):12056–12059. [PubMed] [Google Scholar]
- Wistrand P. J., Knuuttila K. G. Renal membrane-bound carbonic anhydrase. Purification and properties. Kidney Int. 1989 Mar;35(3):851–859. doi: 10.1038/ki.1989.63. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Oshima A., Shimmoto M., Fukuhara Y., Sakuraba H., Yanagisawa N., Suzuki Y. Human beta-galactosidase gene mutations in GM1-gangliosidosis: a common mutation among Japanese adult/chronic cases. Am J Hum Genet. 1991 Aug;49(2):435–442. [PMC free article] [PubMed] [Google Scholar]
- Zhu X. L., Sly W. S. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990 May 25;265(15):8795–8801. [PubMed] [Google Scholar]





