Abstract
It has been suggested that leisure activity and physical exercise can be a protective factor for neuropsychological functions and are associated with a reduced risk of dementia. Thus, the purpose of this study was to investigate the influence of physical exercise and leisure on the neuropsychological functions of healthy older adults. The sample was composed of 51 sedentary female volunteers who were 60–70 years old and were distributed into three groups: A—control, B—leisure, and C—training. Volunteers were submitted to a physical and neuropsychological assessment at baseline and after 6 months. Groups A and B were monitored longitudinally three times a week. Group C improved their neuropsychological functioning and oxygen consumption compared to groups A and B (p = <0.05). The neuropsychological functions of groups A and B were significantly worse after 6 months of monitoring (p = <0.05). The data suggest that physical exercise improves neuropsychological functioning, although leisure activities may also improve this functioning. Thus, an aerobic physical fitness program can partially serve as a non-medication alternative for maintaining and improving these functions in older adults; however, leisure activities should also be considered.
Keywords: Physical exercise, Neuropsychological functions, Leisure activity, Older adults
Introduction
It has been demonstrated that the aging process is accompanied by a decrease in certain neuropsychological functions, such as memory, attention, perception, decision-making, task switching, multitasking, and the speed of information processing (Park et al. 2001; Hedden and Gabrieli 2004).
However, a growing body of literature suggests that age-related declines in neuropsychological aspects can be reduced by using alternative interventions, such as physical exercise (Etnier et al. 2006; Colcombe and Kramer 2003; Kravitz et al. 2012). Furthermore, physical exercise reduces the risk of diseases that impair neuropsychological functions, such as hypertension, diabetes, and cardiovascular disease (Anstey and Christensen 2000; Lacza and Radák 2013).
Several studies have shown that regular physical activity can lead to the enhancement of neuropsychological functions, demonstrating an association between a greater aerobic capacity and improvements in these functions (Angevaren et al. 2007; Langlois et al. 2013; Klusmann et al. 2010; Sofi et al. 2011; Hamer and Chida 2009). Nevertheless, there are other studies that have not found a significant association between neuropsychological functions and physical activity (Denkinger et al. 2012)
Despite the controversies, epidemiological studies suggest that moderately active individuals are at a reduced risk of suffering from mental disorders compared to sedentary individuals; thus, physical exercise provides both physical and neuropsychological benefits (Vaughan et al. 2012), and physically active individuals most likely have faster cognitive processing speeds (Langlois et al. 2013)
However, it is unclear which activities are the most important for neuropsychological maintenance; beyond physical exercise, significant effects have been reported for intellectual stimulation (Hultsch et al. 1999), social engagement, and leisure activities (Wang et al. 2013). Specifically, leisure activity can be a protective factor for neuropsychological functions and is associated with a reduced risk of dementia; this finding is gaining attention because of its possible relationship to physical fitness (Akbaraly et al. 2009). However, controlled trials are required to assess the protective effects of cognitive leisure activities on the risk of dementia (Verghese, et al. 2003). Leisure time physical activities include all muscular activities and may or may not include regular exercise training that is aimed at improving physical fitness (Yu et al. 2000).
Furthermore, it is important to note that many leisure activities that do not include physical activity are gaining importance because they are complex activities on a cognitive level and they include multisensory integration. There are many training regimes that induce improvements in cognitive performance and brain plasticity and do not include physical exercise (Scarmeas et al. 2001; Green and Bavelier 2008; Seinfeld et al. 2013). Therefore, it is believed that both leisure activities and physical exercise are effective for improving the neuropsychological functioning of older adults. Thus, the aim of this study was to investigate the influence of physical exercise and leisure on the neuropsychological functioning of older adults.
Methods
Participants
The leisure group was a convenience sample, and the control and training groups were randomized and consisted of 51 female sedentary volunteers whose demographic data, presented as the mean ± (SD), were as follows: age (years), 64.65 ± (3.56); height (m), 1.68 ± (0.6); weight (kg), 76.3 ± (11.4); body mass index (BMI) (kg/m2), 26.9 ± (3.3); and schooling (years), 8 ± (1.0). According to their self-reported data, no volunteers engaged in alcohol use or illicit drug use or used medications that affect neuropsychological functioning; additionally, no participants had undergone recent surgical intervention, were less than 60 or over 70 years old, or had a low level of schooling. The Mini Mental State Exam (MMSE) was used as a screening tool to exclude subjects with possible mild cognitive impairments or dementia (Folstein et al. 1975).
All the volunteers were distributed into three groups: group A: “control,” which was composed of 17 volunteers (64.82 ± 3.52 years), group B: “leisure,” which was composed of 11 volunteers (65.20 ± 4.04 years), and group C: “training,” which was composed of 23 volunteers (64.04 ± 3.19 years).
Experimental design
Initially, the volunteers were submitted to neuropsychological and physical fitness tests and were reassessed after 6 months using the same initial procedure. This period of 6 months was viewed as being the most appropriate to obtain some physiological response from the volunteers and to simultaneously avoid a learning effect in the neuropsychological testing procedures. The examiners were blinded to the study allocation when collecting the data.
Group A consisted of 17 sedentary volunteers (based on evaluating their aerobic capacity). This group was asked not to vary their everyday activities or to join a new physical fitness program. The volunteers were monitored longitudinally through monthly phone calls to maintain contact and keep the volunteers informed of the progress of the study. They were also informed that although they would not be participating in the fitness program, they could participate in it after the intervention period of the experimental group.
Group B was composed of 11 older adult volunteers. This group participated in a leisure and recreational group (already constituted) twice a week on alternate days. Their activities included dancing and recreational and handcraft activities. This group was also monitored longitudinally with the same purpose as the control group.
Group C was composed of 23 sedentary volunteers (based on evaluating their aerobic capacity) who started a physical fitness program that was centered on aerobic metabolism. The program was composed of 60-min sessions (initially the duration was 20 min, but this amount was gradually increased to a maximum of 60 min) as prescribed after the prior evaluation; this program was conducted three times a week on alternate days for 6 months. The volume and intensity of the physical exercise were prescribed on the basis of the evaluations of aerobic capacity, with estimates of ventilatory threshold 1 (VT-1) and threshold heart frequency. The women participated in stretching and joint flexibility exercises as a supplementary activity. The training sessions were held at the same time of day, and the recommendations were followed by the ACSM (2006).
Prior to participating in this study, all the possible risks that were involved in the experimental procedure were explained to the volunteers, and they participated voluntarily. The study was approved by the Ethics in Research Committee of the Federal University of Uberlândia, Minas Gerais (04/98).
Physical assessment
Prior to the procedure, a medical evaluation including electrocardiograms at rest and with effort was conducted to assess cardiovascular parameters. An assessment of physical performance was conducted with a treadmill stress test while using a modified Bruce protocol (begin with 3 km/h, increase speed by 1.4 km/h after 3 min, and thereafter increase in elevation by 3 % at a constant speed) under continuous ECG monitoring and the assessment of oxygen uptake (ACSM 1995).
Neuropsychological assessment and depressive symptomatology
All the neuropsychological and mood state assessments were administered in a single session, and none lasted for more than an hour and a half. The data were collected by one examiner who was a trained neuropsychologist. All the volunteers were evaluated in the morning (from 8 to 11 a.m.) by the same examiner.
The Geriatric Depression Scale (GDS) is a self-reported scale that evaluates the symptomatology of depression among elderly individuals. The version in this study contained 30 items in the form of yes/no responses and was designed for self-administration (Yesavage et al. 1983).
Digit span numbers were used to evaluate the sustained attention, immediate memory, and verbal working memory of the volunteers and were composed of two stages. In the first stage, the individual was instructed to repeat a sequence of numbers (direct digits), and in the second stage, the individual was required to listen to a sequence of numbers and repeat them in reverse order (inverse digits) (Wechsler 1997). Low values indicated a worsening of attention and mental control.
Logical memory (WMS-III) was used to evaluate episodic memory, verbal memory, and short- and long-term memory. This evaluation was composed of two stories (story A and story B), each of which comprised only a few sentences. After reading each of the stories, the subject was asked to recall as many details as he or she could remember. The examiner marked each of the individual details that the subject spontaneously produced (as per the scoring criteria). Then, after a delay (approximately 30 min that was filled with other unrelated cognitive tests), the participant was asked again to recall each story from memory but without an additional reading or prompting (Wechsler 1997).
A block design was used to evaluate the spatial/visual orientation, speed of execution, perceptual integration, and capacity of planning and organization. The examiners presented the volunteers with a series of nine geometric designs that they reproduced one at a time with a set of red and white blocks. The latter presented subjects with one of three uppercase letters (F, L, or R) that were rotated from 0 to 180° in its either normal or mirror image (i.e., backward) orientation (Wechsler 1981).
The Wisconsin Card Sorting Test (WCST-64) was used to evaluate executive functioning and included four stimulation cards and 64 response cards. Each 8 cm × 8 cm card contained one to four figures, such as a triangle, star, square, and circle, which were colored red, green, yellow, and blue, respectively. The subjects were told only “correct” or “mistake” when they sorted the cards without knowing the principle of classification, and the principle would change after they had eight consecutive corrects. The order of the principles was color-shape-number-color, etc. In this study, we measured the number of correct responses (Spreen and Strauss 1991; Lesak 1995).
Digit symbols were used to evaluate the sustained attention, response speed, and visuomotor coordination. The participant was given a key grid of numbers and matching symbols and a test section with numbers and empty boxes. The test constituted filling in as many empty boxes as possible with a symbol that matched each number. Ninety seconds were given to complete the task. The score was computed as the number of correctly substituted symbols within the 90-s time limit (Wechsler 1981).
Mental control was evaluated by the retrieval of over-learned information and the ability to mentally manipulate information. In addition to the three tasks that comprised the mental control subtest, i.e., counting backward from 20 to 1, reciting the alphabet, and adding serial 3’s, the subtest included four additional tasks: reciting the months of the year forward and backward, an alphabet rhyming task that required the patients to identify letters that rhymed with the word “key,” and an alphabet visualization task that required the volunteers to provide all of the block printed letters that contained curved lines (Wechsler 1997).
The Rey-Osterrieth Complex Figure (ROCF) test was used to evaluate visuospatial constructional ability, visual memory, and learning. The ROCF was administered to all subjects. It consisted of copy, immediate recall, and a 20-min delayed recall of a complex figure. The test included immediate and delayed recalls of a visual stimulus (Spreen and Strauss 1991; Lesak 1995).
The Toulouse-Pieron test was performed to evaluate concentrated attention. This test evaluated the concentrated attention, reaction speed, and accuracy in executing simple tasks. This tool assisted in the investigation of three sectors that are related to attention: indices of correct response, no response + wrong response in executing the test, and the time taken to complete the test (Rainho 1992).
The MMSE was used to evaluate global cognitive functioning, including orientation, immediate memory, attention, calculation, evocation, and language. This assessment was used at baseline (Folstein et al. 1975).
Letter fluency was used to evaluate verbal fluency. The participants were given 60 s each to generate words that began with a specified letter (FAS), excluding proper nouns, numbers, and multiple forms of the same word. We considered the total number of words that were generated as the score of this test. The number of correct responses was summed across the three letters (Lesak 1995).
Raven’s progressive matrices (RPM) test was used to evaluate non-verbal intelligence, visuospatial reasoning, abstract thinking, deductive reasoning, and general intelligence. The RPM is composed of 60 problems that are divided into five subsets of 12. Each person’s total score was the total number that he/she answered correctly. In RPM, a person was shown a matrix of patterns with one pattern missing. The person must discover the rules that govern the patterns and then use those rules to choose the item that best fills in the missing pattern (Raven 1958).
Data analysis
The data are described as the mean ± standard deviation (SD). To assess the differences between the groups, we used a repeated measures ANOVA test followed by a post hoc Tukey test. To perform a comparison between genders, we used Student’s t test for independent samples. Pearson correlation coefficients were calculated between the scores for VO2 max and neuropsychological functions in all groups (control, leisure, and training). The significance level was p ≤ 0.05. The statistical analyses were performed using Statistica 12 software (http://www.statsoft.com).
Results
Over 75 % of the volunteers participated in the training session, and none of the volunteers dropped out. Table 1 shows the means of the control vs training, training vs leisure, and control vs leisure groups, respectively. The scores in the training group were significantly lower in the following tests: in the GDS test, which assessed symptoms of depression (p = 0.01); in the mental control test, which assessed the retrieval of over-learned information and the ability to mentally manipulate information, a low number of correct responses (p = 0.002) and errors (p = 0.04) were observed; in the digit span numbers, which assessed higher levels of sustained attention, immediate memory, and verbal working memory, higher scores were observed for the digit forward (p = 0.0001) and digit backwards (p = 0.00002) tests; in the immediate recall of ROCF test, which assessed visuospatial constructional ability, visual memory and learning (p = 0.01), and delayed recall (p = 0.03); in logical memory, significantly higher levels of episodic memory and verbal, short-, and long-term memory were observed in the immediate recall (p = 0.0005) and delayed recall (p = 0.0003) tests; in the WCST, a significantly higher number of correct responses were observed regarding executive functions (p = 0.0003), and there were also a significantly different number of cancellations (p = 0.0001) and a low number of errors (p = 0.002) in Toulouse-Pieron test, which assessed concentrated attention, reaction speed, and accuracy in executing simple tasks compared to the control group.
Table 1.
Neuropsychological functions and depressive symptoms pre- and post-evaluation
| Measures | Control (N = 17) | Training (N = 23) | Leisure (N = 11) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After 6 months | Baseline | After 6 months | Baseline | After 6 months | ||
| Letter fluency | 21.1 ± 7.5 | 23.2 ± 6.3 | 27.0 ± 4.6 | 26.21 ± 5.4 | 21.9 ± 4.7 | 25.7 ± 8.3d | |
| GDS | 13.3 ± 4.6 | 14.0 ± 3.9a | 10.1 ± 4.2 | 7.6 ± 1.1c | 10.5 ± 2.8 | 10.6 ± 4.0 | |
| Block design | 12.4 ± 4.3 | 11.4 ± 5.1 | 13.6 ± 6.3 | 15.5 ± 4.7c | 14.7 ± 5.1 | 15.1 ± 3.8 | |
| Mental control | Correct responses | 3.7 ± 1.1 | 3.4 ± 0.9ab | 3.8 ± 1.1 | 4.1 ± 1.0b | 4.7 ± 1.8 | 7.8 ± 1.2d |
| Errors | 6.4 ± 2.6 | 7.4 ± 2.2a | 5.0 ± 1.3 | 3.9 ± 1.8c | 5.2 ± 1.8 | 54 ± 2.1 | |
| Digit span | Forward | 3.1 ± 0.5 | 3.8 ± 0.4ab | 4.7 ± 1.2 | 4.9 ± 1.1 | 4.8 ± 0.9 | 4.6 ± 0.8 |
| Backwards | 2.1 ± 0.3 | 2.0 ± 0.6 | 3.3 ± 0.4 | 3.6 ± 0.5 | 3.4 ± 0.6 | 3.2 ± 0.5 | |
| Digit symbol | Correct responses | 11.1 ± 4.0 | 11.2 ± 2.7 | 15.7 ± 5.6 | 17.1 ± 4.7 | 15.9 ± 3.3 | 14.2 ± 5.5 |
| Errors | 1.4 ± 0.2 | 2.0 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.3 | 1.4 ± 0.1 | 1.2 ± 0.4 | |
| MMSE | 25.0 ± 3.95 | 25.4 ± 3.8 | 25.1 ± 3.8 | 25.8 ± 3.1 | 22.5 ± 2.7 | 22.4 ± 2.0 | |
| MMSE drawing | 1.7 ± 0.3 | 1.8 ± 0.3 | 1.8 ± 0.2 | 1.7 ± 0.4 | 1.8 ± 0.2 | 1.7 ± 0.2 | |
| ROCF | Copy | 17.9 ± 8.2 | 18.8 ± 6.7 | 24.0 ± 8.3 | 24.9 ± 6.0 | 23.1 ± 5.3 | 21.5 ± 2.3 |
| Immediate recall | 6.7 ± 1.6 | 7.2 ± 1.1a | 14.1 ± 5.4 | 15.1 ± 3.1 | 7.3 ± 1.3 | 13.4 ± 3.2d | |
| Delayed recall | 6.6 ± 2.2 | 5.5 ± 2.8a | 9.8 ± 3.3 | 10.1 ± 3.5 | 8.8 ± 2.8 | 7.0 ± 2.4 | |
| Raven | 19.9 ± 5.2 | 20.0 ± 4.6 | 21.8 ± 6.4 | 22.0 ± 4.6 | 23.2 ± 6.0 | 22.0 ± 6.2 | |
| Logical memory | Immediate recall | 5.4 ± 0.9 | 4.2 ± 1.7ab | 8.1 ± 2.0 | 8.8 ± 1.8 | 7.1 ± 2.7 | 8.4 ± 2.3 |
| Delayed recall | 4.9 ± 1.0 | 4.2 ± 1.1ab | 5.7 ± 1.7 | 7.0 ± 1.6b | 4.9 ± 1.7d | 8.9 ± 1.6 | |
| WCST | 27.0 ± 5.5 | 22.0 ± 4.8a | 27.4 ± 6.7 | 29.7 ± 7.2 | 25.8 ± 7.7 | 24.6 ± 6.6 | |
| Toulouse | Cancellation numbers | 42.5 ± 4.4 | 41.5 ± 6.9a | 44.9 ± 10.3 | 50.91 ± 10.6 | 45.2 ± 10.5 | 48.3 ± 11.0 |
| Errors | 7.8 ± 5.7 | 6.1 ± 4.6a | 9.7 ± 4.3 | 9.4 ± 2.4 | 9.2 ± 4.5 | 8.9 ± 6.3 | |
Results of the neuropsychological assessment. Data presented as the mean ± standard deviation, and an ANOVA for repeated measurements was followed by a post hoc Tukey test; significant results p ≤ 0.05
WCST Wisconsin Card Sorting Test, ROCF Rey-Osterrieth Complex Figure, MMSE Mini Mental State Exam, GDS Geriatric Depression Scale
aDiffers at 6 months from the training group
bDiffers at 6 months from the leisure group
cDiffers from the baseline conditions in the training group
dDiffers from the baseline conditions in the leisure group
The scores of the training group were significantly lower after 6 months of training (p = 0.0002); the block design test scores, which assessed spatial/visual orientation, speed of execution, perceptual integration, and capacity of planning and organization, were significantly higher after 6 months of training (p = 0.01); additionally, a low number of errors (p = 0.01) were observed in the mental control test, which assessed the retrieval of over-learned information and the ability to mentally manipulate information, compared to the baseline.
The scores of the leisure group were significantly higher, and a higher number of correct responses (p = 0.002) were observed in the mental control test, which assessed the retrieval of over-learned information and the ability to mentally manipulate information; in the logical memory test, which assessed episodic memory and verbal, short-, and long-term memory, significantly higher delayed recall scores (p = 0.02) were present in the leisure group compared to the training group and compared to the baseline (p = 0.001).
The scores of the leisure group were significantly different compared to the control group in the following areas: in the mental control test, which assessed the retrieval of over-learned information and the ability to mentally manipulate information, a low number of correct responses were observed (p = 0.002); in the digit span numbers test, which assessed sustained attention, immediate memory, and verbal working memory, higher scores for the digit forward (p = 0.002) and digit backwards (p = 0.0001) tests were observed, and in the logical memory test, which assessed episodic memory and verbal, short-, and long-term memory, significantly higher scores were present regarding immediate recall (p = 0.03) and delayed recall (p = 0.04) in the leisure group.
The scores in the letter fluency test of the leisure group were significantly higher after 6 months of training compared to the baseline (p = 0.001); the immediate recall of the ROCF test scores, which assessed visuospatial constructional ability, visual memory, and learning, was also significantly different (p = 0.01).
Figure 1 shows the comparison of the results of the VO2 peak in relation to the control, training, and leisure groups. The comparison between the groups showed a significant difference (p = 0.0000). The post hoc test showed a significant increase in the training group compared to the leisure group (p = 0.0001) and the control group (p = 0.002).
Fig. 1.
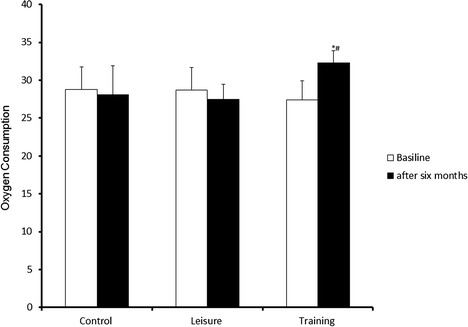
Comparison between the training, leisure, and control groups with respect to oxygen consumption. *P < 0.05. ANOVA followed by a post hoc Tukey test, with values expressed as the mean ± SD. *Differs between training vs control; #differs between training vs leisure
Figure 2 shows positive correlations between VO2 max and neuropsychological functions in the training group. The following positive correlations were observed between VO2 and scores in the training group for the digit symbol test—sustained attention, response speed, and visuomotor coordination (r = 0.75; p = 0.0001) and for the ROCF test—visuospatial constructional ability, visual memory, and learning, which were assessed by the copy, immediate recall, and delayed recall tests: (r = 0.42; p = 0.004) and (r = 0.54; p = 0.002) and (r = 0.42; p = 0.0003), respectively. No significant correlations were observed in the other groups (control or leisure; p > 0.05).
Fig. 2.
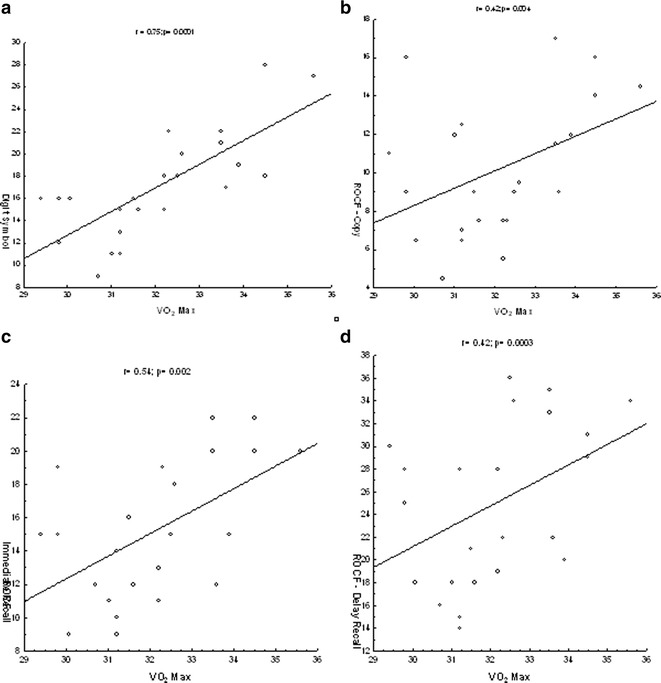
Correlations between the VO2 max and neuropsychological functions. a Positive correlation between the VO2 max and digit symbol test; b positive correlation between the VO2 max and ROCF test for copy; c positive correlation between the VO2 max and ROCF test for immediate recall; and d positive correlation between the VO2 max and ROCF test for recall delay
Discussion
The findings from this study indicate that there is an association between neuropsychological functions and physical exercise training in older adults, which suggests that their neuropsychological status is associated with an increase in aerobic fitness. These findings are compatible with several perspectives that concern the environmental influences of physical exercise behavior on cognition (Van Uffelen et al. 2008).
In the present study, we applied a program of walking as physical exercise at a ventilatory threshold 1 (VT-1) intensity for 6 months, and we observed an improvement in neuropsychological functioning and a reduction in depression symptoms in the training group compared to the control and leisure groups. In general, the training group improved more in their mental control, sustained attention, immediate memory, verbal working memory, response speed, visuomotor coordination, visuospatial constructional ability, visual memory and learning, episodic memory, short- and long-term memory, executive functioning, reaction speed, and accuracy in executing simple tasks compared to the other groups. These results are in agreement with the findings of several reports of improved neuropsychological functioning in individuals who participate in regular physical exercise, and they suggest that a program that constitutes physical exercise could be used to improve neuropsychological aspects in older adults (Etnier et al. 2006).
It is speculated that some mechanisms could explain the improvement in neuropsychological aspects that were observed in the present study, such as the social interaction and increased cerebral blood flow that is dependent on the intensity of exercise (Seeman et al. 2011; Fisher et al. 2013; Endo et al. 2013). Furthermore, it is possible that physical exercise has contributed to neurotransmitter release, which improves neuropsychological performance (Rogers et al. 1990).
We also observed that neuropsychological functioning, including mental control, episodic memory, verbal memory, and short- and long-term memory, was influenced by leisure activity. Epidemiological evidence has suggested that a lifestyle that is characterized by engagement in leisure activities of an intellectual and social nature is associated with slower neuropsychological decline in healthy older adults and could reduce the risk of dementia (Scarmeas et al. 2003). In our study, leisure activity was composed of recreation and leisure activities, specifically dance and handicraft work. It is possible that the stimulus applied by leisure activities was not sufficient to promote major changes in aerobic fitness that could contribute to neuropsychological function enhancements in a similar manner as physical training.
A study has shown that the average intensity of weekly physical exercise is significantly associated with cognitive performance, particularly processing speed, memory, mental flexibility, and performance in overall cognitive function. In this sense, the data of the present study corroborate other findings that show an association between intense physical exercise and neuropsychological functions including sustained attention, response speed, visuomotor coordination, orientation, immediate memory, visual memory, language, visuospatial constructional ability, and mental control (Angevaren et al. 2007).
Furthermore, it is very important to emphasize that for older adults, leisure activities can be posed as an informal educational alternative that assists in sociocultural development regardless of age or social class and contributes to fostering new skills and more social interaction. Leisure activity is an instrument that favors collective and individual well-being, and one of its main characteristics is the freedom of choice among options and the pursuit of the individual’s own interests, with the aim of improving his or her personal satisfaction and quality of life (Toepoel 2013).
However, we observed in the present study that regular physical exercise improves several neuropsychological functions. These findings are similar to reports by some authors that show improvements in neuropsychological functioning in individuals who are involved in this type of activity (De Mello et al. 2013). A number of hypotheses have attempted to explain the mechanisms that can influence neuropsychological functions in individuals who are involved in regular physical exercise. Some studies have suggested that physical training can increase cerebral blood flow, which improves neuropsychological functioning (Vicente-Campos et al. 2012; Birdsill et al. 2013). This influence can be explained by the increase in cerebral blood flow in a specific brain area: the left inferior frontal gyrus (Majerus 2013). This area is associated with several neuropsychological functions, including executive functions, memory, language processing, attention, and others (Wright et al. 2011).
Furthermore, it is important to emphasize that spontaneous slow oscillations occur in cerebral hemodynamics and blood pressure and may reflect neurogenic, metabolic, or myogenic control of the cerebral vasculature. Aging is accompanied by a degeneration of the vascular system, which may have consequences for regional cerebral blood flow and cognitive performance. This degeneration may be reflected by a reduction of spontaneous slow oscillations of cerebral hemodynamics and blood pressure (Vermeij et al. 2014)
In addition to the hypothesis of cerebral blood flow, other studies have suggested that the hematoencephalic barrier becomes permeable to the action of catecholamines during physical exercise, which influences brain metabolism (De Bruin et al. 1990). However, it appears unlikely that these neuromodulator events alone caused the cognitive improvement that was observed in the present study. Diminished scores on the GDS and improved oxygen use might also help explain the overall cognitive improvement of the training group. Regular physical exercise leads to biochemical alterations that involve the release of serotonin and the activation of specific receptors (Beckman and Santos 2013).
Another hypothesis that is related to these alterations is that during physical exercise, the distribution of tryptophan, which is a precursor amino acid for serotonin, is altered by lipolysis, which causes a growing concentration of free fatty acids in the plasma, dislocating tryptophan from albumin-binding sites, and thus raising the levels of free tryptophan that is responsible for synthesizing serotonin (Badawy 2013). There could also be an increase in the reception and oxidation of branched-chain amino acids by the muscles during exercise, and as a result, the bloodstream concentration of these amino acids could be reduced. This whole process can stimulate the brain’s capacity for the reception of free tryptophan and boost both the synthesis and central release of serotonin. Free tryptophan can compete with branched-chain amino acids for the transporter to pass through the hematoencephalic barrier (Melancon et al. 2012).
A limitation of this study was the convenience sample of the group leisure. However, it was possible to observe associations between physical training, leisure activities, and neuropsychological functions, enabling the establishment of our hypothesis. It was possible that the leisure group did not improve in the same manner as the physical exercise group because of a possible ceiling effect from being previously involved in the leisure activities for at least 1 year.
In conclusion, these data suggest that physical exercise improves neuropsychological functions although leisure activities may also improve these functions. Thus, an aerobic physical fitness program can contribute, in part, as a non-medication alternative for maintaining and improving these functions in older adults; however, leisure activities must be considered and not overlooked.
Acknowledgments
All the authors are grateful to the Fundação de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) and the Centro de Estudos em Psicobiologia e Exercício (CEPE).
Conflict of interest
The authors report no conflicts of interest in this work.
References
- ACSM: American College of Sports Medicine, Preventive and Rehabilitative Exercise Committee . Guidelines for exercise testing and prescription. Philadelphia: Lea & Feibiger; 1995. [Google Scholar]
- ACSM: American College of Sports Medicine . In: Guidelines for exercise testing and prescription. Whaley MH, Brubaker PH, Otto RM, editors. Philadelphia: Lea and Febiger; 2006. [Google Scholar]
- Akbaraly TN, Portet F, Fustinoni S, Dartigues JF, Artero S, Rouaud O, Touchon J, Ritchie K, Berr C. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73(11):854–861. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Vanhees L, Wendel-Vos W, Verhaar HJ, Aufdemkampe G, Aleman A, Verschuren WM. Intensity, but not duration, of physical activities is related to cognitive function. Eur Cardiovasc Prev Rehabil. 2007;14(6):825–830. doi: 10.1097/HJR.0b013e3282ef995b. [DOI] [PubMed] [Google Scholar]
- Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology. 2000;46(3):163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- Badawy AA. Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J Psychopharmacol. 2013;27(10):878–893. doi: 10.1177/0269881113499209. [DOI] [PubMed] [Google Scholar]
- Beckman D, Santos LE. The importance of serotonin in exercise-induced adult neurogenesis: new evidence from Tph2-/- mice. J Neurosci. 2013;33(36):14283–14284. doi: 10.1523/JNEUROSCI.2911-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsill AC, Carlsson CM, Willette AA, Okonkwo OC, Johnson SC, Xu G, Oh JM, Gallagher CL, Koscik RL, Jonaitis EM, Hermann BP, LaRue A, Rowley HA, Asthana S, Sager MA, Bendlin BB. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring) 2013;21(7):1313–1320. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- De Bruin LA, Schasfoort EM, Steffens AB, Korf J. Effects of stress and exercise on rat hippocampus and striatum extracellular lactate. Am J Physiol. 1990;259(4 Pt 2):R773–R779. doi: 10.1152/ajpregu.1990.259.4.R773. [DOI] [PubMed] [Google Scholar]
- De Mello MT, Lemos Vde A, Antunes HK, Bittencourt L, Santos-Silva R, Tufik S. Relationship between physical activity and depression and anxiety symptoms: a population study. J Affect Disord. 2013;149(1-3):241–246. doi: 10.1016/j.jad.2013.01.035. [DOI] [PubMed] [Google Scholar]
- Denkinger MD, Nikolaus T, Denkinger C, Lukas A. Physical activity for the prevention of cognitive decline: current evidence from observational and controlled studies. Z Gerontol Geriatr. 2012;45(1):11–16. doi: 10.1007/s00391-011-0262-6. [DOI] [PubMed] [Google Scholar]
- Endo K, Matsukawa K, Liang N, Nakatsuka C, Tsuchimochi H, Okamura H, Hamaoka T. Dynamic exercise improves cognitive function in association with increased prefrontal oxygenation. J Physiol Sci. 2013;63(4):287–298. doi: 10.1007/s12576-013-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52(1):119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Hartwich D, Seifert T, Olesen ND, McNulty CL, Nielsen HB, van Lieshout JJ, Secher NH. Cerebral perfusion, oxygenation and metabolism during exercise in young and elderly individuals. J Physiol. 2013;591(Pt 7):1859–1870. doi: 10.1113/jphysiol.2012.244905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Exercising your brain: a review of human brain plasticity and training-induced learning. Psychol Aging. 2008;23(4):692–701. doi: 10.1037/a0014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39(1):3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14(2):245–263. doi: 10.1037/0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, Dimeo FC. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2010;65(6):680–688. doi: 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- Kravitz E, Schmeidler J, Beeri MS. Cognitive decline and dementia in the oldest-old. Rambam Maimonides Med J. 2012;3(4):e0026. doi: 10.5041/RMMJ.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacza G, Radák Z. Is physical activity an elixir? Orv Hetil. 2013;154(20):764–768. doi: 10.1556/OH.2013.29616. [DOI] [PubMed] [Google Scholar]
- Langlois F, Vu TT, Chassé K, Dupuis G, Kergoat MJ, Bherer L. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68(3):400–404. doi: 10.1093/geronb/gbs069. [DOI] [PubMed] [Google Scholar]
- Lesak MD. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Majerus S. Language repetition and short-term memory: an integrative framework. Front Hum Neurosci. 2013;7:357. doi: 10.3389/fnhum.2013.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon MO, Lorrain D, Dionne IJ. Exercise increases tryptophan availability to the brain in older men age 57-70 years. Med Sci Sports Exerc. 2012;44(5):881–887. doi: 10.1249/MSS.0b013e31823ede8e. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marshuetz C. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues Clin Neurosci. 2001;3(3):151–165. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainho O. Tests of specific aptitudes. In Manual of psychology applied. Rio de Janeiro: CEPA; 1992. [Google Scholar]
- Raven J. Standard progressive matrices. London: HK Lewis; 1958. [Google Scholar]
- Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38(2):123–128. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, Marder KS, Bell KL, Sackeim HA, Van Heertum RL, Moeller JR, Stern Y. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60(3):359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57(12):2236–2242. doi: 10.1212/WNL.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Miller-Martinez DM, Stein Merkin S, Lachman ME, Tun PA, Karlamangla AS. Histories of social engagement and adult cognition: midlife in the U.S. study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i141–i152. doi: 10.1093/geronb/gbq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinfeld S, Figueroa H, Ortiz-Gil J, Sanchez-Vives MV. Effects of music learning and piano practice on cognitive function, mood and quality of life in older adults. Front Psychol. 2013;4:810. doi: 10.3389/fpsyg.2013.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, Macchi C. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University Press; 1991. [Google Scholar]
- Toepoel V. Ageing, leisure, and social connectedness: how could leisure help reduce social isolation of older people? Soc Indic Res. 2013;113(1):355–372. doi: 10.1007/s11205-012-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uffelen JG, Chin A, Paw MJ, Hopman-Rock M, van Mechelen W. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin J Sport Med. 2008;18(6):486–500. doi: 10.1097/JSM.0b013e3181845f0b. [DOI] [PubMed] [Google Scholar]
- Vaughan S, Morris N, Shum D, O’Dwyer S, Polit D. Study protocol: a randomised controlled trial of the effects of a multi-modal exercise program on cognition and physical functioning in older women. BMC Geriatr. 2012;12:60. doi: 10.1186/1471-2318-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Ambrose AF, Sliwinski M, Buschke H. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Vermeij A, Meel-van den Abeelen AS, Kessels RP, van Beek AH, Claassen JA. Very-low-frequency oscillations of cerebral hemodynamics and blood pressure are affected by aging and cognitive load. Neuroimage. 2014;85(Pt 1):608–615. doi: 10.1016/j.neuroimage.2013.04.107. [DOI] [PubMed] [Google Scholar]
- Vicente-Campos D, Mora J, Castro-Piñero J, González-Montesinos JL, Conde-Caveda J, Chicharro JL. Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J Sports Med Phys Fitness. 2012;52(5):537–544. [PubMed] [Google Scholar]
- Wang HX, Jin Y, Hendrie HC, Liang C, Yang L, Cheng Y, Unverzagt FW, Ma F, Hall KS, Murrell JR, Li P, Bian J, Pei JJ, Gao S. Late life leisure activities and risk of cognitive decline. J Gerontol A Biol Sci Med Sci. 2013;68(2):205–213. doi: 10.1093/gerona/gls153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R: manual for the Wechsler Adult Intelligence Scale—revised. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler memory scale. San Antonio: Psychological Corp; 1997. [Google Scholar]
- Wright P, Randall B, Marslen-Wilson WD, Tyler LK. Dissociating linguistic and task-related activity in the left inferior frontal gyrus. J Cogn Neurosci. 2011;23(2):404–413. doi: 10.1162/jocn.2010.21450. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatry Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yu F, Ryan LH, Schaie KW, Willis SL, Kolanowski A. Factors associated with cognition in adults: the Seattle Longitudinal Study. Res Nurs Health. 2000;32(5):540–550. doi: 10.1002/nur.20340. [DOI] [PMC free article] [PubMed] [Google Scholar]


