Abstract
Historically, platelet transfusion has proven a reliable way to treat patients suffering from thrombocytopenia or similar ailments. An undersupply of donors, however, has demanded alternative platelet sources. Scientists have therefore sought to recapitulate the biological events that convert hematopoietic stem cells into platelets in the laboratory. Such platelets have shown good function and potential for treatment. Yet the number manufactured ex vivo falls well short of clinical application. Part of the reason is the remarkable gaps in our understanding of the molecular mechanisms driving platelet formation. Using several stem cell sources, scientists have progressively clarified the chemical signaling and physical microenvironment that optimize ex vivo platelets and reconstituted them in synthetic environments. Key advances in cell reprogramming and the ability to propagate self-renewal have extended the lifetime of megakaryocytes to increase the pool of platelet progenitors.
Keywords: bioreactors, blood platelets, induced pluripotent stem cells, megakaryocytes, polyploidy
Introduction
Platelets are best known for their role in wound repair, but have responsibility in several other functions, including innate immunity, vascular integrity, and neoangiogenesis. The human body has several hundred billion circulating platelets at any one time and recycles its entire platelet population every 10 days [1]. Because of its long history and success, platelet transfusion has remained the most popular way to provide platelets to patients suffering from thrombocytopenia and other ailments that require an external platelet source. However, because platelets must be preserved at room temperature, they risk bacterial contamination, which gives them a shelf life of only a few days. Thus, donors are continuously being sought to replenish a fragile supply that often sees unacceptable proportions go to waste [2]. Improved life spans and medical technologies have only intensified this demand at a rate which platelet donors have not kept up. Indeed, in some nations, it has been estimated that the donor population will underserve by 20% [3]. Thus, alternative sources for platelets have received attention, especially those that generate platelets ex vivo.
One example is hematopoietic stem cells (HSCs), which can be differentiated to megakaryocytes (MKs), the unipotent progenitor of all platelets, and acquired from umbilical cord blood (UCB). A typical platelet unit for transfusion will contain in the neighborhood of 500 billion platelets, and the average MK in the body produces 2000–5000 platelets, but a typical UCB unit only 1 million HSCs [4]. Therefore, assuming 1000 platelets can be gathered from a single MK in the laboratory, HSCs will have to be expanded at least 100 times. However, current ex vivo techniques generate fewer than 100 platelets per MK, meaning the expansion will have to be even greater [5].
To achieve these numbers, scientists have comprehensively investigated the chemical signaling and physical microenvironment that promote MK differentiation, maturation, and the release of platelets into the blood stream. Accordingly, a number of groups have designed bioreactors that recapitulate the microenvironment to promote these events. Complementing this strategy are cell reprogramming methods that take advantage of the limitless proliferation of stem cells to generate self-renewing MKs. In this review, we examine the biological steps considered essential to platelet generation and give attention to methods that promise to acquire sufficient ex vivo platelets for clinical application.
From megakaryocyte to platelet
Megakaryocytes
Platelets are the anucleated fragments of MKs. In the most accepted hierarchical model, HSCs, or CD34+ cells, take the MK lineage through a number of intermediates, with MK-erythroid precursor (MEP) being the penultimate stage [6]. Upon maturation, MKs extend proplatelets, which traverse into the sinusoidal vessels of the bone marrow where they are shred by blood flow into platelets [7]. By this point, the cells will have switched their distinctive markings from CD34+ to CD41a+CD42b+. Strategies for platelet generation ex vivo use this model as the paradigm. Yet as a testament on how much there is still to learn about thrombopoiesis, two recent studies have found that HSCs show surface markings that bias their fate to the MK lineage well before the MEP stage [8,9].
During the differentiation process, a HSC will enter the osteoblast niche and migrate to the perivascular niche to achieve full differentiation. Three transcription factors, GATA1, RUNX1, and NF-E2, are considered the primary determinants of whether MEP will take the MK lineage and proceed with this migration [10]. Thrombopoietin (TPO) is the primary cytokine responsible for the differentiation and binds to c-MPL receptors on CD34+ cells to lead them to the osteoblast niche [11]. The discovery of TPO is considered a cornerstone to our understanding of platelet generation. Indeed, a year after this discovery, the first report to describe in vitro platelet generation was published [12]. The elimination of either TPO or c-MPL receptors results in severe thrombocytopenia and reduces the number of MK progenitors and mature MKs [13]. Once in the osteoblast niche, CD34+ cells interact with collagen I via GPVI and α2β1 [14]. The stability of this niche depends on the protein-tyrosine phosphatases Shp1 and Shp2, which regulate the expression of GPVI and Mpl, respectively [15]. In addition to TPO, several studies have demonstrated that a minimal concoction for CD34+ expansion includes stem cell factor (SCF) and at least one other cytokine [5]. Notch signaling via activation by the Delta-1 ligand has been reported to increase expansion 100 times in vitro, and Stem Regenin 1, a purine derivative, has been found to have a significant positive effect on human CD34+ cells by blocking aryl hydrocarbon receptors [16,17]. More recent studies have found small molecules can have positive effects on CD34+ cell expansion using other mechanisms of action [18,19].
The maturation of MKs is marked by a massive increase in size, as these cells can reach up to 100 μm in diameter. The reason for the large size is that MKs switch from mitosis to endomitosis due to a failure in cytokinesis, which is due to an abnormal contractile ring caused by the absence of myosin II and regulation by RUNX1 [20,21]. While endomitosis explains how fully mature MKs can reach DNA content up to 128N, MKs have been observed to mature using either mitosis or endomitosis, which would explain the variable ploidy in blood and why less ploidy is sufficient for shedding platelets [22]. This increase in size leads to the invaginated membrane system (IMS), which acts as a membrane reservoir for proplatelets. Proplatelets enter the sinusoidal vessels of the bone marrow and are shred into platelets by blood flow at the very last stage of thrombopoiesis. Polyploidy is thought important for MKs to accumulate the cytoplasmic content that will eventually be used for platelet formation. Indeed, it has been argued that ploidy level correlates directly with the number of platelets [23]. Exceptions to this claim are known, however [24,25].
The regulation of the mitosis–endomitosis transition appears to involve multiple mechanisms. Gao et al. [26] showed that GEF-H1 and ECT2 must be downregulated for endomitosis, but that their downregulation occurs sequentially: GEF-H1 occurs in the 2N–4N transition, whereas ECT2 occurs thereafter. Whether these molecules make useful targets for increasing the ploidy of MKs in ex vivo platelets generation has not been explored.
As endomitosis proceeds, so too does the size of the IMS, which will eventually disperse throughout the MK. The forces required for the invaginations are initiated by phosphatidylinositol 4,5-bisphosphate, which activates the WASP–WAVE pathway, which in turn promotes actin assembly [27,28]. Interestingly, despite the ubiquity of the IMS in MKs, its beginnings are localized at one region of the surface membrane, which is marked by GPIb receptors [29]. Not coincidently, GPIb receptors are also markers for MK maturation and the transition to the perivascular niche by the cytokine SDF1 and its receptor CXCR4, which have been observed to accelerate the polyploidization of MKs [30,31]. The perivascular niche is made up of several extracellular matrix proteins including von Willebrand factor (vWF), fibrinogen, and fibronectin and is where MKs will begin to extend proplatelets using the organelles and granules accumulated by endomitosis. GPIb-IX-V is the receptor for vWF, a glycoprotein that facilitates platelet adhesion to the subendothelium. vWF appears to have an important role at the very end stages of platelet generation, as its absence has been associated with fewer platelets being shed from MKs [32,33]. Fibrinogen binds to αIIbβ3 upon MK maturation and can be used to promote proplatelets [34,35]. Finally, fibronectin promotes proplatelet formation by binding to the receptors VLA-4 and VLA-5 [36].
Proplatelets
Visually, proplatelets have numerous swellings that give them the shape of a chain of dumbbells, with each swelling containing the components necessary for a functional platelet. Once in the bloodstream, the proplatelet will be eviscerated at the center of these dumbbells, resulting in two approximately symmetric daughters that can continue to be eviscerated until platelet-sized entities emerge [37]. A single MK will continue to extend proplatelets until finally no more of its cell body remains and its remnants are degraded. It has even been observed that platelets themselves extend protrusions that can be further shed into structures that show surface markings indicative of normal platelets [38]. How the system knows when to end this reductionism is not clear.
Proplatelets are dynamic structures continuously changing their shape [37]. Cytoskeletal proteins are constantly transporting organelles and granules bidirectionally to the proplatelets, which are regularly branching to extend more surface area that will increase the number of platelets [39,40]. However, these dynamics are nothing but inefficient if they do not guide the proplatelet to the sinusoidal blood vessels. Using mutant mice, Zhang et al. [41] showed the important role of sphingosine 1 phosphate (S1P) and its receptor, S1pr1, on proplatelet formation and platelet shedding. Knockdown of S1pr1 led to a significant decrease in platelet count. Furthermore, the mechanisms leading to the last stages of platelet formation appeared unperturbed, as the number of MKs was unchanged, the establishment of the IMS was normal, and MKs retained their strong proximity to the sinusoidal vessels. Rather, what did appear disrupted was the proplatelet preference for the vessels, as the proplatelets protruded in random directions suggesting insensitivity to the S1P gradient between the lumen and blood.
Even if proplatelets are lured to the endothelial wall by the S1P gradient, they must still find a way to penetrate and enter the blood stream. Schachtner et al. [42] found that podosomes on the MK surface can degrade the extracellular matrix, which may provide the gateway for proplatelets to enter the vessels. Podosome activation depends on WASP signaling, as podosomes are actin-rich structures, which is consistent with the degradation being reduced by blebbistatin, a myosin II inhibitor. Interestingly, blebbistatin has been shown to promote platelet generation ex vivo [43,44].
Generating platelets in the laboratory
Immortalized MKs
Using the knowledge gained about the differentiation of MKs and the shedding of platelets, scientists have sought to induce CD34+ cells to the MK lineage. CD34+ cells can be acquired from several sources, including bone marrow, peripheral blood, or UCB. MKs from UCB are generally smaller in size and ploidy [45]. In addition, they shed significantly fewer platelets and show distinctive characteristics from MKs in adult blood [23,46]. Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) are immortal and in theory can provide a limitless number of CD34+ cells. Bluteau et al. [47] showed by global transcriptome analysis the transcription factor network and signaling pathways of megakaryopoiesis in hESCs are more similar to those in HSCs from neonatal blood than those from adult blood, suggesting studies using UCB can be extrapolated to hESCs and hiPSCs.
Gaur et al. [48] were the first to show how human hESCs can be differentiated into MKs by culturing them with OP-9 stromal cells. However, these MKs did not exceed a ploidy of 32N and the number generated per starting hESC was less than one. Lu et al. [49] were able to generate functional platelets that bound to fibrinogen and vWF starting with hESCs cultured in TPO, SCF, and IL-11. Several hESC lines were expanded approximately 100-fold into MKs, but only 15% of these MKs were CD41a+CD42b+. Vascular endothelial growth factor (VEGF) may be another molecule that facilitates expansion, as it was found to generate sac-like structures that seemed to harbor environments more suitable for obtaining a number of hematopoietic progenitors, including MK progenitors, and also maturing MKs that shed functional platelets [50]. Here too, however, the number of platelets is untenable for clinical purposes. Additionally, the platelets observed in these ex vivo studies were larger and more heterogeneous in size than those found in blood. One possible explanation is that the ex vivo generation was carried out in static culture, which did not mimic blood flow eviscerating proplatelets to proper size.
Because they appear to function equivalently, hiPSCs offer a preferred option to hESCs for several reasons, including the absence of ethical controversies and the ability to make any somatic cell in the body a potential source for MKs. One of the more important developments in thrombopoiesis using hiPSCs has been the reporting of immortalized MKs. These cells possess unlimited replication potential while still retaining unipotency, which mitigates the concern of contaminating cells. Nakamura et al. [51] have reported a way to extend the lifetime of hiPSC-derived MKs from 2 months to 5 months, a period that could potentially compensate for the low number of platelets shed by MKs ex vivo. Key to this accomplishment was the manipulation of c-Myc, a transcription factor stimulated by TPO and one of the four Yamanaka factors used to induce pluripotency in somatic cells [52,53]. Overexpression of c-Myc has been found to increase the proliferation of MKs in mice, but at the cost of decreased maturation and polyploidy [54]. Taking advantage of the aforementioned sac-like structures, Takayama et al. [55] found that c-Myc expression was accompanied by an increase in the expressions of p14 (ARF) and p16 (INK4A). Like c-Myc, the activation of these two apoptotic factors has been associated with an inability of MKs to mature and increase their ploidy, perhaps explaining the dysfunctional platelets seen by Nakamura et al. and the need to control the expression of these three factors temporally [56,57]. To resolve this problem, Nakamura et al. used a destabilization domain vector system to more precisely regulate c-Myc expression and incorporated BMI1, a polycomb complex component, to do the same with ARF and INK4A expression [58,59]. However, even then, MKs showed aberrant properties including normal CD41a+ levels but reduced CD42b+ levels due to the activation of caspases. This problem was resolved by adding BCL-XL [60], an anti-apoptosis agent that has been associated with platelet shedding, to the culture. Important was to add BCL-XL sequentially, two to three weeks after introducing c-Myc and BMI1, as mixing all simultaneously had no beneficial effect. The reduced expression of these factors was accompanied by an increase in the expressions of GATA1 and NF-E2. That c-Myc and other factors must be controlled in a relatively small time window suggests the signaling program for platelet generation is an ongoing dynamic process that can be easily perturbed. As an example of this sensitivity, another study has shown that c-Myc and BCL-XL can be used to tip MEP to erythrocyte lineage [61].
However, like others before it, immortalized MKs do not provide the requisite number of platelets, as each only sheds approximately 3–10 platelets [51]. Moreover, the platelets showed less robust response than fresh human platelets in vitro, even though they had all the surface receptor markings of proper function and responded to injury in a mouse model. One possible explanation is the lower ploidy of these MKs. Thus, one strategy could be culturing methods that increase the ploidy number and cell size by aggressive maturation. A very simple way to do this is culturing the cells with nicotinamide [62,63], although such treatment has controversial effects on proplatelet robustness and platelet function [64,65]. If increasing the size and ploidy of MKs does increase the number functional platelets, an ex vivo strategy for this purpose will likely require the identification of a new factor.
Nevertheless, a unique appeal of immortalized MKs is that they can be cryopreserved, which means they can be stored a much longer period than other derived MKs. This feature could mitigate the low platelet yield per MK, as it might be possible to store and access a larger number of MKs when required. This feature would have even more impact as the number of platelets generated per single immortalized MK is increased (Fig.1).
Fig. 1.
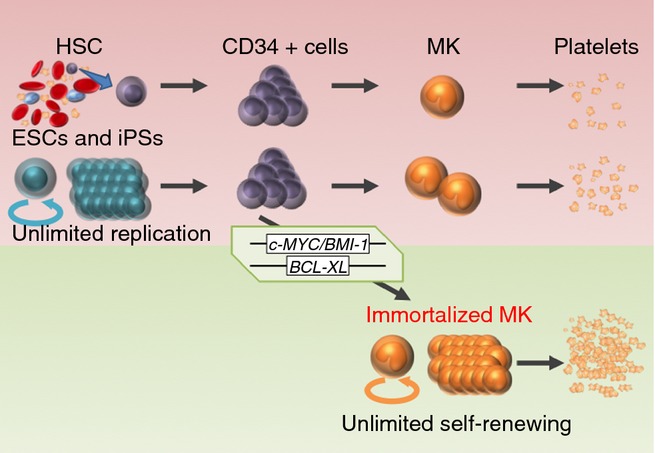
Ex vivo platelet generation has two major obstacles: the expansion of CD34+ cells into megakaryocytes (MKs) and the shedding of platelets from MKs. Prior to embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), hematopoietic stem cells (HSCs) were the only source of CD34+ cells. However, their expansion remains too low for clinical use. Regulating the activation of three transgenes, c-MYC, BMI-1, and BCL-XL, in iPSCs has led to the creation of immortalized MKs. These cells can be cryopreserved and have unlimited replication potential, which offers a solution to the first obstacle.
One caveat to cell reprogramming is the risk of tumors due to the viral transduction of the transcription factors. Platelets, being anucleate, can be irradiated to kill off any contaminating cells. However, it is still preferred to minimize this potential in every step of the differentiation protocol, which is why alternative reprogramming methods, such as transduction by small molecules are being explored [66]. Feng et al. [43] reported that the small molecule iBET151 could be used to inhibit c-Myc expression and still managed to generate immortalized MKs that shed functional platelets. Furthermore, they could improve the yield of CD41a+CD42b+ cells by mild hyperthermia, which has been observed to increase MK differentiation [67]. In addition, they were able to increase the percentage of CD41a+CD42b+ MKs by adding GM6001 or MMP8-I to the culture. These molecules inhibit metalloproteinases such as ADAM17, which are more active in older populations and shed key receptors from the MK surface to compromise platelet response to injury [68,69]. Yet despite these improvements, the platelet yield remains penury. Schlinker et al. [70] proposed a device that could separate platelets from immature MKs, with the latter then recycled in maturation medium to increase their ploidy and platelet yield. Another consideration has the MKs, not platelets, infused and shed platelets in the blood stream resulting in in vivo generation [71].
Recapitulating the microenvironment
The potential of immortal MKs may be enhanced with better recapitulation of the natural microenvironment in which platelets are shed. One very simple adjustment is switching the culture from two dimensions to three dimensions, which has been reported to increase the number of progenitors [72]. Furthermore, three dimensions make for more surface area, which could permit more proplatelets to engage with the endothelial wall and therefore enhance the number of platelets acquired. Sullenbarger et al. [73] prepared a 3D perfusion bioreactor using scaffolds made of polyester fabric or hydrogel. They showed that coating the scaffold with TPO or fibronectin increased the number of platelets. The platelets, however, were heterogeneous, with a number showing abnormal shape and size. Building on this system, Pallotta et al. [74] designed a bioreactor that included both the osteoblast and perivascular niches between which MKs could migrate by coating silk microtubes with the appropriate extracellular matrix proteins and growth factors. An important advantage of this system was the incorporation of fluorescence imaging to observe MK migration and platelet shedding live. The number of platelets per MK was 200, which is within a magnitude of blood, but only 7% of MKs extended proplatelets toward the pseudo-endothelial wall, suggesting ways that bias the proplatelets to the surface would be advantageous.
The osteoblast niche is a relatively hypoxic environment, a condition that is thought to preserve the pluripotent state and regulate hematopoiesis [75,76]. Therefore, bioreactors that are slightly hypoxic could help expand CD34+ cells. Switching the microenvironment from 5% oxygen at early-stage culturing to 20% at late stage was shown to promote residence in the osteoblast niche and platelet generation [77]. One theory for the stability is that hypoxia causes less expression of vascular cell adhesion molecule 1 and thus denies transition to the perivascular niche [78]. The osteoblast niche can also be stabilized using certain alkenes that enhance the expansion of CD34+ cells [79].
Shear stress is an important factor in platelet number. Thon et al. [80] have built a microfluidic bioreactor that along with considering bone marrow stiffness, the extracellular matrix composition, and other factors also incorporates shear stress. They found that the inclusion of shear stress resulted in much faster rates of MK activation, as proplatelets began emerging within seconds of trapping compared with the several hours seen in static conditions and grew at much faster rates, reaching velocities that were comparable with those observed in living mice. The shear stress in their system was generated by parallel flows running around the scaffolds. Nakagawa et al. [81] found, however, that a confluent system may be more effective, as they reported that flow intersecting at 60° achieves a 3.6-fold increase from a single-flow system. The reasons for this angle are unclear, but incorporating such a feature into future bioreactors should not be a major challenge.
The impact of flow may be further amplified by extracellular matrix proteins. Results from a mouse model suggest that vWF enhances the evisceration of proplatelets by blood flow [33]. Additionally, shear stress increases the expression of RUNX1 in CD41a+ cells, indicating that biomechanical forces can promote hematopoietic development [82]. Consistently, another biomechanical property, elasticity, was found to enhance the expansion of CD34+ cells [83].
Conclusions
A major challenge in the field of platelet research is generating the massive number of platelets needed for viable patient care. Despite advances in ex vivo techniques, there are still ways to go before seeing ex vivo platelets reach the clinic. Key advances in the field, however, are bringing this goal closer to reality. Bioreactors that better recapitulate the MK environment by including key extracellular matrix proteins and the effects of shear stress have shown that platelet totals can be increased by several factors. Perhaps more important is the invention of immortalized MKs. These cells can be maintained for several months, which reduces the storage concern of platelets. Introducing immortalized MKs into advanced bioreactors may contribute to the significant leap needed in ex vivo platelet generation.
Disclosure of Conflict of Interests
K. Eto has submitted patents related to reference 51 and 81.
References
- 1.Deutsch VR, Tomer A. Megakaryocyte development and platelet production. Br J Haematol. 2006;134:453–66. doi: 10.1111/j.1365-2141.2006.06215.x. [DOI] [PubMed] [Google Scholar]
- 2.Whitaker BI, Hinkins S. The 2011 National Blood Collection and Utilization Survey Report. Washington DC: The United States Department of Health and Human Services; 2011. [Google Scholar]
- 3.Japan Ministry of Health Law. Simulation of Future Number of Blood Donors. [Internet]. http://www.mhlw.go.jp/stf/shingi2/0000070616.html. Japanese. Accessed 23 January, 2015.
- 4.Avanzi MP, Mitchell WB. Ex vivo production of platelets from stem cells. Br J Haematol. 2014;165:237–47. doi: 10.1111/bjh.12764. [DOI] [PubMed] [Google Scholar]
- 5.Lee EJ, Godara P, Haylock D. Biomanufacture of human platelets for transfusion: Rationale and approaches. Exp Hematol. 2014;42:332–46. doi: 10.1016/j.exphem.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Tozawa K, Ono-Uruga Y, Matsubara Y. Megakaryopoiesis. Clin Exp Thromb Hemost. 2014;1:5. [Google Scholar]
- 7.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Jr, Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 8.Sanjuan-Pla A, Macaulay IC, Jensen CT, Woll PS, Luis TC, Mead A, Moore S, Carella C, Matsuoka S, Bouriez Jones T, Chowdhury O, Stenson L, Lutteropp M, Green JC, Facchini R, Boukarabila H, Grover A, Gambardella A, Thongjuea S, Carrelha J, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–6. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, Ema H, Nakauchi H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–26. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Takayama M, Fujita R, Suzuki M, Okuyama R, Aiba S, Motohashi H, Yamamoto M. Genetic analysis of hierarchical regulation for Gata1 and NF-E2 p45 gene expression in megakaryopoiesis. Mol Cell Biol. 2010;30:2668–80. doi: 10.1128/MCB.01304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, Forstrom JW, Buddle MM, Oort PJ, Hagen FS, Roth GJ, Papayannopoulou T, Foster DC. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–71. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 12.Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85:402–13. [PubMed] [Google Scholar]
- 13.Solar GP, Kerr WG, Zeigler FC, Hess D, Donahue C, de Sauvage FJ, Eaton DL. Role of c-mpl in early hematopoiesis. Blood. 1998;92:4–10. [PubMed] [Google Scholar]
- 14.Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P, Dominici M, Horwitz EM. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121:5238–49. doi: 10.1182/blood-2012-10-463414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazharian A, Mori J, Wang YJ, Heising S, Neel BG, Watson SP, Senis YA. Megakaryocyte-specific deletion of the protein-tyrosine phosphatases Shp1 and Shp2 causes abnormal megakaryocyte development, platelet production, and function. Blood. 2013;121:4205–20. doi: 10.1182/blood-2012-08-449272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–8. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, Csaszar E, Knapp DJ, Miller P, Ngom M, Imren S, Roy DC, Watts KL, Kiem HP, Herrington R, Iscove NN, Humphries RK, Eaves CJ, Cohen S, Marinier A, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345:1509–12. doi: 10.1126/science.1256337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmud N, Petro B, Baluchamy S, Li X, Taioli S, Lavelle D, Quigley JG, Suphangul M, Araki H. Differential effects of epigenetic modifiers on the expansion and maintenance of human cord blood stem/progenitor cells. Biol Blood Marrow Transplant. 2014;20:480–9. doi: 10.1016/j.bbmt.2013.12.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lordier L, Jalil A, Aurade F, Larbret F, Larghero J, Debili N, Vainchenker W, Chang Y. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood. 2008;112:3164–74. doi: 10.1182/blood-2008-03-144956. [DOI] [PubMed] [Google Scholar]
- 21.Lordier L, Bluteau D, Jalil A, Legrand C, Pan J, Rameau P, Jouni D, Bluteau O, Mercher T, Leon C, Gachet C, Debili N, Vainchenker W, Raslova H, Chang Y. RUNX1-induced silencing of non-muscle myosin heavy chain IIB contributes to megakaryocyte polyploidization. Nat Commun. 2012;3:717. doi: 10.1038/ncomms1704. [DOI] [PubMed] [Google Scholar]
- 22.Leysi-Derilou Y, Robert A, Duchesne C, Garnier A, Boyer L, Pineault N. Polyploid megakaryocytes can complete cytokinesis. Cell Cycle. 2010;9:2589–99. doi: 10.4161/cc.9.13.12078. [DOI] [PubMed] [Google Scholar]
- 23.Mattia G, Vulcano F, Milazzo L, Barca A, Macioce G, Giampaolo A, Hassan HJ. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–97. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- 24.Machlus KR, Italiano JE., Jr The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–96. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sola-Visner MC, Christensen RD, Hutson AD, Rimsza LM. Megakaryocyte size and concentration in the bone marrow of thrombocytopenic and nonthrombocytopenic neonates. Pediatr Res. 2007;61:479–84. doi: 10.1203/pdr.0b013e3180332c18. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Smith E, Ker E, Campbell P, Cheng EC, Zou S, Lin S, Wang L, Halene S, Krause DS. Role of RhoA-specific guanine exchange factors in regulation of endomitosis in megakaryocytes. Dev Cell. 2012;22:573–84. doi: 10.1016/j.devcel.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze H, Korpal M, Hurov J, Kim SW, Zhang J, Cantley LC, Graf T, Shivdasani RA. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107:3868–75. doi: 10.1182/blood-2005-07-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eto K, Nishikii H, Ogaeri T, Suetsugu S, Kamiya A, Kobayashi T, Yamazaki D, Oda A, Takenawa T, Nakauchi H. The WAVE2/Abi1 complex differentially regulates megakaryocyte development and spreading: implications for platelet biogenesis and spreading machinery. Blood. 2007;110:3637–47. doi: 10.1182/blood-2007-04-085860. [DOI] [PubMed] [Google Scholar]
- 29.Eckly A, Heijnen H, Pertuy F, Geerts W, Proamer F, Rinckel JY, Leon C, Lanza F, Gachet C. Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood. 2014;123:921–30. doi: 10.1182/blood-2013-03-492330. [DOI] [PubMed] [Google Scholar]
- 30.Guerriero R, Mattia G, Testa U, Chelucci C, Macioce G, Casella I, Samoggia P, Peschle C, Hassan HJ. Stromal cell-derived factor 1alpha increases polyploidization of megakaryocytes generated by human hematopoietic progenitor cells. Blood. 2001;97:2587–95. doi: 10.1182/blood.v97.9.2587. [DOI] [PubMed] [Google Scholar]
- 31.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 32.Balduini A, Pallotta I, Malara A, Lova P, Pecci A, Viarengo G, Balduini CL, Torti M. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6:1900–7. doi: 10.1111/j.1538-7836.2008.03132.x. [DOI] [PubMed] [Google Scholar]
- 33.Poirault-Chassac S, Nguyen KA, Pietrzyk A, Casari C, Veyradier A, Denis CV, Baruch D. Terminal platelet production is regulated by von Willebrand factor. PLoS ONE. 2013;8:e63810. doi: 10.1371/journal.pone.0063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eto K, Murphy R, Kerrigan SW, Bertoni A, Stuhlmann H, Nakano T, Leavitt AD, Shattil SJ. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A. 2002;99:12819–24. doi: 10.1073/pnas.202380099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin alpha IIb beta3. Blood. 2006;108:1509–14. doi: 10.1182/blood-2005-11-011957. [DOI] [PubMed] [Google Scholar]
- 36.Matsunaga T, Fukai F, Kameda T, Shide K, Shimoda H, Torii E, Kamiunten A, Sekine M, Yamamoto S, Hidaka T, Kubuki Y, Yokokura S, Uemura M, Matsuoka A, Waki F, Matsumoto K, Kanaji N, Ishii T, Imataki O, Dobashi H, et al. Potentiated activation of VLA-4 and VLA-5 accelerates proplatelet-like formation. Ann Hematol. 2012;91:1633–43. doi: 10.1007/s00277-012-1498-y. [DOI] [PubMed] [Google Scholar]
- 37.Thon JN, Montalvo A, Patel-Hett S, Devine MT, Richardson JL, Ehrlicher A, Larson MK, Hoffmeister K, Hartwig JH, Italiano JE., Jr Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191:861–74. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwertz H, Koster S, Kahr WH, Michetti N, Kraemer BF, Weitz DA, Blaylock RC, Kraiss LW, Greinacher A, Zimmerman GA, Weyrich AS. Anucleate platelets generate progeny. Blood. 2010;115:3801–9. doi: 10.1182/blood-2009-08-239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson JL, Shivdasani RA, Boers C, Hartwig JH, Italiano JE., Jr Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood. 2005;106:4066–75. doi: 10.1182/blood-2005-06-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–54. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, Schulz C, von Bruhl ML, Tirniceriu A, Gaertner F, Proia RL, Graf T, Bolz SS, Montanez E, Prinz M, Muller A, von Baumgarten L, Billich A, Sixt M, Fassler R, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165–81. doi: 10.1084/jem.20121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schachtner H, Calaminus SD, Sinclair A, Monypenny J, Blundell MP, Leon C, Holyoake TL, Thrasher AJ, Michie AM, Vukovic M, Gachet C, Jones GE, Thomas SG, Watson SP, Machesky LM. Megakaryocytes assemble podosomes that degrade matrix and protrude through basement membrane. Blood. 2013;121:2542–52. doi: 10.1182/blood-2012-07-443457. [DOI] [PubMed] [Google Scholar]
- 43.Feng Q, Shabrani N, Thon JN, Huo H, Thiel A, Machlus KR, Kim K, Brooks J, Li F, Luo C, Kimbrel EA, Wang J, Kim KS, Italiano J, Cho J, Lu SJ, Lanza R. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Reports. 2014;3:817–31. doi: 10.1016/j.stemcr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin JW, Swift J, Spinler KR, Discher DE. Myosin-II inhibition and soft 2D matrix maximize multinucleation and cellular projections typical of platelet-producing megakaryocytes. Proc Natl Acad Sci U S A. 2011;108:11458–63. doi: 10.1073/pnas.1017474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazaki R, Ogata H, Iguchi T, Sogo S, Kushida T, Ito T, Inaba M, Ikehara S, Kobayashi Y. Comparative analyses of megakaryocytes derived from cord blood and bone marrow. Br J Haematol. 2000;108:602–9. doi: 10.1046/j.1365-2141.2000.01854.x. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs DA, McGinn SG, Cantu CL, Klein RR, Sola-Visner MC, Rimsza LM. Developmental differences in megakaryocyte size in infants and children. Am J Clin Pathol. 2012;138:140–5. doi: 10.1309/AJCP4EMTJYA0VGYE. [DOI] [PubMed] [Google Scholar]
- 47.Bluteau O, Langlois T, Rivera-Munoz P, Favale F, Rameau P, Meurice G, Dessen P, Solary E, Raslova H, Mercher T, Debili N, Vainchenker W. Developmental changes in human megakaryopoiesis. J Thromb Haemost. 2013;11:1730–41. doi: 10.1111/jth.12326. [DOI] [PubMed] [Google Scholar]
- 48.Gaur M, Kamata T, Wang S, Moran B, Shattil SJ, Leavitt AD. Megakaryocytes derived from human embryonic stem cells: a genetically tractable system to study megakaryocytopoiesis and integrin function. J Thromb Haemost. 2006;4:436–42. doi: 10.1111/j.1538-7836.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 49.Lu SJ, Li F, Yin H, Feng Q, Kimbrel EA, Hahm E, Thon JN, Wang W, Italiano JE, Cho J, Lanza R. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res. 2011;21:530–45. doi: 10.1038/cr.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takayama N, Nishikii H, Usui J, Tsukui H, Sawaguchi A, Hiroyama T, Eto K, Nakauchi H. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, Fujita K, Koike T, Harimoto K, Dohda T, Watanabe A, Okita K, Takahashi N, Sawaguchi A, Yamanaka S, Nakauchi H, Nishimura S, Eto K. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014;14:535–48. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Chanprasert S, Geddis AE, Barroga C, Fox NE, Kaushansky K. Thrombopoietin (TPO) induces c-myc expression through a PI3K- and MAPK-dependent pathway that is not mediated by Akt, PKCzeta or mTOR in TPO-dependent cell lines and primary megakaryocytes. Cell Signal. 2006;18:1212–8. doi: 10.1016/j.cellsig.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Thompson A, Zhang Y, Kamen D, Jackson CW, Cardiff RD, Ravid K. Deregulated expression of c-myc in megakaryocytes of transgenic mice increases megakaryopoiesis and decreases polyploidization. J Biol Chem. 1996;271:22976–82. doi: 10.1074/jbc.271.38.22976. [DOI] [PubMed] [Google Scholar]
- 55.Takayama N, Nishimura S, Nakamura S, Shimizu T, Ohnishi R, Endo H, Yamaguchi T, Otsu M, Nishimura K, Nakanishi M, Sawaguchi A, Nagai R, Takahashi K, Yamanaka S, Nakauchi H, Eto K. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207:2817–30. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo Y, Niu C, Breslin P, Tang M, Zhang S, Wei W, Kini AR, Paner GP, Alkan S, Morris SW, Diaz M, Stiff PJ, Zhang J. c-Myc-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Blood. 2009;114:2097–106. doi: 10.1182/blood-2009-01-197947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takayama N, Eto K. Pluripotent stem cells reveal the developmental biology of human megakaryocytes and provide a source of platelets for clinical application. Cell Mol Life Sci. 2012;69:3419–28. doi: 10.1007/s00018-012-0995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oguro H, Iwama A, Morita Y, Kamijo T, van Lohuizen M, Nakauchi H. Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice. J Exp Med. 2006;203:2247–53. doi: 10.1084/jem.20052477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Josefsson EC, James C, Henley KJ, Debrincat MA, Rogers KL, Dowling MR, White MJ, Kruse EA, Lane RM, Ellis S, Nurden P, Mason KD, O'Reilly LA, Roberts AW, Metcalf D, Huang DC, Kile BT. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J Exp Med. 2011;208:2017–31. doi: 10.1084/jem.20110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirose S, Takayama N, Nakamura S, Nagasawa K, Ochi K, Hirata S, Yamazaki S, Yamaguchi T, Otsu M, Sano S, Takahashi N, Sawaguchi A, Ito M, Kato T, Nakauchi H, Eto K. Immortalization of erythroblasts by c-MYC and BCL-XL enables large-scale erythrocyte production from human pluripotent stem cells. Stem Cell Reports. 2013;1:499–508. doi: 10.1016/j.stemcr.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avanzi MP, Goldberg F, Davila J, Langhi D, Chiattone C, Mitchell WB. Rho kinase inhibition drives megakaryocyte polyploidization and proplatelet formation through MYC and NFE2 downregulation. Br J Haematol. 2014;164:867–76. doi: 10.1111/bjh.12709. [DOI] [PubMed] [Google Scholar]
- 63.Giammona LM, Panuganti S, Kemper JM, Apostolidis PA, Lindsey S, Papoutsakis ET, Miller WM. Mechanistic studies on the effects of nicotinamide on megakaryocytic polyploidization and the roles of NAD+ levels and SIRT inhibition. Exp Hematol. 2009;37:1340–52.e3. doi: 10.1016/j.exphem.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leysi-Derilou Y, Duchesne C, Garnier A, Pineault N. Single-cell level analysis of megakaryocyte growth and development. Differentiation. 2012;83:200–9. doi: 10.1016/j.diff.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Giammona LM, Fuhrken PG, Papoutsakis ET, Miller WM. Nicotinamide (vitamin B3) increases the polyploidisation and proplatelet formation of cultured primary human megakaryocytes. Br J Haematol. 2006;135:554–66. doi: 10.1111/j.1365-2141.2006.06341.x. [DOI] [PubMed] [Google Scholar]
- 66.Karagiannis P, Yamanaka S. The fate of cell reprogramming. Nat Methods. 2014;11:1006–8. doi: 10.1038/nmeth.3109. [DOI] [PubMed] [Google Scholar]
- 67.Pineault N, Boucher JF, Cayer MP, Palmqvist L, Boyer L, Lemieux R, Proulx C. Characterization of the effects and potential mechanisms leading to increased megakaryocytic differentiation under mild hyperthermia. Stem Cells Dev. 2008;17:483–93. doi: 10.1089/scd.2007.0149. [DOI] [PubMed] [Google Scholar]
- 68.Bergmeier W, Piffath CL, Cheng G, Dole VS, Zhang Y, von Andrian UH, Wagner DD. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates GPIbalpha shedding from platelets in vitro and in vivo. Circ Res. 2004;95:677–83. doi: 10.1161/01.RES.0000143899.73453.11. [DOI] [PubMed] [Google Scholar]
- 69.Nishikii H, Eto K, Tamura N, Hattori K, Heissig B, Kanaji T, Sawaguchi A, Goto S, Ware J, Nakauchi H. Metalloproteinase regulation improves in vitro generation of efficacious platelets from mouse embryonic stem cells. J Exp Med. 2008;205:1917–27. doi: 10.1084/jem.20071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlinker AC, Radwanski K, Wegener C, Min K, Miller WM. Separation of in-vitro-derived megakaryocytes and platelets using spinning-membrane filtration. Biotechnol Bioeng. 2015;112:788–800. doi: 10.1002/bit.25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuentes R, Wang Y, Hirsch J, Wang C, Rauova L, Worthen GS, Kowalska MA, Poncz M. Infusion of mature megakaryocytes into mice yields functional platelets. J Clin Invest. 2010;120:3917–22. doi: 10.1172/JCI43326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Ma T, Kniss DA, Yang ST, Lasky LC. Human cord cell hematopoiesis in three-dimensional nonwoven fibrous matrices: in vitro simulation of the marrow microenvironment. J Hematother Stem Cell Res. 2001;10:355–68. doi: 10.1089/152581601750288966. [DOI] [PubMed] [Google Scholar]
- 73.Sullenbarger B, Bahng JH, Gruner R, Kotov N, Lasky LC. Prolonged continuous in vitro human platelet production using three-dimensional scaffolds. Exp Hematol. 2009;37:101–10. doi: 10.1016/j.exphem.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pallotta I, Lovett M, Kaplan DL, Balduini A. Three-dimensional system for the in vitro study of megakaryocytes and functional platelet production using silk-based vascular tubes. Tissue Eng Part C Methods. 2011;17:1223–32. doi: 10.1089/ten.tec.2011.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 76.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Lasky LC, Sullenbarger B. Manipulation of oxygenation and flow-induced shear stress can increase the in vitro yield of platelets from cord blood. Tissue Eng Part C Methods. 2011;17:1081–8. doi: 10.1089/ten.tec.2011.0108. [DOI] [PubMed] [Google Scholar]
- 78.Pallotta I, Lovett M, Rice W, Kaplan DL, Balduini A. Bone marrow osteoblastic niche: a new model to study physiological regulation of megakaryopoiesis. PLoS ONE. 2009;4:e8359. doi: 10.1371/journal.pone.0008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chua KN, Chai C, Lee PC, Ramakrishna S, Leong KW, Mao HQ. Functional nanofiber scaffolds with different spacers modulate adhesion and expansion of cryopreserved umbilical cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2007;35:771–81. doi: 10.1016/j.exphem.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, Feng Q, Lu S, Lanza R, Neeves KB, Weitz DA, Italiano JE., Jr Platelet bioreactor-on-a-chip. Blood. 2014;124:1857–67. doi: 10.1182/blood-2014-05-574913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakagawa Y, Nakamura S, Nakajima M, Endo H, Dohda T, Takayama N, Nakauchi H, Arai F, Fukuda T, Eto K. Two differential flows in a bioreactor promoted platelet generation from human pluripotent stem cell-derived megakaryocytes. Exp Hematol. 2013;41:742–8. doi: 10.1016/j.exphem.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, Garcia-Cardena G, Daley GQ. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–5. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, Rasko JE. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28:1123–8. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]


