Abstract
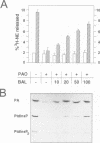
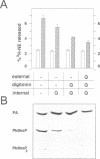
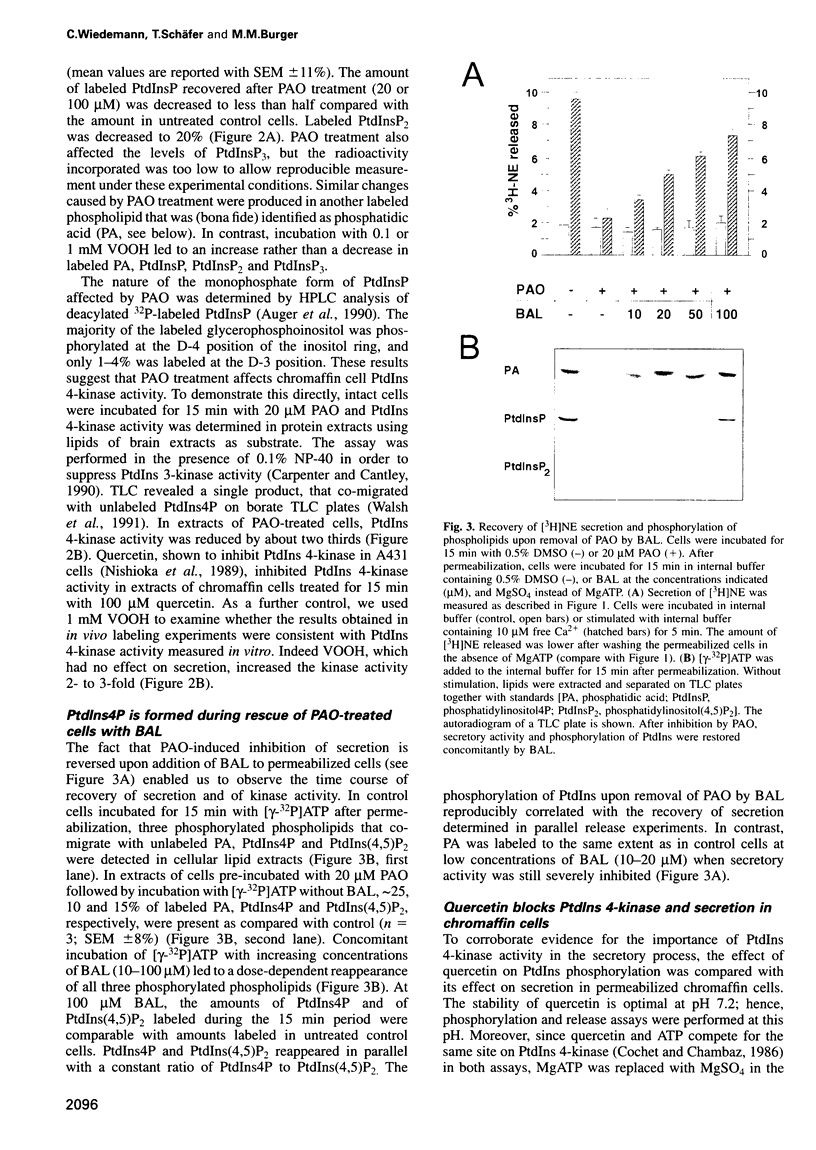
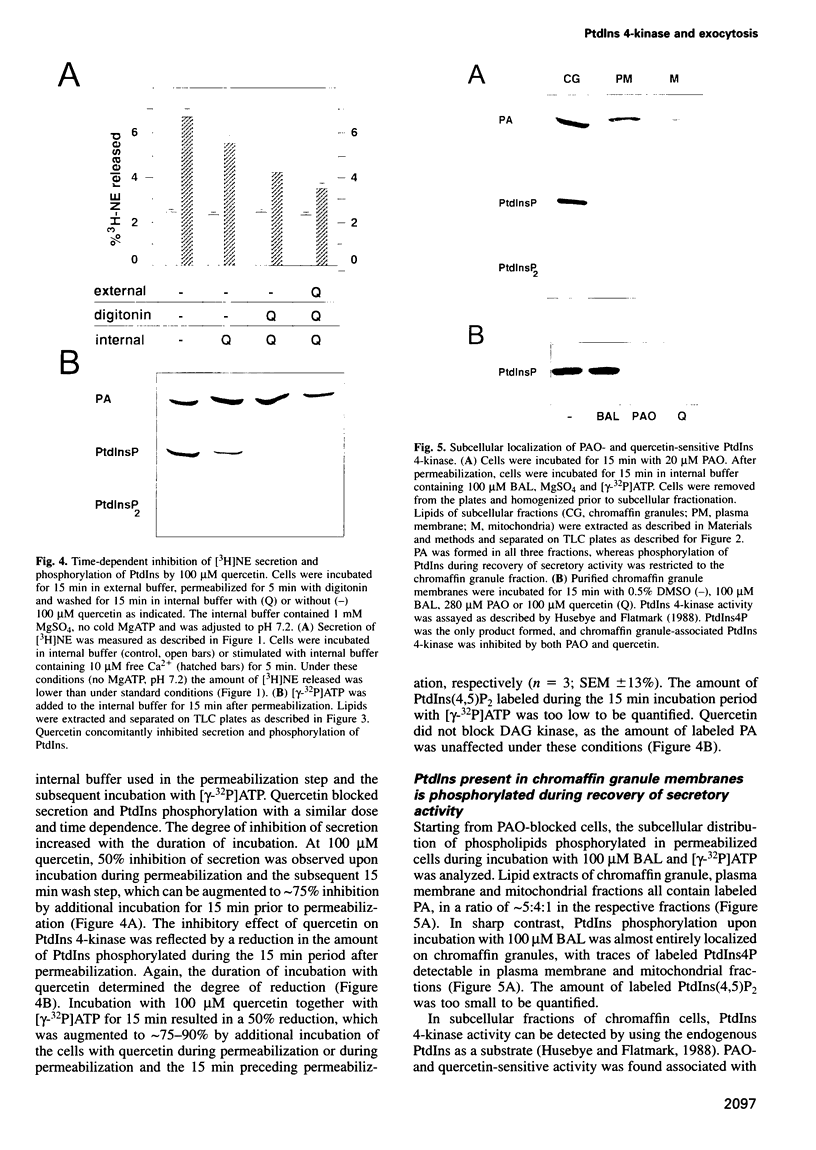
Permeabilized bovine adrenal chromaffin cells have been used to characterize the MgATP requirement of processes preceding exocytosis. Incubation of primary cultures with the membrane-permeable phenylarsine oxide (PAO) at 20 microM inhibited the phosphorylation of phosphatidylinositol (PtdIns) and completely blocked secretion. This block could be reversed by addition of 2,3-dimercaptopropanol to the permeabilized cells. Simultaneous addition of [gamma32P]ATP and 2,3-dimercaptopropanol permitted a comparison between recovery of secretion and phosphorylation of intracellular components. Recovery of secretion closely correlated with phosphorylation of PtdIns and PtdIns4P. Subcellular fractionation of permeabilized cells after recovery of secretion revealed that the majority of newly phosphorylated PtdIns4P was localized on the chromaffin granules. In accordance with these results, PtdIns 4-kinase activity was found in protein extracts of permeabilized cells as well as associated with purified chromaffin granules, sensitive in both cases to PAO. Additionally, PtdIns 4-kinase activity in these two assays was inhibited by quercetin. In permeabilized cells, quercetin decreased the levels of labeled PtdIns4P and Ptdlns(4,5)P2 and inhibited secretion. Our data suggest that a chromaffin granule-associated PtdIns 4-kinase acts in the priming of exocytosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittner M. A., Holz R. W. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J Biol Chem. 1992 Aug 15;267(23):16219–16225. [PubMed] [Google Scholar]
- Buckley J. T., Lefebvre Y. A., Hawthorne J. N. Identification of an actively phosphorylated component of adrenal medulla chromaffin granules. Biochim Biophys Acta. 1971 Sep 1;239(3):517–519. doi: 10.1016/0005-2760(71)90047-6. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C., Chambaz E. M. Catalytic properties of a purified phosphatidylinositol-4-phosphate kinase from rat brain. Biochem J. 1986 Jul 1;237(1):25–31. doi: 10.1042/bj2370025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio R. L., Pilch P. F. Phosphatidylinositol 4-kinase is a component of glucose transporter (GLUT 4)-containing vesicles. J Biol Chem. 1991 Jul 15;266(20):13278–13283. [PubMed] [Google Scholar]
- Eberhard D. A., Cooper C. L., Low M. G., Holz R. W. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem J. 1990 May 15;268(1):15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endemann G. C., Graziani A., Cantley L. C. A monoclonal antibody distinguishes two types of phosphatidylinositol 4-kinase. Biochem J. 1991 Jan 1;273(Pt 1):63–66. doi: 10.1042/bj2730063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Morales P., Minami Y., Luong E., Klausner R. D., Samelson L. E. Tyrosine phosphorylation in T cells is regulated by phosphatase activity: studies with phenylarsine oxide. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9255–9259. doi: 10.1073/pnas.87.23.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. E., Hajduk P. J., Yoon H. S., Fesik S. W. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994 Sep 8;371(6493):168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995 Mar 9;374(6518):173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Martin T. F. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993 Dec 9;366(6455):572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Martin T. F. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J Cell Biol. 1992 Oct;119(1):139–151. doi: 10.1083/jcb.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel A., Schäfer T., Gerosa D., Burger M. M. In chromaffin cells, the mammalian Sec1p homologue is a syntaxin 1A-binding protein associated with chromaffin granules. J Biol Chem. 1994 Mar 25;269(12):8623–8626. [PubMed] [Google Scholar]
- Holz R. W., Bittner M. A., Peppers S. C., Senter R. A., Eberhard D. A. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J Biol Chem. 1989 Apr 5;264(10):5412–5419. [PubMed] [Google Scholar]
- Horrigan F. T., Bookman R. J. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994 Nov;13(5):1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Hughes P. J., Michell R. H. Novel inositol containing phospholipids and phosphates: their synthesis and possible new roles in cellular signalling. Curr Opin Neurobiol. 1993 Jun;3(3):383–400. doi: 10.1016/0959-4388(93)90132-i. [DOI] [PubMed] [Google Scholar]
- Husebye E. S., Flatmark T. Phosphatidylinositol kinase of bovine adrenal chromaffin granules: kinetic properties and inhibition by low concentrations of Ca2+. Biochim Biophys Acta. 1988 Feb 22;968(2):261–265. doi: 10.1016/0167-4889(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh A., Thomas G. M., Ball A., Prosser S., Cunningham E., Cockcroft S., Hsuan J. J. Requirement for phosphatidylinositol transfer protein in epidermal growth factor signaling. Science. 1995 May 26;268(5214):1188–1190. doi: 10.1126/science.7761838. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B., Chen K. S. Phosphatidylinositol-3-kinase in isolated rat adipocytes. Activation by insulin and subcellular distribution. J Biol Chem. 1992 Feb 15;267(5):3423–3428. [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Levenson R. M., Blackshear P. J. Insulin-stimulated protein tyrosine phosphorylation in intact cells evaluated by giant two-dimensional gel electrophoresis. J Biol Chem. 1989 Nov 25;264(33):19984–19993. [PubMed] [Google Scholar]
- Liao K., Hoffman R. D., Lane M. D. Phosphotyrosyl turnover in insulin signaling. Characterization of two membrane-bound pp15 protein tyrosine phosphatases from 3T3-L1 adipocytes. J Biol Chem. 1991 Apr 5;266(10):6544–6553. [PubMed] [Google Scholar]
- Liscovitch M., Chalifa V., Pertile P., Chen C. S., Cantley L. C. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem. 1994 Aug 26;269(34):21403–21406. [PubMed] [Google Scholar]
- Marandici A., Monder C. Purification and characterization of corticosteroid side chain isomerase. Biochemistry. 1990 Feb 6;29(5):1147–1154. doi: 10.1021/bi00457a008. [DOI] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Imoto M., Sawa T., Hamada M., Naganawa H., Takeuchi T., Umezawa K. Screening of phosphatidylinositol kinase inhibitors from Streptomyces. J Antibiot (Tokyo) 1989 May;42(5):823–825. doi: 10.7164/antibiotics.42.823. [DOI] [PubMed] [Google Scholar]
- Peppers S. C., Holz R. W. Catecholamine secretion from digitonin-treated PC12 cells. Effects of Ca2+, ATP, and protein kinase C activators. J Biol Chem. 1986 Nov 5;261(31):14665–14669. [PubMed] [Google Scholar]
- Phillips J. H. Phosphatidylinositol kinase. A component of the chromaffin-granule membrane. Biochem J. 1973 Nov;136(3):579–587. doi: 10.1042/bj1360579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Faure R., Burgess J. W., Bevan A. P., Lachance D., Zhang-Sun G., Fantus I. G., Ng J. B., Hall D. A., Lum B. S. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem. 1994 Feb 11;269(6):4596–4604. [PubMed] [Google Scholar]
- Purkiss J., Murrin R. A., Owen P. J., Boarder M. R. Lack of phospholipase D activity in chromaffin cells: bradykinin-stimulated phosphatidic acid formation involves phospholipase C in chromaffin cells but phospholipase D in PC12 cells. J Neurochem. 1991 Sep;57(3):1084–1087. doi: 10.1111/j.1471-4159.1991.tb08262.x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
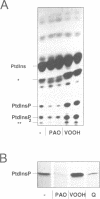
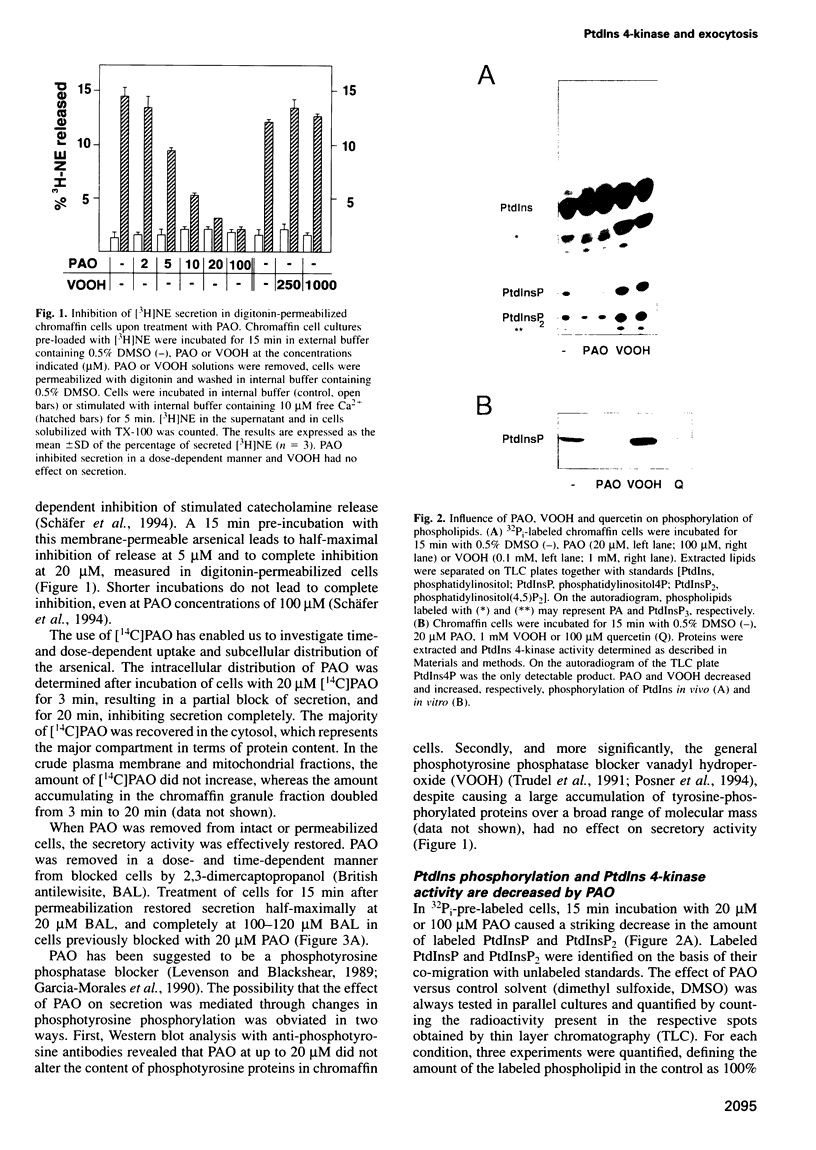
- Schaefer T., Wiedemann C., Gitler C., Burger M. M. Effects of arsenicals on the secretory process in chromaffin cells. Ann N Y Acad Sci. 1994 Mar 9;710:356–367. doi: 10.1111/j.1749-6632.1994.tb26642.x. [DOI] [PubMed] [Google Scholar]
- Schäfer T., Karli U. O., Gratwohl E. K., Schweizer F. E., Burger M. M. Digitonin-permeabilized cells are exocytosis competent. J Neurochem. 1987 Dec;49(6):1697–1707. doi: 10.1111/j.1471-4159.1987.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Stack J. H., Emr S. D. Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI 3-kinase activities. J Biol Chem. 1994 Dec 16;269(50):31552–31562. [PubMed] [Google Scholar]
- Susa M., Keeler M., Varticovski L. Platelet-derived growth factor activates membrane-associated phosphatidylinositol 3-kinase and mediates its translocation from the cytosol. Detection of enzyme activity in detergent-solubilized cell extracts. J Biol Chem. 1992 Nov 15;267(32):22951–22956. [PubMed] [Google Scholar]
- Trudel S., Pâquet M. R., Grinstein S. Mechanism of vanadate-induced activation of tyrosine phosphorylation and of the respiratory burst in HL60 cells. Role of reduced oxygen metabolites. Biochem J. 1991 Jun 15;276(Pt 3):611–619. doi: 10.1042/bj2760611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. P., Caldwell K. K., Majerus P. W. Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9184–9187. doi: 10.1073/pnas.88.20.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler W. L., Cleland W. W. A specific and sensitive assay for disulfides. J Biol Chem. 1968 Feb 25;243(4):716–719. [PubMed] [Google Scholar]