Abstract
The preprotein translocase of the outer mitochondrial membrane is a multi-subunit complex with receptors and a general import pore. We report the molecular identification of Tom7, a small subunit of the translocase that behaves as an integral membrane protein. The deletion of TOM7 inhibited the mitochondrial import of the outer membrane protein porin, whereas the import of preproteins destined for the mitochondrial interior was impaired only slightly. However, protein import into the mitochondrial interior was strongly inhibited when it occurred in two steps: preprotein accumulation at the outer membrane in the absence of a membrane potential and subsequent further import after the re-establishment of a membrane potential. The delay of protein import into tom7delta mitochondria seemed to occur after the binding of preproteins to the outer membrane receptor sites. A lack of Tom7 stabilized the interaction between the receptors Tom20 and Tom22 and the import pore component Tom40. This indicated that Tom7 exerts a destabilizing effect on part of the outer membrane translocase, whereas Tom6 stabilizes the interaction between the receptors and the import pore. Synthetic growth defects of the double mutants tom7delta tom20delta and tom7delta tom6delta provided genetic evidence for the functional relationship of Tom7 with Tom20 and Tom6. These results suggest that (i) Tom7 plays a role in sorting and accumulation of the preproteins at the outer membrane, and (ii) Tom7 and Tom6 perform complementary functions in modulating the dynamics of the outer membrane translocase.
Full text
PDF
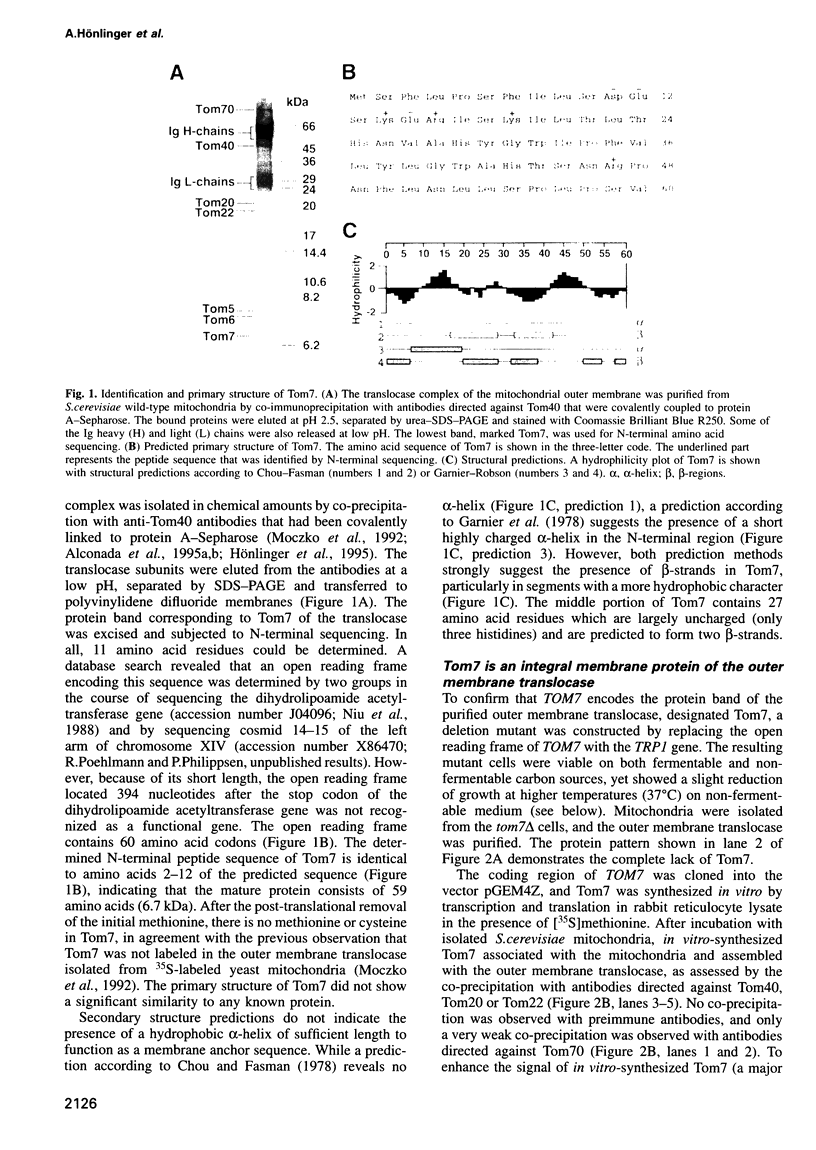
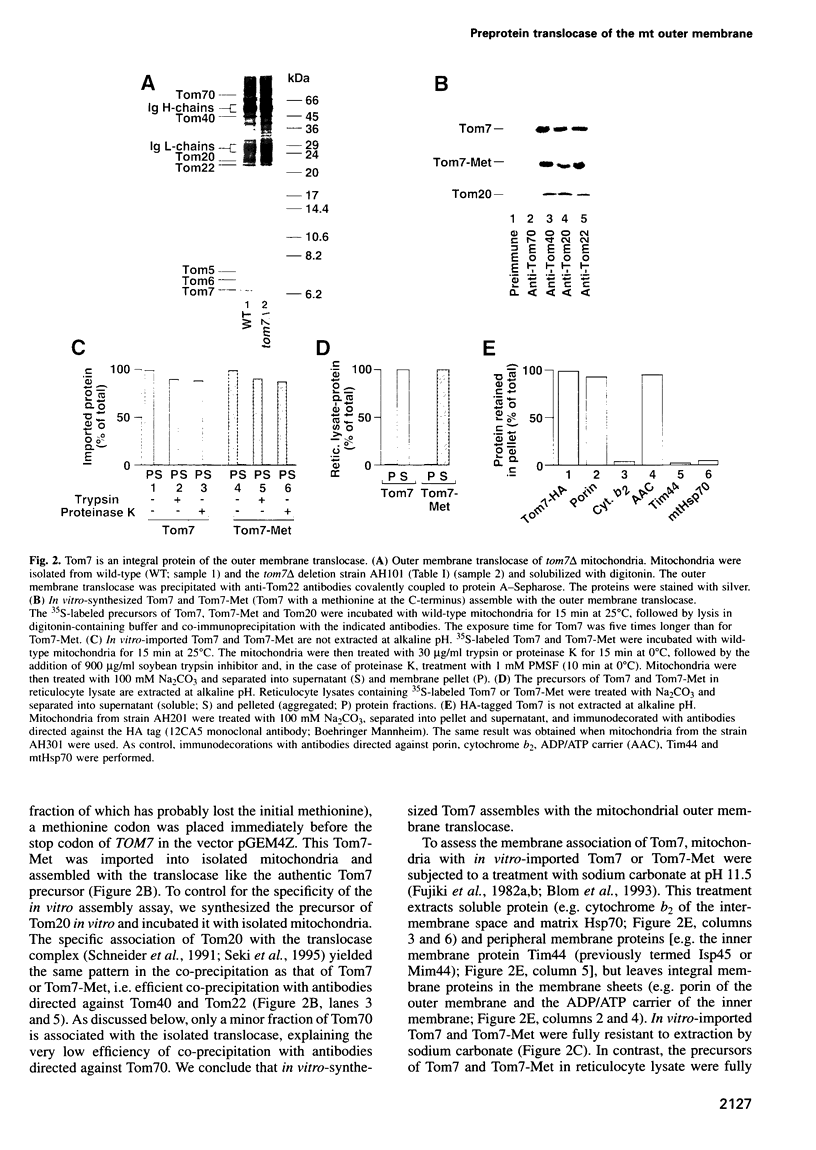
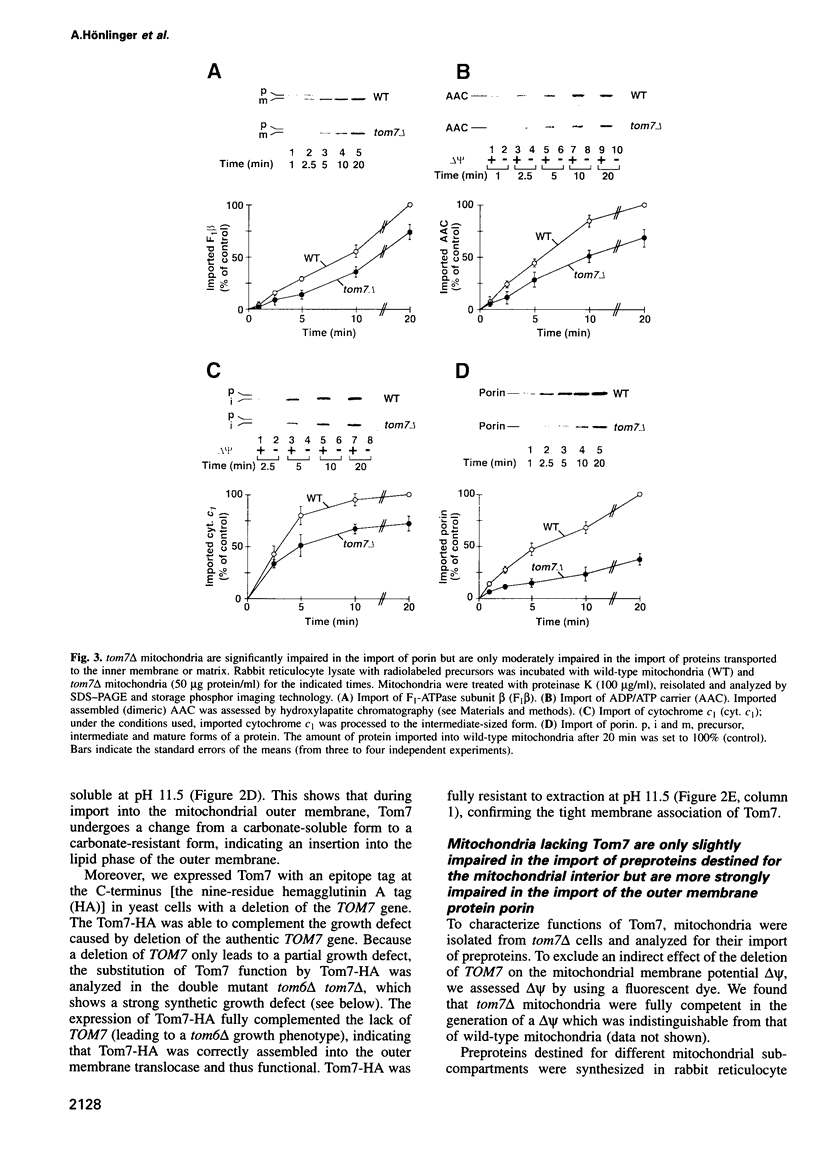
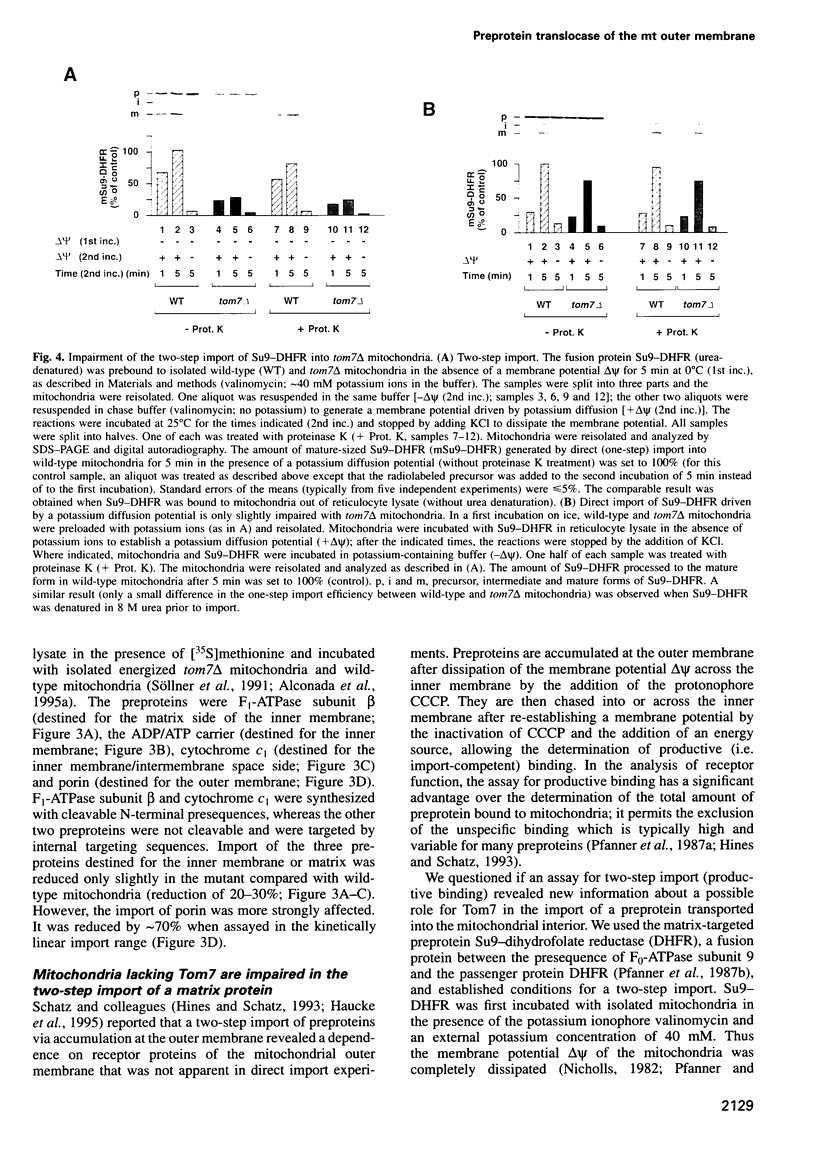

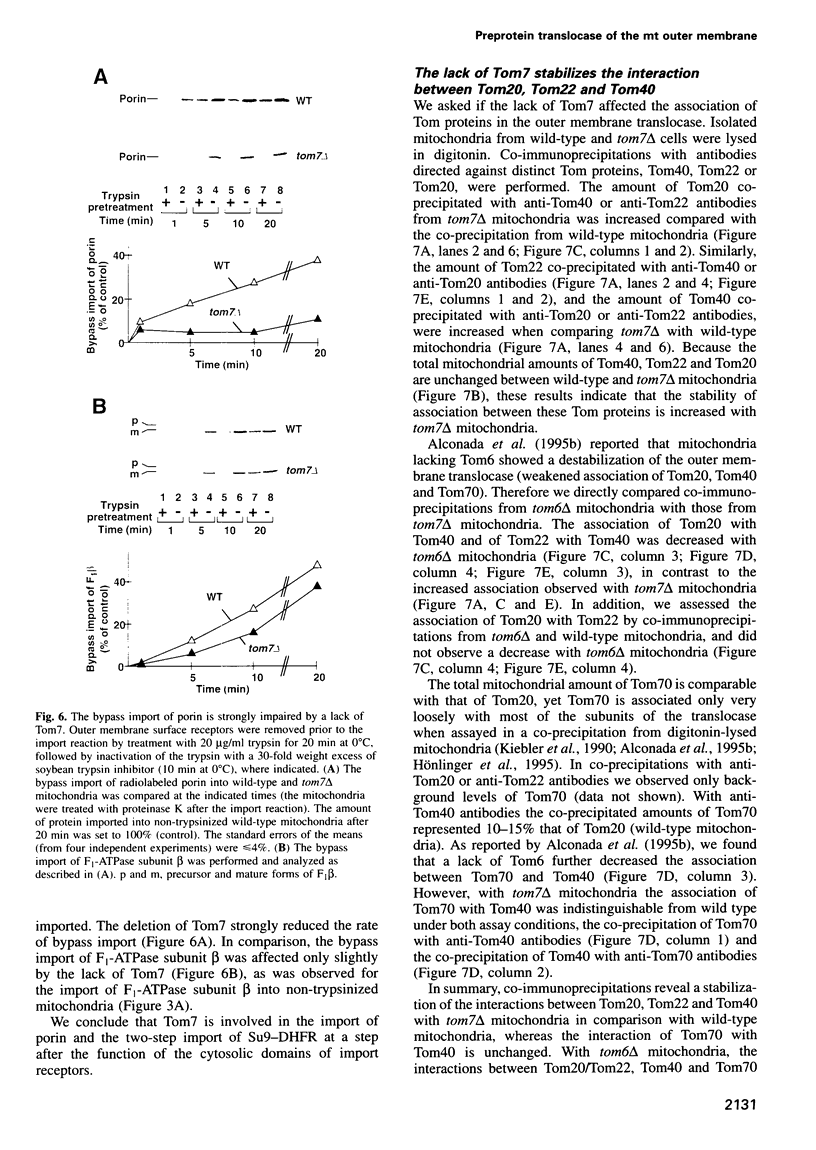





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alconada A., Gärtner F., Hönlinger A., Kübrich M., Pfanner N. Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 1995;260:263–286. doi: 10.1016/0076-6879(95)60144-9. [DOI] [PubMed] [Google Scholar]
- Alconada A., Kübrich M., Moczko M., Hönlinger A., Pfanner N. The mitochondrial receptor complex: the small subunit Mom8b/Isp6 supports association of receptors with the general insertion pore and transfer of preproteins. Mol Cell Biol. 1995 Nov;15(11):6196–6205. doi: 10.1128/mcb.15.11.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Berthold J., Bauer M. F., Schneider H. C., Klaus C., Dietmeier K., Neupert W., Brunner M. The MIM complex mediates preprotein translocation across the mitochondrial inner membrane and couples it to the mt-Hsp70/ATP driving system. Cell. 1995 Jun 30;81(7):1085–1093. doi: 10.1016/s0092-8674(05)80013-3. [DOI] [PubMed] [Google Scholar]
- Blom J., Kübrich M., Rassow J., Voos W., Dekker P. J., Maarse A. C., Meijer M., Pfanner N. The essential yeast protein MIM44 (encoded by MPI1) is involved in an early step of preprotein translocation across the mitochondrial inner membrane. Mol Cell Biol. 1993 Dec;13(12):7364–7371. doi: 10.1128/mcb.13.12.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J. L., Goeckeler J., Schekman R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9643–9646. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Douglas M. G. Biogenesis of ISP6, a small carboxyl-terminal anchored protein of the receptor complex of the mitochondrial outer membrane. J Biol Chem. 1995 Mar 10;270(10):5674–5679. doi: 10.1074/jbc.270.10.5674. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Eilers M., Oppliger W., Schatz G. Both ATP and an energized inner membrane are required to import a purified precursor protein into mitochondria. EMBO J. 1987 Apr;6(4):1073–1077. doi: 10.1002/j.1460-2075.1987.tb04860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Fowler S., Shio H., Hubbard A. L., Lazarow P. B. Polypeptide and phospholipid composition of the membrane of rat liver peroxisomes: comparison with endoplasmic reticulum and mitochondrial membranes. J Cell Biol. 1982 Apr;93(1):103–110. doi: 10.1083/jcb.93.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambill B. D., Voos W., Kang P. J., Miao B., Langer T., Craig E. A., Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993 Oct;123(1):109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gratzer S., Lithgow T., Bauer R. E., Lamping E., Paltauf F., Kohlwein S. D., Haucke V., Junne T., Schatz G., Horst M. Mas37p, a novel receptor subunit for protein import into mitochondria. J Cell Biol. 1995 Apr;129(1):25–34. doi: 10.1083/jcb.129.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner F., Bömer U., Guiard B., Pfanner N. The sorting signal of cytochrome b2 promotes early divergence from the general mitochondrial import pathway and restricts the unfoldase activity of matrix Hsp70. EMBO J. 1995 Dec 1;14(23):6043–6057. doi: 10.1002/j.1460-2075.1995.tb00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner F., Voos W., Querol A., Miller B. R., Craig E. A., Cumsky M. G., Pfanner N. Mitochondrial import of subunit Va of cytochrome c oxidase characterized with yeast mutants. J Biol Chem. 1995 Feb 24;270(8):3788–3795. doi: 10.1074/jbc.270.8.3788. [DOI] [PubMed] [Google Scholar]
- Hachiya N., Mihara K., Suda K., Horst M., Schatz G., Lithgow T. Reconstitution of the initial steps of mitochondrial protein import. Nature. 1995 Aug 24;376(6542):705–709. doi: 10.1038/376705a0. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Ostermann J., Guiard B., Neupert W. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell. 1987 Dec 24;51(6):1027–1037. doi: 10.1016/0092-8674(87)90589-7. [DOI] [PubMed] [Google Scholar]
- Haucke V., Lithgow T., Rospert S., Hahne K., Schatz G. The yeast mitochondrial protein import receptor Mas20p binds precursor proteins through electrostatic interaction with the positively charged presequence. J Biol Chem. 1995 Mar 10;270(10):5565–5570. doi: 10.1074/jbc.270.10.5565. [DOI] [PubMed] [Google Scholar]
- Hines V., Brandt A., Griffiths G., Horstmann H., Brütsch H., Schatz G. Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS70. EMBO J. 1990 Oct;9(10):3191–3200. doi: 10.1002/j.1460-2075.1990.tb07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines V., Schatz G. Precursor binding to yeast mitochondria. A general role for the outer membrane protein Mas70p. J Biol Chem. 1993 Jan 5;268(1):449–454. [PubMed] [Google Scholar]
- Horst M., Hilfiker-Rothenfluh S., Oppliger W., Schatz G. Dynamic interaction of the protein translocation systems in the inner and outer membranes of yeast mitochondria. EMBO J. 1995 May 15;14(10):2293–2297. doi: 10.1002/j.1460-2075.1995.tb07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker T. C., Hoyt M. A., Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. doi: 10.1146/annurev.ge.21.120187.001355. [DOI] [PubMed] [Google Scholar]
- Hönlinger A., Kübrich M., Moczko M., Gärtner F., Mallet L., Bussereau F., Eckerskorn C., Lottspeich F., Dietmeier K., Jacquet M. The mitochondrial receptor complex: Mom22 is essential for cell viability and directly interacts with preproteins. Mol Cell Biol. 1995 Jun;15(6):3382–3389. doi: 10.1128/mcb.15.6.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990 May 18;61(4):723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kassenbrock C. K., Cao W., Douglas M. G. Genetic and biochemical characterization of ISP6, a small mitochondrial outer membrane protein associated with the protein translocation complex. EMBO J. 1993 Aug;12(8):3023–3034. doi: 10.1002/j.1460-2075.1993.tb05971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler M., Keil P., Schneider H., van der Klei I. J., Pfanner N., Neupert W. The mitochondrial receptor complex: a central role of MOM22 in mediating preprotein transfer from receptors to the general insertion pore. Cell. 1993 Aug 13;74(3):483–492. doi: 10.1016/0092-8674(93)80050-o. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Pfaller R., Söllner T., Griffiths G., Horstmann H., Pfanner N., Neupert W. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature. 1990 Dec 13;348(6302):610–616. doi: 10.1038/348610a0. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Kronidou N. G., Oppliger W., Bolliger L., Hannavy K., Glick B. S., Schatz G., Horst M. Dynamic interaction between Isp45 and mitochondrial hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12818–12822. doi: 10.1073/pnas.91.26.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübrich M., Dietmeier K., Pfanner N. Genetic and biochemical dissection of the mitochondrial protein-import machinery. Curr Genet. 1995 Apr;27(5):393–403. doi: 10.1007/BF00311207. [DOI] [PubMed] [Google Scholar]
- Lithgow T., Glick B. S., Schatz G. The protein import receptor of mitochondria. Trends Biochem Sci. 1995 Mar;20(3):98–101. doi: 10.1016/s0968-0004(00)88972-0. [DOI] [PubMed] [Google Scholar]
- Lithgow T., Junne T., Suda K., Gratzer S., Schatz G. The mitochondrial outer membrane protein Mas22p is essential for protein import and viability of yeast. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11973–11977. doi: 10.1073/pnas.91.25.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Mahlke K., Pfanner N. Role of an energized inner membrane in mitochondrial protein import. Delta psi drives the movement of presequences. J Biol Chem. 1991 Sep 25;266(27):18051–18057. [PubMed] [Google Scholar]
- Mayer A., Lill R., Neupert W. Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J Cell Biol. 1993 Jun;121(6):1233–1243. doi: 10.1083/jcb.121.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Nargang F. E., Neupert W., Lill R. MOM22 is a receptor for mitochondrial targeting sequences and cooperates with MOM19. EMBO J. 1995 Sep 1;14(17):4204–4211. doi: 10.1002/j.1460-2075.1995.tb00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko M., Dietmeier K., Söllner T., Segui B., Steger H. F., Neupert W., Pfanner N. Identification of the mitochondrial receptor complex in Saccharomyces cerevisiae. FEBS Lett. 1992 Oct 5;310(3):265–268. doi: 10.1016/0014-5793(92)81345-m. [DOI] [PubMed] [Google Scholar]
- Moczko M., Ehmann B., Gärtner F., Hönlinger A., Schäfer E., Pfanner N. Deletion of the receptor MOM19 strongly impairs import of cleavable preproteins into Saccharomyces cerevisiae mitochondria. J Biol Chem. 1994 Mar 25;269(12):9045–9051. [PubMed] [Google Scholar]
- Moczko M., Gärtner F., Pfanner N. The protein import receptor MOM19 of yeast mitochondria. FEBS Lett. 1993 Jul 12;326(1-3):251–254. doi: 10.1016/0014-5793(93)81801-6. [DOI] [PubMed] [Google Scholar]
- Niu X. D., Browning K. S., Behal R. H., Reed L. J. Cloning and nucleotide sequence of the gene for dihydrolipoamide acetyltransferase from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7546–7550. doi: 10.1073/pnas.85.20.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Panzner S., Dreier L., Hartmann E., Kostka S., Rapoport T. A. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995 May 19;81(4):561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Pfaller R., Pfanner N., Neupert W. Mitochondrial protein import. Bypass of proteinaceous surface receptors can occur with low specificity and efficiency. J Biol Chem. 1989 Jan 5;264(1):34–39. [PubMed] [Google Scholar]
- Pfaller R., Steger H. F., Rassow J., Pfanner N., Neupert W. Import pathways of precursor proteins into mitochondria: multiple receptor sites are followed by a common membrane insertion site. J Cell Biol. 1988 Dec;107(6 Pt 2):2483–2490. doi: 10.1083/jcb.107.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Douglas M. G., Endo T., Hoogenraad N. J., Jensen R. E., Meijer M., Neupert W., Schatz G., Schmitz U. K., Shore G. C. Uniform nomenclature for the protein transport machinery of the mitochondrial membranes. Trends Biochem Sci. 1996 Feb;21(2):51–52. [PubMed] [Google Scholar]
- Pfanner N., Müller H. K., Harmey M. A., Neupert W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in the binding to receptor sites. EMBO J. 1987 Nov;6(11):3449–3454. doi: 10.1002/j.1460-2075.1987.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Distinct steps in the import of ADP/ATP carrier into mitochondria. J Biol Chem. 1987 Jun 5;262(16):7528–7536. [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J. 1985 Nov;4(11):2819–2825. doi: 10.1002/j.1460-2075.1985.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Söllner T., Neupert W. Mitochondrial import receptors for precursor proteins. Trends Biochem Sci. 1991 Feb;16(2):63–67. doi: 10.1016/0968-0004(91)90026-r. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Ramage L., Junne T., Hahne K., Lithgow T., Schatz G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 1993 Nov;12(11):4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J., Maarse A. C., Krainer E., Kübrich M., Müller H., Meijer M., Craig E. A., Pfanner N. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994 Dec;127(6 Pt 1):1547–1556. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. R., Jensen R. E. Protein translocation across mitochondrial membranes: what a long, strange trip it is. Cell. 1995 Nov 17;83(4):517–519. doi: 10.1016/0092-8674(95)90089-6. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of ADP/ATP carrier into mitochondria. Precursor imported in vitro acquires functional properties of the mature protein. J Biol Chem. 1984 Mar 25;259(6):3487–3491. [PubMed] [Google Scholar]
- Schlossmann J., Dietmeier K., Pfanner N., Neupert W. Specific recognition of mitochondrial preproteins by the cytosolic domain of the import receptor MOM72. J Biol Chem. 1994 Apr 22;269(16):11893–11901. [PubMed] [Google Scholar]
- Schneider H., Söllner T., Dietmeier K., Eckerskorn C., Lottspeich F., Trülzsch B., Neupert W., Pfanner N. Targeting of the master receptor MOM19 to mitochondria. Science. 1991 Dec 13;254(5038):1659–1662. doi: 10.1126/science.1661031. [DOI] [PubMed] [Google Scholar]
- Schnell D. J., Kessler F., Blobel G. Isolation of components of the chloroplast protein import machinery. Science. 1994 Nov 11;266(5187):1007–1012. doi: 10.1126/science.7973649. [DOI] [PubMed] [Google Scholar]
- Seki N., Moczko M., Nagase T., Zufall N., Ehmann B., Dietmeier K., Schäfer E., Nomura N., Pfanner N. A human homolog of the mitochondrial protein import receptor Mom19 can assemble with the yeast mitochondrial receptor complex. FEBS Lett. 1995 Nov 20;375(3):307–310. doi: 10.1016/0014-5793(95)01229-8. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Steger H. F., Söllner T., Kiebler M., Dietmeier K. A., Pfaller R., Trülzsch K. S., Tropschug M., Neupert W., Pfanner N. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J Cell Biol. 1990 Dec;111(6 Pt 1):2353–2363. doi: 10.1083/jcb.111.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Griffiths G., Pfaller R., Pfanner N., Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989 Dec 22;59(6):1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Wiedmann M., Schlossmann J., Keil P., Neupert W., Pfanner N. Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature. 1992 Jan 2;355(6355):84–87. doi: 10.1038/355084a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Brunner J., Baker A., Schatz G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989 Sep 21;341(6239):205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Voos W., Gambill B. D., Laloraya S., Ang D., Craig E. A., Pfanner N. Mitochondrial GrpE is present in a complex with hsp70 and preproteins in transit across membranes. Mol Cell Biol. 1994 Oct;14(10):6627–6634. doi: 10.1128/mcb.14.10.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wickner W. T. How ATP drives proteins across membranes. Science. 1994 Nov 18;266(5188):1197–1198. doi: 10.1126/science.7973701. [DOI] [PubMed] [Google Scholar]