Abstract
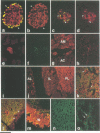
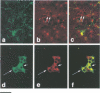
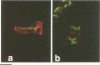
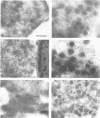
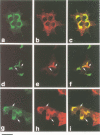
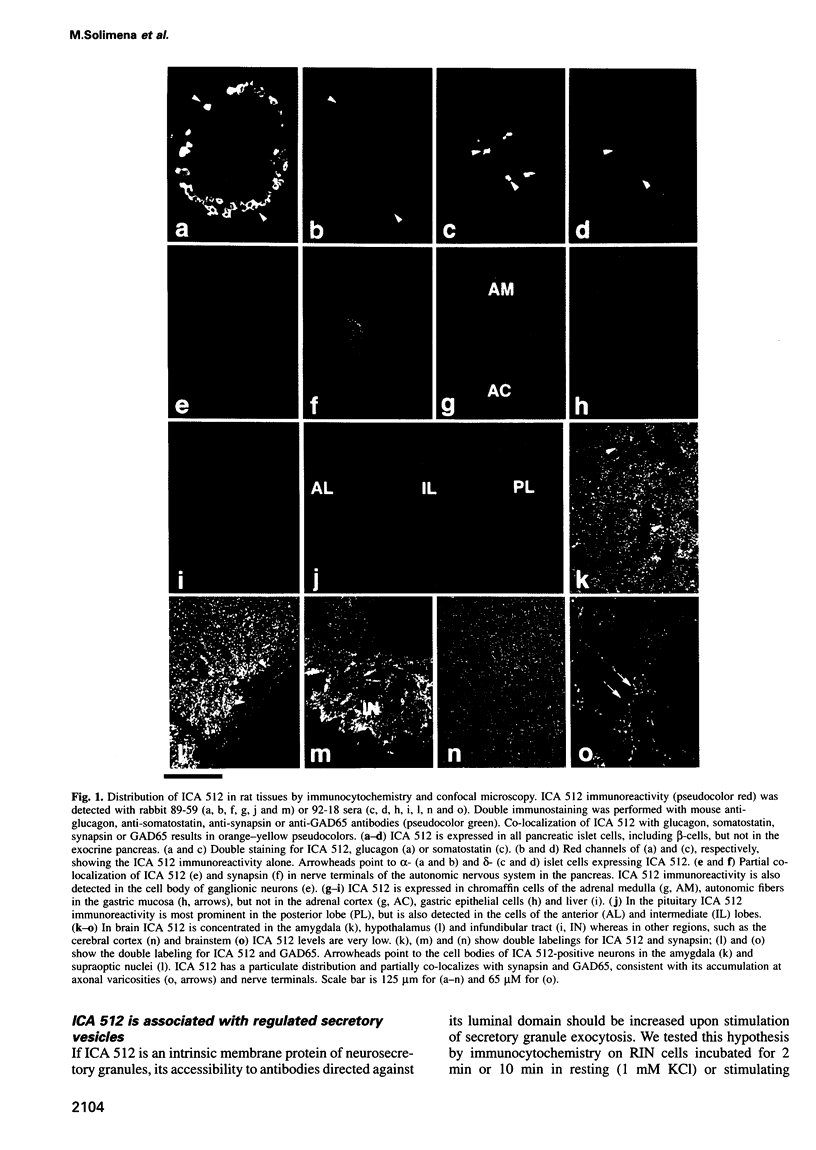
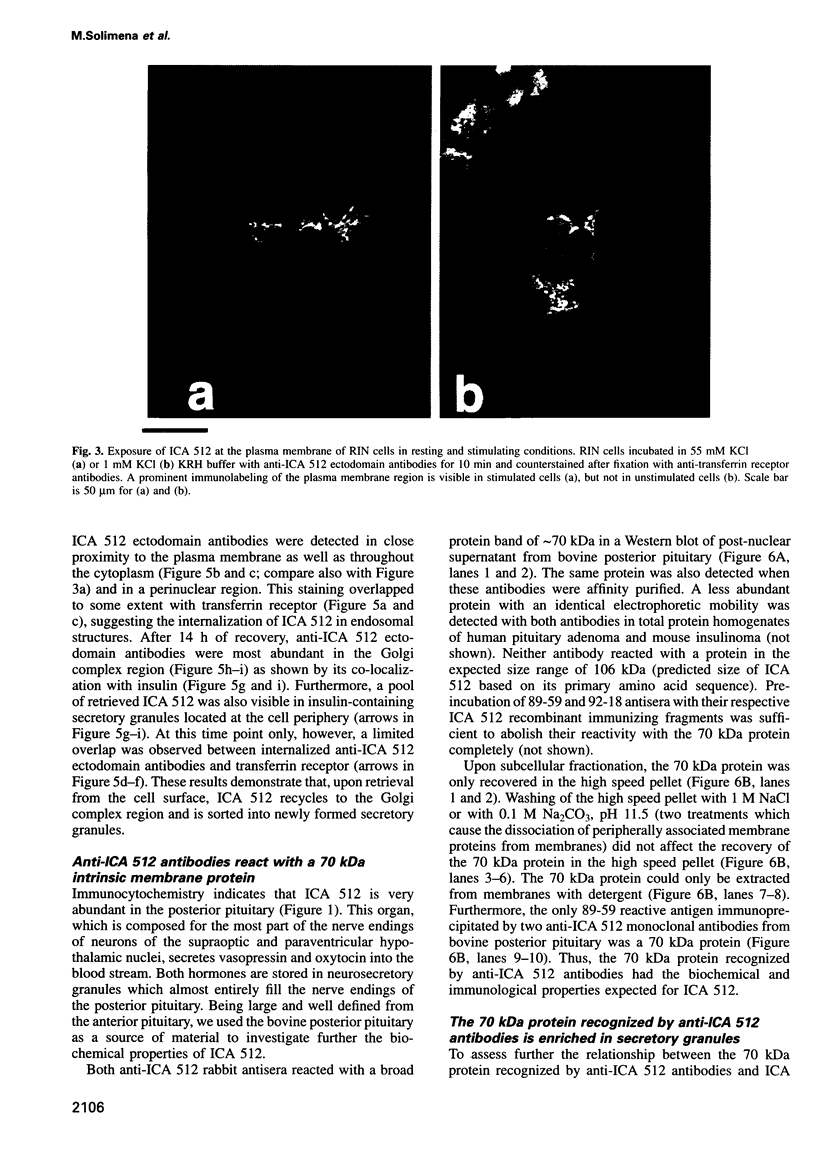
Islet cell autoantigen (ICA) 512 is a novel autoantigen of insulin-dependent diabetes mellitus (IDDM) which is homologous to receptor-type protein tyrosine phosphatases (++PTPases). We show that ICA 512 is an intrinsic membrane protein of secretory granules expressed in insulin-producing pancreatic beta-cells as well as in virtually all other peptide-secreting endocrine cells and neurons containing neurosecretory granules. ICA 512 is cleaved at its luminal domain and, following exposure at the cell surface, recycles to the Golgi complex region and is sorted into newly formed secretory granules. By immunoprecipitation, anti-ICA 512 autoantibodies were detected in 15/17 (88%) newly diagnosed IDDM patients, but not in 10/10 healthy subjects. These results suggest that tyrosine phosphorylation participates in some aspect of secretory granule function common to all neuroendocrine cells and that a subset of autoantibodies in IDDM is directed against an integral membrane protein of insulin-containing granules.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. A., Bowman M. A., Campbell L., Darrow B. L., Kaufman D. L., Maclaren N. K. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994 Nov;94(5):2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Bergman B., Haskins K. Islet-specific T-cell clones from the NOD mouse respond to beta-granule antigen. Diabetes. 1994 Feb;43(2):197–203. doi: 10.2337/diab.43.2.197. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay S. M., Tonks N. K. Identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTP mu. J Biol Chem. 1994 Nov 11;269(45):28472–28477. [PubMed] [Google Scholar]
- Brand S. H., Castle J. D. SCAMP 37, a new marker within the general cell surface recycling system. EMBO J. 1993 Oct;12(10):3753–3761. doi: 10.1002/j.1460-2075.1993.tb06053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. H., Laurie S. M., Mixon M. B., Castle J. D. Secretory carrier membrane proteins 31-35 define a common protein composition among secretory carrier membranes. J Biol Chem. 1991 Oct 5;266(28):18949–18957. [PubMed] [Google Scholar]
- Buckley K. M., Floor E., Kelly R. B. Cloning and sequence analysis of cDNA encoding p38, a major synaptic vesicle protein. J Cell Biol. 1987 Dec;105(6 Pt 1):2447–2456. doi: 10.1083/jcb.105.6.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño L., Russo E., Zhou L., Lipes M. A., Eisenbarth G. S. Identification and cloning of a granule autoantigen (carboxypeptidase-H) associated with type I diabetes. J Clin Endocrinol Metab. 1991 Dec;73(6):1197–1201. doi: 10.1210/jcem-73-6-1197. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chilcote T. J., Galli T., Mundigl O., Edelmann L., McPherson P. S., Takei K., De Camilli P. Cellubrevin and synaptobrevins: similar subcellular localization and biochemical properties in PC12 cells. J Cell Biol. 1995 Apr;129(1):219–231. doi: 10.1083/jcb.129.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift-O'Grady L., Linstedt A. D., Lowe A. W., Grote E., Kelly R. B. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J Cell Biol. 1990 May;110(5):1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Benfenati F., Valtorta F., Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Cameron R., Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983 May;96(5):1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Milgram S. L., Husten E. J., Yun H. Y., Mains R. E. Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains. Protein Sci. 1993 Apr;2(4):489–497. doi: 10.1002/pro.5560020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. D., Eiden L. E., Hoffman B. J. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany M. B., Lee S., Edwards R. H., Buckley K. M. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992 Sep 4;70(5):861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Stahl B., Walch-Solimena C., Takei K., Daniels L., Khoklatchev A., De Camilli P., Südhof T. C., Jahn R. Localization of Rab5 to synaptic vesicles identifies endosomal intermediate in synaptic vesicle recycling pathway. Eur J Cell Biol. 1994 Dec;65(2):319–326. [PubMed] [Google Scholar]
- Harrison L. C., De Aizpurua H., Loudovaris T., Campbell I. L., Cebon J. S., Tait B. D., Colman P. G. Reactivity to human islets and fetal pig proislets by peripheral blood mononuclear cells from subjects with preclinical and clinical insulin-dependent diabetes. Diabetes. 1991 Sep;40(9):1128–1133. doi: 10.2337/diab.40.9.1128. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. P., Wang H., D'Eustachio P., Musacchio J. M., Schlessinger J., Sap J. Cloning and characterization of R-PTP-kappa, a new member of the receptor protein tyrosine phosphatase family with a proteolytically cleaved cellular adhesion molecule-like extracellular region. Mol Cell Biol. 1993 May;13(5):2942–2951. doi: 10.1128/mcb.13.5.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. J. Cellular immunity to human insulin in individuals at high risk for the development of type I diabetes mellitus. J Autoimmun. 1990 Jun;3(3):321–327. doi: 10.1016/0896-8411(90)90150-q. [DOI] [PubMed] [Google Scholar]
- Kostron H., Winkler H., Peer L. J., König P. Uptake of adenosine triphosphate by isolated adrenal chromaffin granules: a carrier-mediated transport. Neuroscience. 1977;2(1):159–166. doi: 10.1016/0306-4522(77)90077-x. [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M. S., Lu J., Goto Y., Notkins A. L. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994 May;13(5):505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- Lowe A. W., Madeddu L., Kelly R. B. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J Cell Biol. 1988 Jan;106(1):51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Notkins A. L., Lan M. S. Isolation, sequence and expression of a novel mouse brain cDNA, mIA-2, and its relatedness to members of the protein tyrosine phosphatase family. Biochem Biophys Res Commun. 1994 Oct 28;204(2):930–936. doi: 10.1006/bbrc.1994.2549. [DOI] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Mundigl O., Matteoli M., Daniell L., Thomas-Reetz A., Metcalf A., Jahn R., De Camilli P. Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of Brefeldin A in axon and dendrites. J Cell Biol. 1993 Sep;122(6):1207–1221. doi: 10.1083/jcb.122.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navone F., Di Gioia G., Jahn R., Browning M., Greengard P., De Camilli P. Microvesicles of the neurohypophysis are biochemically related to small synaptic vesicles of presynaptic nerve terminals. J Cell Biol. 1989 Dec;109(6 Pt 2):3425–3433. doi: 10.1083/jcb.109.6.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Lill H. Porters and neurotransmitter transporters. J Exp Biol. 1994 Nov;196:213–228. doi: 10.1242/jeb.196.1.213. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Obendorf D., Schwarzenbrunner U., Fischer-Colbrie R., Laslop A., Winkler H. Immunological characterization of a membrane glycoprotein of chromaffin granules: its presence in endocrine and exocrine tissues. Neuroscience. 1988 Apr;25(1):343–351. doi: 10.1016/0306-4522(88)90030-9. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987 Jun 19;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Asplin C. M., Clemons P., Lyen K., Tatpati O., Raghu P. K., Paquette T. L. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983 Dec 23;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P., Lang R., van Endert P. M., Benazzi E., Felix A. M., Pastore R. M., Spinas G. A., Sinigaglia F. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med. 1995 May 1;181(5):1923–1927. doi: 10.1084/jem.181.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo M., Castaño L., Babu S., Buelow R., Kuo Y. L., Martin S., Martin A., Powers A. C., Prochazka M., Naggert J. Islet cell autoantigen 69 kD (ICA69). Molecular cloning and characterization of a novel diabetes-associated autoantigen. J Clin Invest. 1993 Jul;92(1):359–371. doi: 10.1172/JCI116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin D. U., Pleasic S. M., Palmer-Crocker R., Shapiro J. A. Cloning and expression of IDDM-specific human autoantigens. Diabetes. 1992 Feb;41(2):183–186. doi: 10.2337/diab.41.2.183. [DOI] [PubMed] [Google Scholar]
- Rabin D. U., Pleasic S. M., Shapiro J. A., Yoo-Warren H., Oles J., Hicks J. M., Goldstein D. E., Rae P. M. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol. 1994 Mar 15;152(6):3183–3188. [PubMed] [Google Scholar]
- Reetz A., Solimena M., Matteoli M., Folli F., Takei K., De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991 May;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich E. P., Sherwin R. S., Kanagawa O., Janeway C. A., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature. 1989 Sep 28;341(6240):326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- Roep B. O., Arden S. D., de Vries R. R., Hutton J. C. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature. 1990 Jun 14;345(6276):632–634. doi: 10.1038/345632a0. [DOI] [PubMed] [Google Scholar]
- Roep B. O., Kallan A. A., Duinkerken G., Arden S. D., Hutton J. C., Bruining G. J., de Vries R. R. T-cell reactivity to beta-cell membrane antigens associated with beta-cell destruction in IDDM. Diabetes. 1995 Mar;44(3):278–283. doi: 10.2337/diab.44.3.278. [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Hester L. D., Snyder S. H. Primary culture of neonatal rat olfactory neurons. J Neurosci. 1991 May;11(5):1243–1255. doi: 10.1523/JNEUROSCI.11-05-01243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Streuli M., Krueger N. X., Itoh M., Tsai A. Y. CD45 and a family of receptor-linked protein tyrosine phosphatases. Biochem Soc Trans. 1992 Feb;20(1):165–169. doi: 10.1042/bst0200165. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Chrétien M., Day R. The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie. 1994;76(3-4):197–209. doi: 10.1016/0300-9084(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Serra-Pages C., Saito H., Streuli M. Mutational analysis of proprotein processing, subunit association, and shedding of the LAR transmembrane protein tyrosine phosphatase. J Biol Chem. 1994 Sep 23;269(38):23632–23641. [PubMed] [Google Scholar]
- Sibley R. K., Sutherland D. E., Goetz F., Michael A. F. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985 Aug;53(2):132–144. [PubMed] [Google Scholar]
- Silsand T., Flatmark T. Purification of cytochrome b-561. An integral heme protein of the adrenal chromaffin granule membrane. Biochim Biophys Acta. 1974 Aug 8;359(2):257–266. [PubMed] [Google Scholar]
- Solimena M., De Camilli P. Diabetes. Spotlight on a neuronal enzyme. Nature. 1993 Nov 4;366(6450):15–17. doi: 10.1038/366015a0. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Ariniello P. D., Tang M., Munro J. M., Blattler W. A., Adler D. A., Disteche C. M., Saito H. Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region. EMBO J. 1992 Mar;11(3):897–907. doi: 10.1002/j.1460-2075.1992.tb05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C., Takei K., Marek K. L., Midyett K., Südhof T. C., De Camilli P., Jahn R. Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J Neurosci. 1993 Sep;13(9):3895–3903. doi: 10.1523/JNEUROSCI.13-09-03895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster P., Vanacore L., Nairn A. C., Marino C. R. Subcellular localization of CFTR to endosomes in a ductal epithelium. Am J Physiol. 1994 Aug;267(2 Pt 1):C340–C348. doi: 10.1152/ajpcell.1994.267.2.C340. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Xu T., Caron L. A., Fehon R. G., Artavanis-Tsakonas S. The involvement of the Notch locus in Drosophila oogenesis. Development. 1992 Aug;115(4):913–922. doi: 10.1242/dev.115.4.913. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Yasuda K. Monoclonal antibody to a common antigen of secretory granule membranes: intracellular localization and recycling of the antigen after secretion. J Histochem Cytochem. 1992 Jun;40(6):793–806. doi: 10.1177/40.6.1588026. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Y., Wang Y., Dixon J. E. Dissecting the catalytic mechanism of protein-tyrosine phosphatases. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1624–1627. doi: 10.1073/pnas.91.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]